Use of Autotransfusion following Percutaneous Thrombectomy for Cardiogenic Shock Due to Pulmonary Embolism in a Single Session—A Case Report
Abstract
:1. Introduction
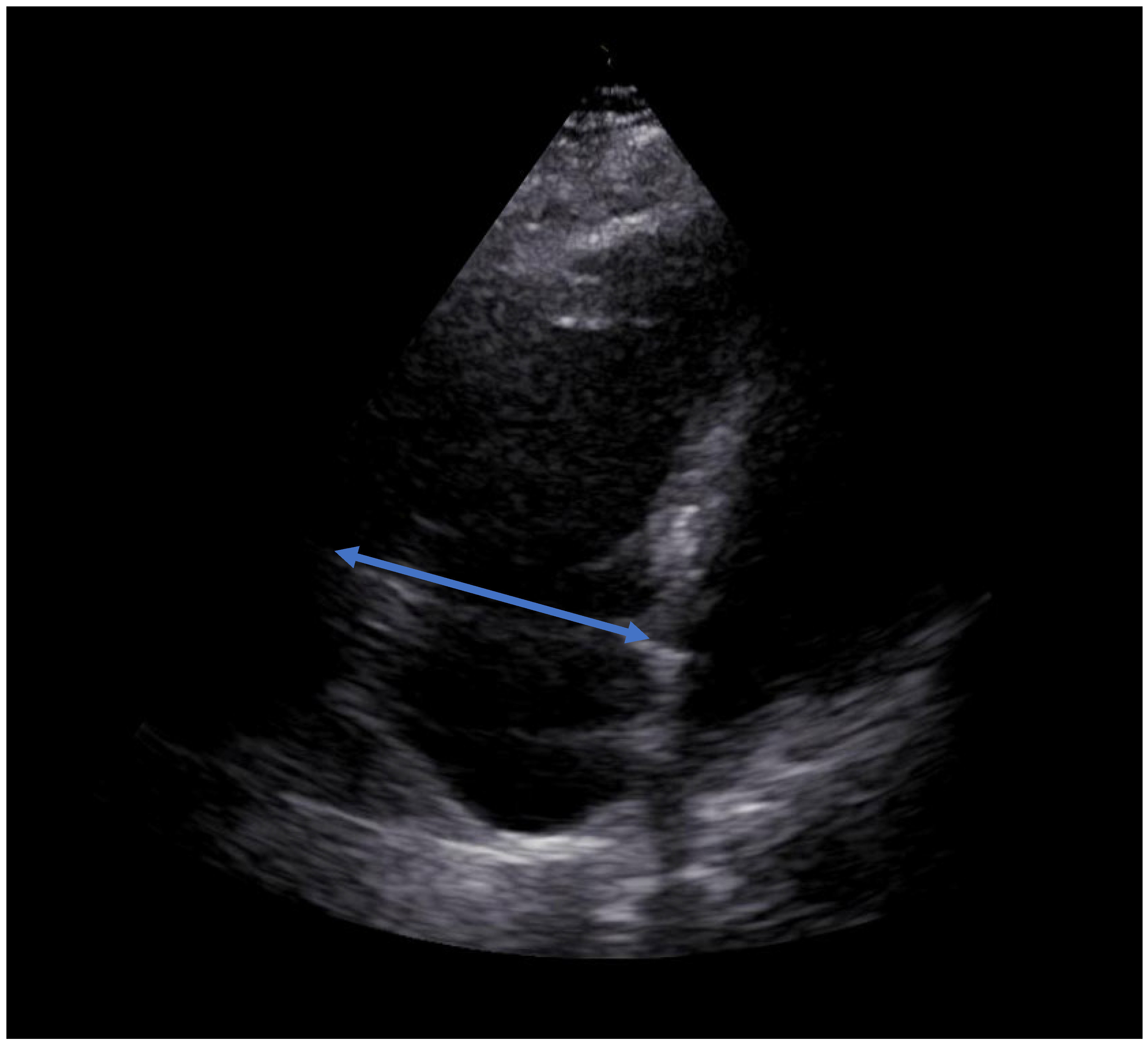
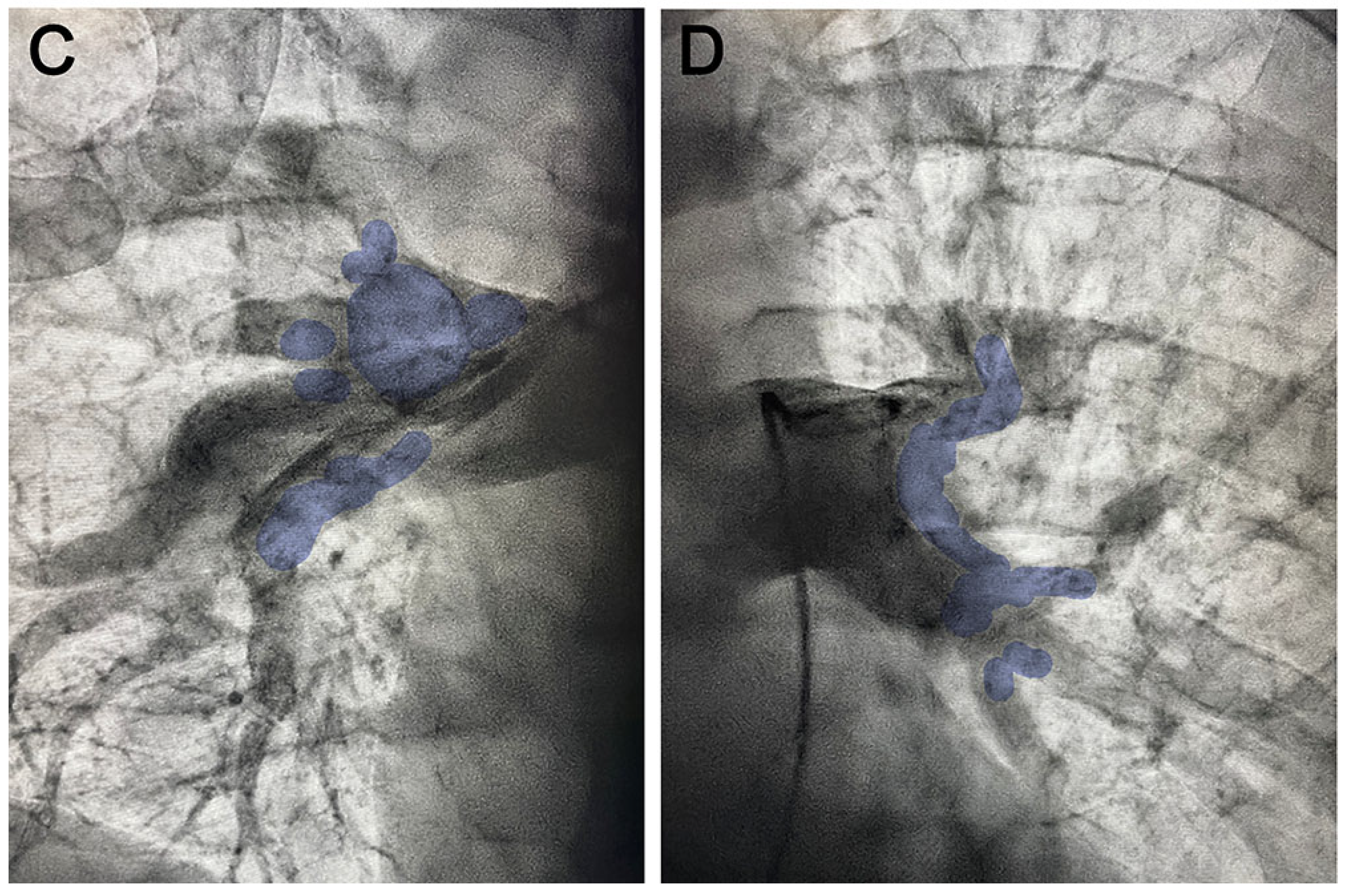
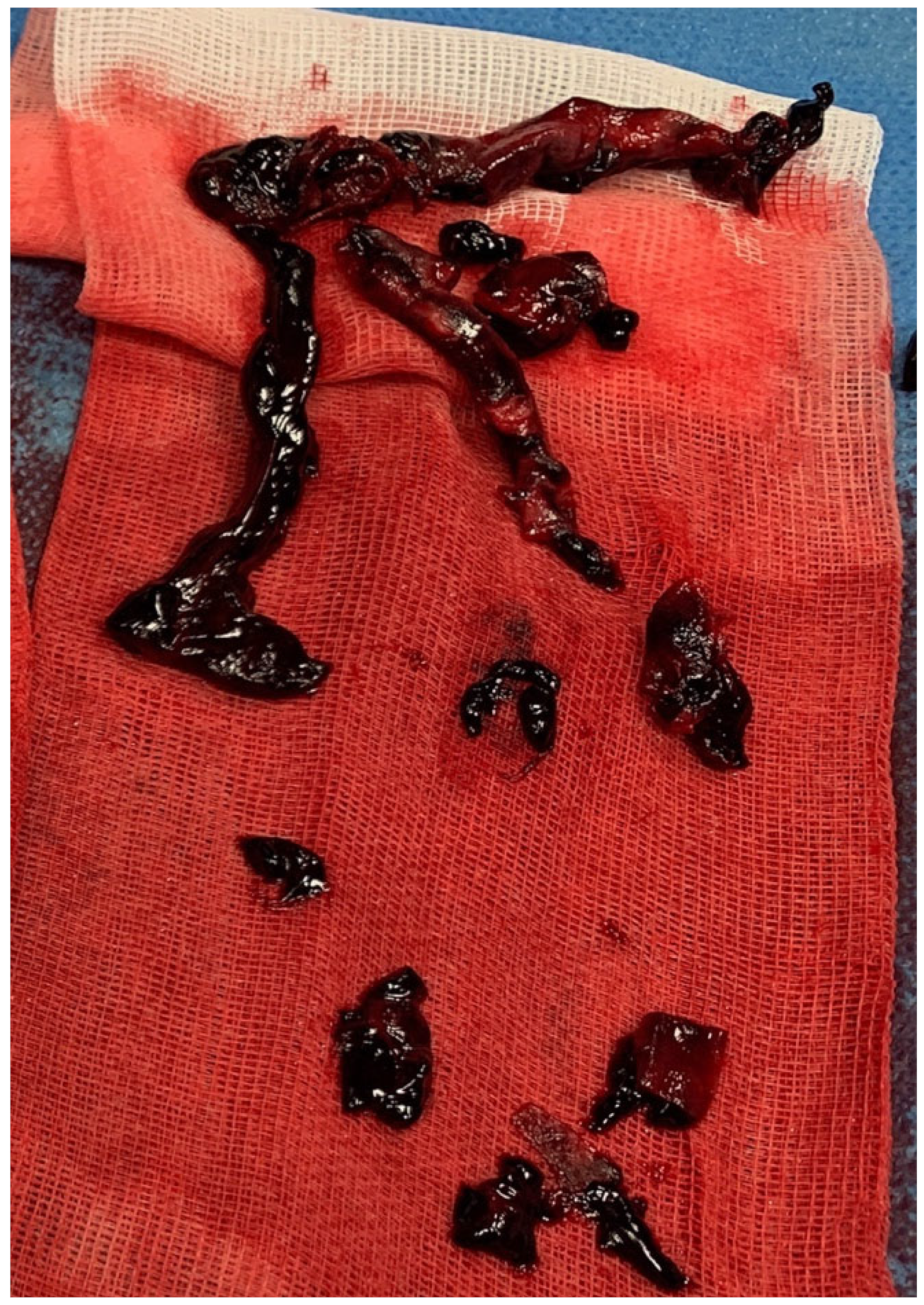
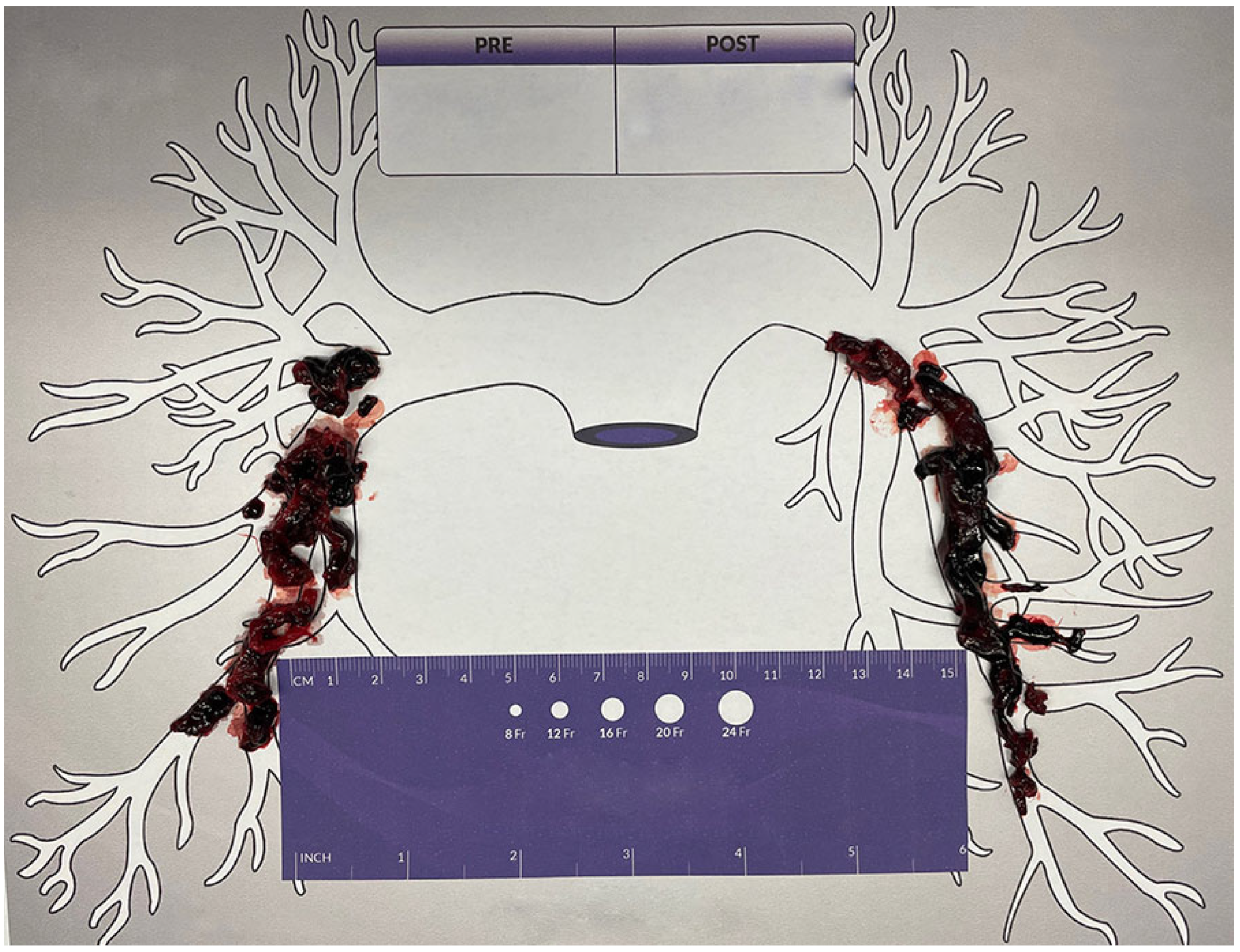
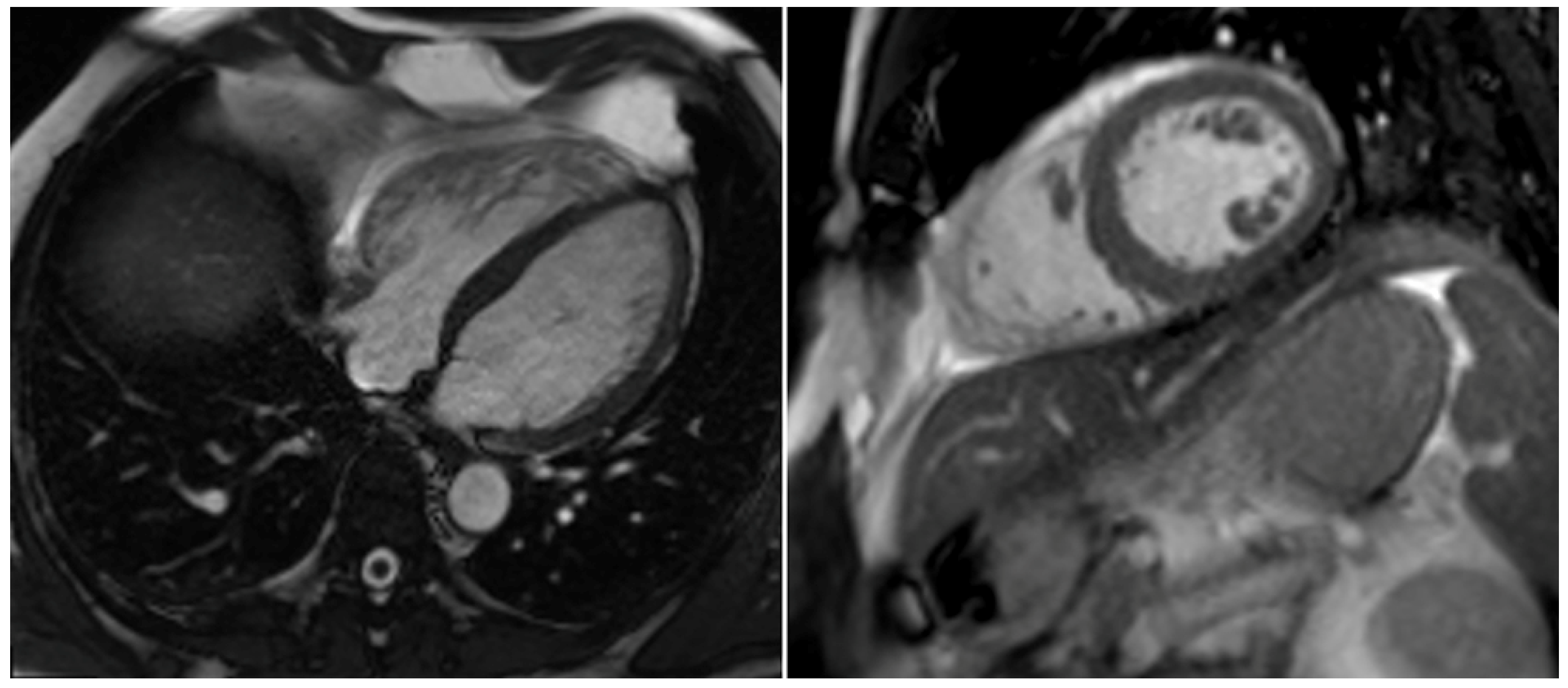
2. Case Report
3. Discussion
4. Autotransfusion Procedure
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorse, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [PubMed] [Green Version]
- Tarbox, A.K.; Swaroop, M. Symposium: Embolism in the intensive care unit. Int. J. Crit. Illn. Inj. Sci. 2013, 3, 69–72. [Google Scholar] [PubMed]
- Rivera-Lebron, B.; Mcdaniel, M.; Ahrar, K.; Alrifai, A.; Dudzinski, D.M.; Fanola, C.; Blais, D.; Janicke, D.; Melamed, R.; Mohrien, K.; et al. Diagnosis, Treatment and Follow Up of Acute Pulmonary Embolism: Consensus Practice from the PERT Consortium. Clin. Appl. Thromb. Hemost. 2019, 25, 1076029619853037. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.N.; Devarapally, S.R.; Lee, L.S.; Gupta, N. Catheter Directed Thrombolysis Of Pulmonary Embolism; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ucar, E.Y. Update on Thrombolytic Therapy in Acute Pulmonary Thromboembolism. Eurasian J. Med. 2019, 51, 186–190. [Google Scholar]
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jimenez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef]
- Meinel, F.G.; Nance, J.W., Jr.; Schoepf, U.J.; Hoffmann, V.S.; Thierfelder, K.M.; Costello, P.; Goldhaber, S.Z.; Bamberg, F. Predictive Value of Computed Tomography in Acute Pulmonary Embolism: Systematic Review and Meta-analysis. Am. J. Med. 2015, 128, 747–759.e742. [Google Scholar] [CrossRef]
- Agarwal, S.; Saum, J.; Chanamalou, S.; Cole, W.; Patel, S.M. When Harry Met Sally: Single-Session INARI FlowTriever and Impella RP. J. Cardiol. Cases 2021, 23, 57–60. [Google Scholar] [CrossRef]
- Toma, C.; Bunte, M.C.; Cho, K.H.; Jaber, W.A.; Chambers, J.; Stegman, B.; Gondi, S.; Leung, D.A.; Savin, M.; Khandhar, S.; et al. Percutaneous mechanical thrombectomy in a real-world pulmonary embolism population: Interim results of the FLASH registry. Catheter Cardiovasc. Interv. 2022, 99, 1345–1355. [Google Scholar] [CrossRef]
- Tu, T.; Toma, C.; Tapson, V.F.; Adams, C.; Jaber, W.A.; Silver, M.; Khandhar, S.; Amin, R.; Weinberg, M.; Engelhardt, T.; et al. A Prospective, Single-Arm, Multicenter Trial of Catheter-Directed Mechanical Thrombectomy for Intermediate-Risk Acute Pulmonary Embolism: The FLARE Study. JACC Cardiovasc. Interv. 2019, 12, 859–869. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [Green Version]
- Mathbout, M.F.; Hennawi, H.A.; Khedr, A.; Bidwell, K.; Todoran, T.M. Inari large-bore mechanical thrombectomy in intermediate-high risk submassive PE patients: Case series and literature review. Glob. Cardiol. Sci. Pract. 2022, 2022, e202208. [Google Scholar] [CrossRef]
- Nadler, S.B.; Hidalgo, J.H.; Bloch, T. Prediction of blood volume in normal human adults. Surgery 1962, 51, 224–232. [Google Scholar]
- Hooper, N.; Armstrong, T.J. Hemorrhagic Shock; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Carless, P.A.; Henry, D.A.; Moxey, A.J.; O’connell, D.; Brown, T.; Fergusson, D.A. Cell salvage for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst. Rev. 2010, 2010, Cd001888. [Google Scholar]
- Klein, A.A.; Bailey, C.R.; Charlton, A.J.; Evans, E.; Guckian-Fisher, M.; Mccrossan, R.; Nimmo, A.F.; Payne, S.; Shreeve, K.; Smith, J.; et al. Association of Anaesthetists guidelines: Cell salvage for peri-operative blood conservation 2018. Anaesthesia 2018, 73, 1141–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, J.H.; Coelho, G.R.; Feitosa Neto, B.A.; Nogueira, E.A.; Teixeira, C.C.; Mesquita, D.F. Liver transplantation in Jehovah’s Witnesses patients in a center of northeastern Brazil. Arq. Gastroenterol. 2013, 50, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.V.; Nagababu, E.; Johnson, D.J.; Kebaish, K.M.; Lipsitz, J.A.; Dwyer, I.M.; Zuckerberg, G.S.; Barodka, V.M.; Berkowitz, D.E.; Frank, S.M. 2,3-Diphosphoglycerate Concentrations in Autologous Salvaged Versus Stored Red Blood Cells and in Surgical Patients After Transfusion. Anesth. Analg. 2016, 122, 616–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paravicini, D.; Wasylewski, A.H.; Rassat, J.; Thys, J. Red blood cell survival and morphology during and after intraoperative autotransfusion. Acta Anaesthesiol. Belg. 1984, 35, 43–49. [Google Scholar]
- Waters, J.H. Indications and contraindications of cell salvage. Transfusion 2004, 44 (Suppl. S12), 40S–44S. [Google Scholar] [CrossRef]
- Ottesen, S.; Froysaker, T. Use of Haemonetics Cell Saver for autotransfusion in cardiovascular surgery. Scand. J. Thorac. Cardiovasc. Surg. 1982, 16, 263–268. [Google Scholar] [CrossRef]
- Esper, S.A.; Waters, J.H. Intra-operative cell salvage: A fresh look at the indications and contraindications. Blood Transfus. 2011, 9, 139–147. [Google Scholar]
- Spahn, D.R.; Bouillon, B.; Cerny, V.; Coats, T.J.; Duranteau, J.; Fernandez-Mondejar, E.; Filipescu, D.; Hunt, B.J.; Komadina, R.; Nardi, G.; et al. Management of bleeding and coagulopathy following major trauma: An updated European guideline. Crit. Care 2013, 17, R76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, G.; Melnyk, V.; Facco, F.L.; Waters, J.H.; Smith, K.J. Cost-effectiveness Analysis of Intraoperative Cell Salvage for Obstetric Hemorrhage. Anesthesiology 2018, 128, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Waters, J.H. Intraoperative blood recovery. ASAIO J. 2013, 59, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Waters, J.H.; Lukauskiene, E.; Anderson, M.E. Intraoperative blood salvage during cesarean delivery in a patient with beta thalassemia intermedia. Anesth. Analg. 2003, 97, 1808–1809. [Google Scholar] [CrossRef]






| Preinterventional | Immediately after the Procedure | At Discharge 6 Days after the Procedure | |
|---|---|---|---|
| Clinical data | |||
| Dyspnea [NYHA] | IV | II–III | 0 |
| Arterial blood pressure [systolic/diastolic/mean, mmHg] | 73/44/70 | 103/70/84 | 122/61/81 |
| Heart rate [beats/minute] | 100 | 81 | 64 |
| SpO2 [%] | 90 | 95 | 97 |
| Oxygen supplementation [liters/minute] | 10 | 3 | 0 |
| Respiratory rate [breaths/minute] | 29 | 16 | 12–14 |
| Echocardiographic data | |||
| RVEDD [mm] | 65 | 49 | 37 |
| TAPSE [mm] | 12 | 16 | 21 |
| TASV [cm/s] | 8 | 12 | 17 |
| sPAP [mmHg] | 55 | 15 | 14 |
| LVEF [%] | 43 | 54 | 56 |
| D- Sign | Yes | No | No |
| McConnell‘s sign | Yes | Yes | No |
| Systolic notching of PWD over pulmonary valve | Yes | Slightly | No |
| PAC hemodynamics | |||
| PAP [systolic/diastolic/mean, mmHg] | 44/23/29 | 25/10/16 | Not available |
| Laboratory data | |||
| D-Dimer [µg/L] | 41,531 | Not available | Not available |
| Troponin [pg/mL] | 75.20 | 505 | 21.8 |
| Myoglobin [µg/L] | 80 | 36 | Not available |
| NT-Pro-BNP [pg/mL] | 207 | 1062 | <50 |
| Arterial lactate [mmol/L] | 3.5 | 1.5 | Not available |
| Hb [mmol/l] | 10 | 8.2 | 9.7 |
| Thrombocytes [Gpt/l] | 175 | 142 | 315 |
| Hematocrit [%] | 47 | 38 | 42 |
| Therapy data | |||
| Noradrenalin [µg/kg/min] | 0.47 | 0 | 0 |
| Dobutamine [µg/kg/min] | 8.3 | 0 | 0 |
| Unfractionated intravenous heparin | Yes | Yes | No |
| NOAC | No | No | Yes (Apixaban) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haertel, F.; Baez, L.; Franz, M.; Bogoviku, J.; Klein, F.; Dannberg, G.; Schulze, P.C.; Möbius-Winkler, S. Use of Autotransfusion following Percutaneous Thrombectomy for Cardiogenic Shock Due to Pulmonary Embolism in a Single Session—A Case Report. Diagnostics 2023, 13, 1392. https://doi.org/10.3390/diagnostics13081392
Haertel F, Baez L, Franz M, Bogoviku J, Klein F, Dannberg G, Schulze PC, Möbius-Winkler S. Use of Autotransfusion following Percutaneous Thrombectomy for Cardiogenic Shock Due to Pulmonary Embolism in a Single Session—A Case Report. Diagnostics. 2023; 13(8):1392. https://doi.org/10.3390/diagnostics13081392
Chicago/Turabian StyleHaertel, Franz, Laura Baez, Marcus Franz, Jurgen Bogoviku, Friederike Klein, Gudrun Dannberg, P. Christian Schulze, and Sven Möbius-Winkler. 2023. "Use of Autotransfusion following Percutaneous Thrombectomy for Cardiogenic Shock Due to Pulmonary Embolism in a Single Session—A Case Report" Diagnostics 13, no. 8: 1392. https://doi.org/10.3390/diagnostics13081392
APA StyleHaertel, F., Baez, L., Franz, M., Bogoviku, J., Klein, F., Dannberg, G., Schulze, P. C., & Möbius-Winkler, S. (2023). Use of Autotransfusion following Percutaneous Thrombectomy for Cardiogenic Shock Due to Pulmonary Embolism in a Single Session—A Case Report. Diagnostics, 13(8), 1392. https://doi.org/10.3390/diagnostics13081392






