Influence of Adipose Tissue on Early Metabolic Programming: Conditioning Factors and Early Screening
Abstract
:1. Introduction
1.1. Fetal Programming
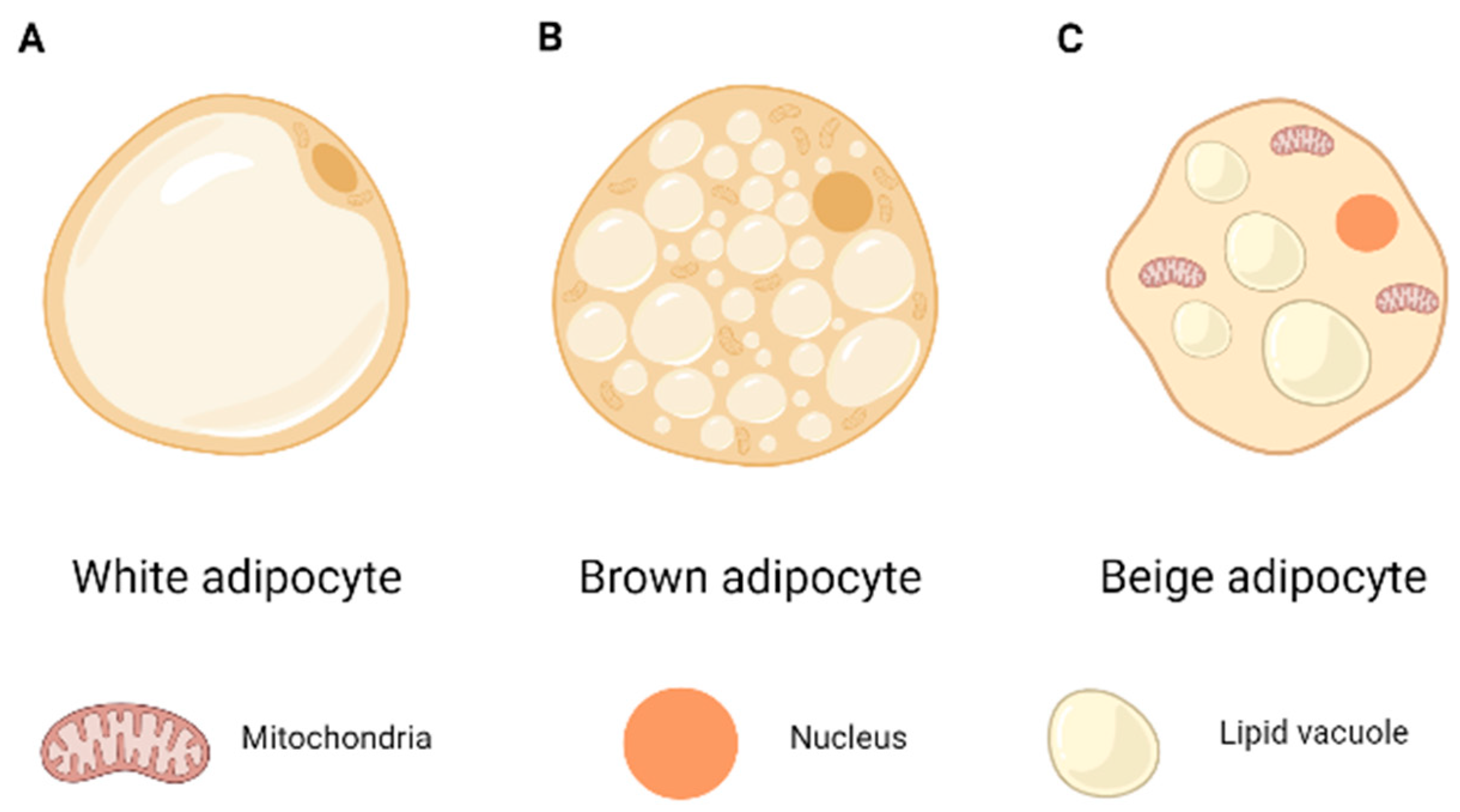
1.2. Adipose Tissue
1.3. Endocrine Function
1.4. Role of the Placenta in Adipose Tissue
2. Materials and Methods
3. Results and Discussion
3.1. Endocrine Factors Regulating Adipose Tissue Programming
3.2. Nutritional Factors and the Adipose Tissue Programming
3.3. THE Link between Breastfeeding and the Adipose Tissue
3.4. Environmental Factors Regulating Adipose Tissue Programming
3.5. Early Screening for Risks Associated with High Adipose Tissue in Offspring
3.6. Limitations
| Authors and Year | Sample Information | Study Design | Major Findings |
|---|---|---|---|
| Endocrine factors regulating adipose tissue programming | |||
| Luo ZC et al. (2012) [40] | 307 women from Canada: 27 GDM 280 no diabetics | Observational | Higher IGF-I levels indicate increased placental and fetal growth. Higher maternal and fetal IGF-I levels may explain fetal hypertrophy in children of mothers with GDM. |
| Hawkes CP et al. (2019) [41] | 601 children born to healthy mothers (Ireland) 317 boys 284 girls | Observational | Increased IGF-1 concentrations are associated with higher fat mass and higher free fat mass at birth. IGF-2 is associated with reduced lean mass. |
| Kadakia R et al. (2016) [42] | 112 pairs of women-children, healthy mothers, from Chicago | Observational | IGF-1 is an important and independent factor affecting neonatal body composition. |
| Putet G et al. (2016) [43] | 238 healthy full-term infants
|
| Factors different than IGF-1 may play a role in determining growth velocity. |
| Horan MK et al. (2014) [44] | 280 mother-children. ROLO clinical trial
| Low glycemic index dietary advice vs. usual prenatal care |
|
| Donnelly JM et al. (2015) [45] |
| Low glycemic index diet during pregnancy vs. normal dietary guidelines | Fetal leptin in the umbilical cord significantly associated with birth weight. |
| Solis-Paredes M et al. (2016) [46] | 67 women at term that gave birth by caesarean | Observational | Maternal adiposity status may play an active role in regulating fetal lipid profile and, consequently, fetal programming. |
| Crume TL et al. (2015) [47] | 804 women-children from Colorado | Observational | Insulin resistance in early pregnancy is an independent predictor of neonatal adiposity. Maternal circulating lipid levels have a relatively modest impact on neonatal fat accumulation and adiposity. |
| O’Brien CM et al. (2019) [48] | 911 women in the “Standard care” group of the LIMIT, OW or OB clinical trial. | Standard healthcare recommendation from their hospitals. | Increased adiponectin concentrations are associated with a smaller abdominal circumference in women with OW or OB. |
| Barbour LA et al. (2018) [49] | 54 women from Colorado
| Observational | Postprandial TGs are positively related to excess weight in the newborn and therefore their control during pregnancy may prevent excess adiposity in the NB and decrease the risk of obesity. |
| Heerwagen MJR et al. (2018) [50] | 20 women from the Colorado Multiple Institutional Review COMIRB Board
| Observational | Placental LPL activity correlates positively with adiposity in the newborn. |
| Nutritional factors and adipose tissue programming | |||
| Chatzi L et al. (2018) [51] |
| Observational | Greater adherence to the Mediterranean diet during pregnancy is associated with lower adiposity in offspring. |
| Grivell RM et al. (2016) [52] | Australian women OW or OB
|
| Lower thigh fat mass and lower subscapular adiposity in children born to obese mothers with diet and lifestyle intervention. |
| Claesson IM et al. (2016) [53] | 302 children-obese mother from Sweden
| Weekly visits with midwife during pregnancy to change nutrition and fitness habits + aqua aerobics classes. | Dietary advices and practicing physical exercise during pregnancy are not related to the BMI of children at 5 years old. |
| O’Brien CM et al. (2018) [54] | 721 pregnant women in the “Standard Care” group. LIMIT clinical trial. | Usual health recommendation from their hospitals. | No association between maternal diet and fetal adiposity at 28 and 36 weeks’ gestation. |
| Foster BA et al. (2017) [55] | 63 obese women or women with GDM (double-blind clinical trial) | Supplementation with 800 mg/day of DHA. | DHA supplementation during pregnancy is associated with lower adiposity in the offspring. |
| Horan MK et al. (2016) [56] | 280 mother-children. ROLO trial
| Low glycemic index dietary advice vs. usual prenatal care | Diet during pregnancy, especially saturated fat and sodium is associated with infant adiposity at 6 months after birth. |
| Blumfield ML et al. (2014) [57] | 179 Australian pregnant women from “Women and Their Children’s Healthy Study” | Observational | The body composition of the fetus may be modulated by the mother’s nutritional intervention during pregnancy. |
| Crume TL et al. (2016) [58] | 1410 healthy pregnant women from Colorado | Observational | Regardless of pregestational BMI, increased intake of macronutrients except protein is associated with an increase in neonatal fat mass. |
| Brei C et al. (2018) [59] | 208 healthy pregnant women |
|
|
| Donnelly JM et al. (2015) [60] |
| Low glycemic index diet during pregnancy vs. normal dietary guidelines | Low GI diet is associated with a smaller thigh circumference in neonates, but not with adiposity at 6 months of age. |
| Geraghty AA et al. (2016) [61] | 331 mothers-children ROLO clinical trial | Low glycemic index diet during pregnancy vs. normal dietary guidelines. | Blood lipid concentrations at the end of pregnancy and in the cord are associated with offspring anthropometry. Maternal TG concentrations are associated with birth weight. |
| Berglund SK et al. (2016) [62] | PREOBE clinical trial (cohorts)
| Observational | Children born to obese or overweight mothers have higher birth weights than those born to normal weight mothers. |
| Poprzeczny AJ et al. (2018) [63] | 912 women in the Standard Care group of the LIMIT study (102 developed GDM) | Guidelines and usual recommendations of their hospitals for pregnancy. | Increased maternal BMI in overweight/obese women is associated with increased fetal growth, but not with increased fetal adiposity. |
| Nehab SR et al. (2020) [64] | 124 women-children from Rio de Janeiro | Observational | Women with excessive weight gain have children with higher body mass and higher fat mass, compared to mothers with adequate or insufficient weight gain in pregnancy. |
| Savage JS et al. (2019) [65] | 26 women from USA with OW or OB
| Dietary advice through a weekly interview with a nutritionist, and physical activity advice. | Individualized feeding in women with OW or OB shows preventive effects on fetal growth velocity and thus lower risk of OW or OB of the baby. |
| Blackwell SC et al. (2016) [66] | 841 women from a clinical trial of women with GDM. Texas
| Dietary advice and treatment for GDM. | Excessive weight gain is associated with an increase in the baby’s body mass, risk of being large for gestational age, and higher total birth weight. |
| Ásbjörnsdóttir B et al. (2019) [67] | 219 women with DM2 in Copenhagen
|
| There is a trend towards a lower prevalence of fetal overgrowth when encouraged to improve adherence to a healthy diet in addition to routine diabetes care, in women with DM2 and the children born to them. |
| Link between breastfeeding and the adipose tissue | |||
| Martin RM et al. (2013) [68] | 17,046 newborns from PROBIT clinical trial recruited between 1996 and 1997. (Belarus)
|
| An intervention to improve the duration and duration of breastfeeding did not prevent overweight or obesity, nor did it affect IGF-I levels, among these children at 11.5 years. |
| M Martin, et al. (2017) [69] | 17,046 newborns from PROBIT clinical trial recruited between 1996–97. (Belarus)
|
| Increasing the duration and exclusivity of breastfeeding did not decrease adiposity in children at 16 years of age or blood pressure. |
| Schwartz R et al. (2015) [70] | 323 adolescent mothers in Brazil and their children
| The intervention group received advice on breastfeeding and exclusive breastfeeding and on how to introduce complementary feeding at 6 months. | Breastfeeding and advice on how to start the complementary feeding does not cause a decrease in overweight/obesity prevalence. |
| Inostroza J et al. (2014) [71] | 248 Chilean children born to obese mothers
|
| Infants fed with low-protein formula have lower weight gain than those fed with a normal formula. |
| Environmental factors regulating adipose tissue programming | |||
| Iguacel I et al. (2018) [72] | 1031 Spanish children | Observational | Rapid infant weight gain, parental overweight/obesity, maternal smoking, and origin/ethnicity predict childhood overweight/obesity and have cumulative effects. |
| Taylor RW et al. (2017) [76] | 206 women at the end of pregnancy—their babies | Recommendations for following an introduction of complementary feeding based on ‘baby-led weaning’. | No differences were shown, although the intervention group showed less anxiety about food. |
| Early screening for risks associated with high adipose tissue in offspring | |||
| Mitanchez D et al. (2015) [77] | Mini Review | The current definition of GDM does not allow identifying pregestational diabetes from true GDM. | |
| Tam WH et al. (2017) [78] | 970 mothers from Hyperglycemia and Adverse Pregnancy Outcome study and their children. | Reevaluations 7 years after delivery. | Maternal hyperglycemia in pregnancy is independently associated with offspring’s risk of abnormal glucose tolerance, obesity, and higher BP at 7 years of age. |
| McIntyre HD et al. (2015) [79] | Review | To evaluate the different ways of DMG diagnosis | |
| Corcoran SM et al. (2018) [82] | 248 women deemed at risk of GDM before 15 weeks | First trimester measurement of Adiponectin and 1,5-Anhydroglucitol are potential early biomarkers for the later onset of GDM. | |
| Thagaard IN et al. (2017) [83] | 2590 pregnant women, categorized into normal weight, moderately obese, or severely obese. | Low adiponectin measured in the first trimester is associated with the development of GDM; higher BMI was associated with lower performance of adiponectin, though this was insignificant. Leptin had an inverse relationship with GDM in severely obese women and did not improve the ability to predict GDM. | |
| Thaware PK et al. (2019) [84] | 100 women in early pregnancy from Belfast, UK. | Observational | Ultrasonography-measured visceral adipose tissue in early pregnancy is a potential clinical tool for improving sensitivity of selective screening for gestational diabetes, which, compared with universal oral glucose tolerance testing, is likely to reduce by half the numbers requiring this test. |
| Alves JG et al. (2020) [85] | 627 pregnant women from Brazil. | Prospective cohort | There is an increased risk of GDM in relation to VAD measured in early pregnancy. This association remained so after adjusting for BMI, and VAD was more predictive of GDM than pre-pregnancy BMI. |
| D’Ambrosi F et al. (2020) [86] |
| Cohorts | Sonographic thickness of maternal visceral adipose tissue was greater in women with GDM than in non-diabetic patients, independently of other known risk factors associated with GDM in the 1st and in the 2nd trimester of pregnancy. Thus, this measurement may be considered of clinical use in 1st trimester screening. |
| Ma’ayeh M et al. (2020) [94] | Review | Contemporary research into prophylactic and therapeutic interventions for preeclampsia are providing novel and promising modalities. | |
| Kuchenbecker WK et al. (2014) [97] | 53 women with obesity and infertility | Prospective cohort | In women with obesity and infertility, measuring IAF by US is in good agreement with the CT scan methodology but the measurement of SAF by US is unreliable. |
| Pétursdóttir Maack H et al. (2021) [96] | 3777 women at around 18 gestational weeks. | Greater subcutaneous adipose tissue thickness measured with second trimester ultrasound is associated with increased risk of developing pre-eclampsia. The measurement may improve prediction models for pre-eclampsia. | |
| Association | Target Population | Cut-Offs for GMD Diagnosis |
|---|---|---|
| FIGO (at any time of gestation) IADPSG (24–28 weeks of gestation) | Universal Risk-based | Fasting glycemia: 92–125 mg/dL (5.1–6.9 mM) Glycemia 1 h after overload ≥ 180 mg/dL (10.0 mM) Glycemia 2 h after overload: 153–199 mg/dL (8.5–11.0 mM) |
| ACOG NIH (24–28 weeks of gestation) | Risk-based | Step 1: If glycemia ≥ 130 mg/dL (7.8 mM), proceed with Step 2: Fasting glycemia ≥ 95 mg/dL (5.3 mM) Glycemia 1 h after overload ≥ 180 mg/dL (10.0 mM) Glycemia 2 h after overload ≥ 155 mg/dL (8.6 mM) Glycemia 3 h after overload ≥ 140 mg/dL (7.8 mM) |
| ADA 2020 (24–28 weeks of gestation) | Universal | One step method: 75 g OGTT, fasting postprandial glucose ≥ 92 mg/dL (≥5.1 mmol/L); 1 h: ≥ 180 mg/dL (≥10 mmol/L); 2 h: 153–199 mg/dL (≥8.5 mmol/L). Two step method: 1. First OGTT 50 g of glucose load: fasting postprandial glucose ≥ 95 mg/dL (≥5.3 mmol/L); 1 h: ≥ 180 mg/dL (≥10 mmol/L); 2 h: ≥155 mg/dL/≥8.6 mmlo/L); 3 h: ≥140 mg/dL (≥7.8 mmol/L). |
| WHO (at any time of gestation) | Universal | One-step method: OGTT (75 g glucose) Two steps method: 1. first OGTT with 50 g of glucose load; >7.8 mmol/L (>1.4 g/L) after 1 h. 2. Second OGTT with 75 g of glucose and evaluation as standard OGTT. |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desoye, G.; Herrera, E. Adipose tissue development and lipid metabolism in the human fetus: The 2020 perspective focusing on maternal diabetes and obesity. Prog. Lipid Res. 2021, 81, 101082. [Google Scholar] [CrossRef]
- Levy-Marchal, C. Adipose tissue development. From animal models to clinical conditions. Endocr. Dev. 2010, 19, vii–ix. [Google Scholar]
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, K.M. Maternal regulation of fetal development and health in adult life. Eur. J. Obstet. Gynecol. Reprod. Biol. 1998, 78, 141–150. [Google Scholar] [CrossRef]
- Casanello, P.; Krause, B.J.; Castro-Rodriguez, J.A.; Uauy, R. Fetal Programming of Chronic Diseases: Current Concepts and Epigenetics. Rev. Chil. Pediatr. 2015, 86, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Mennitti, L.V.; Oliveira, J.L.; Morais, C.A.; Estadella, D.; Oyama, L.M.; Nascimento, C.M.O.D.; Pisani, L.P. Type of fatty acids in maternal diets during pregnancy and/or lactation and metabolic consequences of the offspring. J. Nutr. Biochem. 2015, 26, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.; Godfrey, K.; Gluckman, P.; Harding, J.; Owens, J.; Robinson, J. Fetal nutrition and cardiovascular disease in adult life. Lancet 1993, 341, 938–941. [Google Scholar] [CrossRef]
- OMS. Obesidad Y Sobrepeso. Available online: https://www.who.int/es/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 7 March 2023).
- Moreno-Mendez, E.; Quintero-Fabian, S.; Fernandez-Mejia, C.; Lazo-De-La-Vega-Monroy, M.-L. Early-life programming of adipose tissue. Nutr. Res. Rev. 2020, 33, 244–259. [Google Scholar] [CrossRef]
- Sanchez-Gurmaches, J.; Guertin, D.A. Adipocyte lineages: Tracing back the origins of fat. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2014, 1842, 340–351. [Google Scholar] [CrossRef]
- Song, T.; Kuang, S. Adipocyte dedifferentiation in health and diseases. Clin. Sci. 2019, 133, 2107–2119. [Google Scholar] [CrossRef]
- Bartness, T.J.; Liu, Y.; Shrestha, Y.B.; Ryu, V. Neural innervation of white adipose tissue and the control of lipolysis. Front. Neuroendocr. 2014, 35, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef] [PubMed]
- Cristancho, A.G.; Lazar, M.A. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Frontini, A.; Cinti, S. Distribution and Development of Brown Adipocytes in the Murine and Human Adipose Organ. Cell Metab. 2010, 11, 253–256. [Google Scholar] [CrossRef]
- Cypess, A.M.; White, A.P.; Vernochet, C.; Schulz, T.J.; Xue, R.; Sass, C.A.; Huang, T.L.; Roberts-Toler, C.; Weiner, L.S.; Sze, C.; et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat. Med. 2013, 19, 635–639. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Brown Adipose Tissue: Function and Physiological Significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Tseng, Y.-H.; Cypess, A.M.; Kahn, C.R. Cellular bioenergetics as a target for obesity therapy. Nat. Rev. Drug Discov. 2010, 9, 465–482. [Google Scholar] [CrossRef]
- Nedergaard, J.; Bengtsson, T.; Cannon, B. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Metab. 2007, 293, E444–E452. [Google Scholar] [CrossRef]
- Ishibashi, J.; Seale, P. Beige Can Be Slimming. Science 2010, 328, 1113–1114. [Google Scholar] [CrossRef]
- Poissonnet, C.M.; Burdi, A.R.; Garn, S.M. The chronology of adipose tissue appearance and distribution in the human fetus. Early Hum. Dev. 1984, 10, 1–11. [Google Scholar] [CrossRef]
- Liang, X.; Yang, Q.; Fu, X.; Rogers, C.J.; Wang, B.; Pan, H.; Zhu, M.J.; Nathanielsz, P.W.; Du, M. Maternal Obesity Epige-netically Alters Visceral Fat Progenitor Cell Properties in Male Offspring Mice. J. Physiol. 2016, 594, 4453–4466. [Google Scholar] [CrossRef]
- Halaas, J.L.; Gajiwala, K.S.; Maffei, M.; Cohen, S.L.; Chait, B.T.; Rabinowitz, D.; Lallone, R.L.; Burley, S.K.; Friedman, J.M. Weight-Reducing Effects of the Plasma Protein Encoded by the obese Gene. Science 1995, 269, 543–546. [Google Scholar] [CrossRef]
- Fasshauer, M.; Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.M.; Byrne, J.; Mahony, R.M.; Foley, M.E.; McAuliffe, F.M. Leptin, fetal growth and insulin resistance in non-diabetic pregnancies. Early Hum. Dev. 2014, 90, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Sivan, E.; Mazaki-Tovi, S.; Pariente, C.; Efraty, Y.; Schiff, E.; Hemi, R.; Kanety, H. Adiponectin in Human Cord Blood: Re-lation to Fetal Birth Weight and Gender. J. Clin. Endocrinol. Metab. 2003, 88, 5656–5660. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Dong, M.; Fang, Q.; He, J.; Wang, Z.; Yang, X. Alterations of serum resistin in normal pregnancy and pre-eclampsia. Clin. Sci. 2005, 108, 81–84. [Google Scholar] [CrossRef]
- Merrill, D.C.; Karoly, M.; Chen, K.; Ferrario, C.M.; Brosnihan, K.B. Angiotensin-(1-7) in Normal and Preeclamptic Pregnancy. Endocrine 2002, 18, 239–246. [Google Scholar] [CrossRef]
- Aye, I.L.; Jansson, T.; Powell, T.L. Tnf-A Stimulates System a Amino Acid Transport in Primary Human Trophoblast Cells Mediated by P38 Mapk Signaling. Physiol. Rep. 2015, 3, e12594. [Google Scholar] [CrossRef]
- Houra, M.; Nazem-Kazerani, F.; Mortazavi, M.; Hadavi, M.; Moosavi, S.; Arababadi, M. The roles played by IL-10, IL-23 and IL-17A in term delivery. J. Neonatal-Perinatal Med. 2021, 14, 85–93. [Google Scholar] [CrossRef]
- Hunt, J.S.; Chen, H.L.; Miller, L. Tumor Necrosis Factors: Pivotal Components of Pregnancy? Biol. Reprod. 1996, 54, 554–562. [Google Scholar] [CrossRef]
- Rull, A.; Camps, J.; Alonso-Villaverde, C.; Joven, J. Insulin Resistance, Inflammation, and Obesity: Role of Monocyte Chemoattractant Protein-1 (or Ccl2) in the Regulation of Metabolism. Mediat. Inflamm. 2010, 2010, 326580. [Google Scholar] [CrossRef] [PubMed]
- Kierson, J.A.; DiMatteo, D.M.; Locke, R.G.; Mackley, A.B.; Spear, M.L. Ghrelin and cholecystokinin in term and preterm human breast milk. Acta Paediatr. 2006, 95, 991–995. [Google Scholar] [CrossRef]
- Boucher, J.; Masri, B.; Daviaud, D.; Gesta, S.; Guigné, C.; Mazzucotelli, A.; Castan-Laurell, I.; Tack, I.; Knibiehler, B.; Carpéné, C.; et al. Apelin, a Newly Identified Adipokine up-Regulated by Insulin and Obesity. Endocrinology 2005, 146, 1764–1771. [Google Scholar] [CrossRef]
- Cianflone, K.; Xia, Z.; Chen, L.Y. Critical review of acylation-stimulating protein physiology in humans and rodents. Biochim. Biophys. Acta (BBA) Biomembr. 2003, 1609, 127–143. [Google Scholar] [CrossRef]
- Østensen, M.; Marhaug, G.; Husby, G. Amyloid-related serum protein (saa) during and after pregnancy in healthy women and women with rheumatic disease. Acta Pathol. Microbiol. Scand. Ser. C Immunol. 1985, 93C, 1–5. [Google Scholar] [CrossRef]
- Jayabalan, N.; Nair, S.; Nuzhat, Z.; Rice, G.E.; Zuñiga, F.A.; Sobrevia, L.; Leiva, A.; Sanhueza, C.; Gutiérrez, J.A.; Lappas, M.; et al. Cross Talk between Adipose Tissue and Placenta in Obese and Gestational Diabetes Mellitus Pregnancies via Exosomes. Front. Endocrinol. 2017, 8, 239. [Google Scholar] [CrossRef]
- Jansson, N.; Nilsfelt, A.; Gellerstedt, M.; Wennergren, M.; Rossander-Hultheén, L.; Powell, T.L.; Jansson, T. Maternal hormones linking maternal body mass index and dietary intake to birth weight. Am. J. Clin. Nutr. 2008, 87, 1743–1749. [Google Scholar] [CrossRef] [PubMed]
- Dimasuay, K.G.; Boeuf, P.; Powell, T.L.; Jansson, T. Placental Responses to Changes in the Maternal Environment De-termine Fetal Growth. Front. Physiol. 2017, 7, 12. [Google Scholar]
- Luo, Z.C.; Nuyt, A.M.; Delvin, E.; Audibert, F.; Girard, I.; Shatenstein, B.; Cloutier, A.; Cousineau, J.; Djemli, A.; Deal, C.; et al. Maternal and Fetal Igf-I and Igf-Ii Levels, Fetal Growth, and Gestational Diabetes. J. Clin. Endocrinol. Metab. 2012, 97, 1720–1728. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.P.; Grimberg, A.; Kenny, L.C.; Kiely, M.; Hourihane, J.O.B.; Irvine, A.; McPhaul, M.J.; Caulfield, M.P.; Zemel, B.S.; Murray, D.M. The relationship between IGF-I and -II concentrations and body composition at birth and over the first 2 months. Pediatr. Res. 2019, 85, 687–692. [Google Scholar] [CrossRef]
- Kadakia, R.; Ma, M.; Josefson, J.L. Neonatal adiposity increases with rising cord blood IGF-1 levels. Clin. Endocrinol. 2016, 85, 70–75. [Google Scholar] [CrossRef]
- Putet, G.; Labaune, J.-M.; Mace, K.; Steenhout, P.; Grathwohl, D.; Raverot, V.; Morel, Y.; Picaud, J.-C. Effect of dietary protein on plasma insulin-like growth factor-1, growth, and body composition in healthy term infants: A randomised, double-blind, controlled trial (Early Protein and Obesity in Childhood (EPOCH) study). Br. J. Nutr. 2016, 115, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Horan, M.K.; McGowan, C.A.; Gibney, E.R.; Donnelly, J.M.; McAuliffe, F.M. Maternal Low Glycaemic Index Diet, Fat Intake and Postprandial Glucose Influences Neonatal Adiposity--Secondary Analysis from the Rolo Study. Nutr. J. 2014, 13, 78. [Google Scholar] [CrossRef]
- Donnelly, J.M.; Lindsay, K.L.; Walsh, J.M.; Horan, M.; Molloy, E.J.; McAuliffe, F.M. Fetal metabolic influences of neonatal anthropometry and adiposity. BMC Pediatr. 2015, 15, 175. [Google Scholar] [CrossRef]
- Solis-Paredes, M.; Espino, Y.S.S.; Estrada-Gutierrez, G.; Nava-Salazar, S.; Ortega-Castillo, V.; Rodriguez-Bosch, M.; Bra-vo-Flores, E.; Espejel-Nuñez, A.; Tolentino-Dolores, M.; Gaona-Estudillo, R.; et al. Ma-ternal and Fetal Lipid and Adipokine Profiles and Their Association with Obesity. Int. J. Endocrinol. 2016, 2016, 7015626. [Google Scholar] [CrossRef]
- Crume, T.L.; Shapiro, A.L.; Brinton, J.T.; Glueck, D.H.; Martinez, M.; Kohn, M.; Harrod, C.; Friedman, J.E.; Dabelea, D. Maternal Fuels and Metabolic Measures During Pregnancy and Neonatal Body Composition: The Healthy Start Study. J. Clin. Endocrinol. Metab. 2015, 100, 1672–1680. [Google Scholar] [CrossRef]
- O’brien, C.M.; Louise, J.; Deussen, A.; Dodd, J.M. Maternal cardiometabolic markers are associated with fetal growth: A secondary exploratory analysis of the LIMIT randomised trial. BMC Endocr. Disord. 2019, 19, 97. [Google Scholar] [CrossRef]
- Barbour, L.A.; Farabi, S.S.; Friedman, J.E.; Hirsch, N.M.; Reece, M.S.; Van Pelt, R.E.; Hernandez, T.L. Postprandial Tri-glycerides Predict Newborn Fat More Strongly Than Glucose in Women with Obesity in Early Pregnancy. Obesity 2018, 26, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Heerwagen, M.J.; Gumina, D.L.; Hernandez, T.L.; Van Pelt, R.E.; Kramer, A.W.; Janssen, R.C.; Jensen, D.R.; Powell, T.L.; Friedman, J.E.; Winn, V.D.; et al. Placental lipoprotein lipase activity is positively associated with newborn adiposity. Placenta 2018, 64, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Chatzi, L.; Rifas-Shiman, S.L.; Georgiou, V.; Joung, K.E.; Koinaki, S.; Chalkiadaki, G.; Margioris, A.; Sarri, K.; Vassilaki, M.; Vafeiadi, M.; et al. Adherence to the Mediterranean diet during pregnancy and offspring adiposity and cardiometabolic traits in childhood. Pediatr. Obes. 2017, 12 (Suppl. S1), 47–56. [Google Scholar] [CrossRef]
- Grivell, R.; Yelland, L.; Deussen, A.; Crowther, C.; Dodd, J. Antenatal dietary and lifestyle advice for women who are overweight or obese and the effect on fetal growth and adiposity: The LIMIT randomised trial. BJOG Int. J. Obstet. Gynaecol. 2016, 123, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Claesson, I.-M.; Josefsson, A.; Olhager, E.; Oldin, C.; Sydsjö, G. Effects of a gestational weight gain restriction program for obese women: Sibling pairs’ weight development during the first five years of life. Sex. Reprod. Health 2018, 17, 65–74. [Google Scholar] [CrossRef]
- O’brien, C.M.; Louise, J.; Deussen, A.; Dodd, J.M. In Overweight or Obese Pregnant Women, Maternal Dietary Factors are not Associated with Fetal Growth and Adiposity. Nutrients 2018, 10, 870. [Google Scholar] [CrossRef]
- Foster, B.A.; Escaname, E.; Powell, T.L.; Larsen, B.; Siddiqui, S.K.; Menchaca, J.; Aquino, C.; Ramamurthy, R.; Hale, D.E. Randomized Controlled Trial of DHA Supplementation during Pregnancy: Child Adiposity Outcomes. Nutrients 2017, 9, 566. [Google Scholar] [CrossRef] [PubMed]
- Horan, M.K.; McGowan, C.A.; Gibney, E.R.; Byrne, J.; Donnelly, J.M.; McAuliffe, F.M. Maternal Nutrition and Glycaemic Index during Pregnancy Impacts on Offspring Adiposity at 6 Months of Age—Analysis from the ROLO Randomised Controlled Trial. Nutrients 2016, 8, 7. [Google Scholar] [CrossRef]
- Blumfield, M.L.; Hure, A.J.; MacDonald-Wicks, L.K.; Smith, R.; Simpson, S.J.; Giles, W.B.; Raubenheimer, D.; E Collins, C. Dietary balance during pregnancy is associated with fetal adiposity and fat distribution. Am. J. Clin. Nutr. 2012, 96, 1032–1041. [Google Scholar] [CrossRef]
- Crume, T.L.; Brinton, J.T.; Shapiro, A.; Kaar, J.; Glueck, D.H.; Siega-Riz, A.M.; Dabelea, D. Maternal dietary intake during pregnancy and offspring body composition: The Healthy Start Study. Am. J. Obstet. Gynecol. 2016, 215, 609.e1–609.e8. [Google Scholar] [CrossRef]
- Brei, C.; Stecher, L.; Meyer, D.M.; Young, V.; Much, D.; Brunner, S.; Hauner, H. Impact of Dietary Macronutrient Intake During Early and Late Gestation on Offspring Body Composition at Birth, 1, 3, and 5 Years of Age. Nutrients 2018, 10, 579. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.M.; Walsh, J.M.; Byrne, J.; Molloy, E.J.; McAuliffe, F.M. Impact of maternal diet on neonatal anthropometry: A randomized controlled trial. Pediatr. Obes. 2014, 10, 52–56. [Google Scholar] [CrossRef]
- Geraghty, A.A.; Alberdi, G.; O’Sullivan, E.J.; O’Brien, E.C.; Crosbie, B.; Twomey, P.J.; McAuliffe, F.M. Maternal Blood Lipid Profile During Pregnancy and Associations with Child Adiposity: Findings from the Rolo Study. PLoS ONE 2016, 11, e0161206. [Google Scholar] [CrossRef]
- Berglund, S.K.; García-Valdés, L.; Torres-Espinola, F.J.; Segura, M.T.; Martínez-Zaldívar, C.; Aguilar, M.J.; Agil, A.; Lorente, J.A.; Florido, J.; Padilla, C.; et al. Maternal, Fetal and Perinatal Alterations Associated with Obesity, Overweight and Gestational Diabetes: An Observational Cohort Study (Preobe). BMC Public Health 2016, 16, 207. [Google Scholar] [CrossRef]
- Poprzeczny, A.J.; Louise, J.; Deussen, A.R.; Dodd, J. The mediating effects of gestational diabetes on fetal growth and adiposity in women who are overweight and obese: Secondary analysis of the LIMIT randomised trial. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Nehab, S.R.; Villela, L.D.; Soares, F.V.M.; Abranches, A.D.; Araújo, D.M.R.; da Silva, L.M.L.; Amaral, Y.N.V.; Junior, S.C.G.; Meio, M.D.B.B.; Moreira, M.E. Gestational weight gain and body composition of full-term newborns and infants: A cohort study. BMC Pregnancy Childbirth 2020, 20, 474. [Google Scholar] [CrossRef] [PubMed]
- Savage, J.S.; Hohman, E.E.; McNitt, K.M.; Pauley, A.M.; Leonard, K.S.; Turner, T.; Pauli, J.M.; Gernand, A.D.; Rivera, D.E.; Downs, D.S. Uncontrolled Eating during Pregnancy Predicts Fetal Growth: The Healthy Mom Zone Trial. Nutrients 2019, 11, 899. [Google Scholar] [CrossRef]
- Blackwell, S.C.; Landon, M.B.; Mele, L.; Reddy, U.M.; Casey, B.M.; Wapner, R.; Varner, M.W.; Rouse, D.J.; Thorp, J.M.; Sciscione, A.; et al. Relationship Between Excessive Gestational Weight Gain and Neonatal Adiposity in Women With Mild Gestational Diabetes Mellitus. Obstet. Gynecol. 2016, 128, 1325–1332. [Google Scholar] [CrossRef]
- Sbjörnsdóttir, B.; Vestgaard, M.; Ringholm, L.; Andersen, L.L.T.; Jensen, D.M.; Damm, P.; Mathiesen, E.R. Effect of Mo-tivational Interviewing on Gestational Weight Gain and Fetal Growth in Pregnant Women with Type 2 Diabetes. BMJ Open Diabetes Res. Care 2019, 7, e000733. [Google Scholar] [CrossRef]
- Martin, R.M.; Patel, R.; Kramer, M.S.; Guthrie, L.; Vilchuck, K.; Bogdanovich, N.; Sergeichick, N.; Gusina, N.; Foo, Y.; Palmer, T.; et al. Effects of Promoting Longer-term and Exclusive Breastfeeding on Adiposity and Insulin-like Growth Factor-I at Age 11.5 Years. JAMA 2013, 309, 1005–1013. [Google Scholar] [CrossRef]
- Martin, R.M.; Kramer, M.S.; Patel, R.; Rifas-Shiman, S.L.; Thompson, J.; Yang, S.; Vilchuck, K.; Bogdanovich, N.; Hameza, M.; Tilling, K.; et al. Effects of Promoting Long-Term, Exclusive Breastfeeding on Adolescent Adiposity, Blood Pressure, and Growth Trajectories: A Secondary Analysis of a Randomized Clinical Trial. JAMA Pediatr. 2017, 171, e170698. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.; Vigo, Á.; de Oliveira, L.D.; Giugliani, E.R.J. The Effect of a Pro-Breastfeeding and Healthy Comple-mentary Feeding Intervention Targeting Adolescent Mothers and Grandmothers on Growth and Prevalence of Overweight of Preschool Children. PLoS ONE 2015, 10, e0131884. [Google Scholar] [CrossRef]
- Inostroza, J.; Haschke, F.; Steenhout, P.; Grathwohl, D.; Nelson, S.E.; Ziegler, E.E. Low-Protein Formula Slows Weight Gain in Infants of Overweight Mothers. J. Craniofacial Surg. 2014, 59, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Iguacel, I.; On behalf of the CALINA study group; Escartín, L.; Fernández-Alvira, J.M.; Iglesia, I.; Labayen, I.; Moreno, L.A.; Samper, M.P.; Rodríguez, G. Early life risk factors and their cumulative effects as predictors of overweight in Spanish children. Int. J. Public Health 2018, 63, 501–512. [Google Scholar] [CrossRef]
- Moreno-Fernandez, J.; Ochoa, J.; Ojeda, M.L.; Nogales, F.; Carreras, O.; Díaz-Castro, J. Inflammation and oxidative stress, the links between obesity and COVID-19: A narrative review. J. Physiol. Biochem. 2022, 78, 581–591. [Google Scholar] [CrossRef]
- Eskenazi, B.; Rauch, S.; Iurlaro, E.; Gunier, R.B.; Rego, A.; Gravett, M.G.; Cavoretto, P.I.; Deruelle, P.; García-May, P.K.; Mhatre, M.; et al. Diabetes mellitus, maternal adiposity, and insulin-dependent gestational diabetes are associated with COVID-19 in pregnancy: The INTERCOVID study. Am. J. Obstet. Gynecol. 2022, 227, 74.e1–74.e16. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Conti, C.P.S.; Gunier, R.B.; Ariff, S.; Craik, R.; Cavoretto, P.I.; Rauch, S.; Gandino, S.; Nieto, R.; Winsey, A.; et al. Pregnancy Outcomes and Vaccine Effectiveness During the Period of Omicron as the Variant of Concern, Intercovid-2022: A Multinational, Observational Study. Lancet 2023, 401, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.W.; Williams, S.M.; Fangupo, L.J.; Wheeler, B.J.; Taylor, B.J.; Daniels, L.; Fleming, E.A.; McArthur, J.; Morison, B.; Erickson, L.W.; et al. Effect of a Baby-Led Approach to Complementary Feeding on Infant Growth and Overweight: A Randomized Clinical Trial. JAMA Pediatr. 2017, 171, 838–846. [Google Scholar] [CrossRef]
- Mitanchez, D.; Yzydorczyk, C.; Simeoni, U. What Neonatal Complications Should the Pediatrician Be Aware of in Case of Maternal Gestational Diabetes? World J. Diabetes 2015, 6, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Tam, W.H.; Ma, R.C.W.; Ozaki, R.; Li, A.M.; Chan, M.H.M.; Yuen, L.Y.; Lao, T.T.H.; Yang, X.; Ho, C.S.; Tutino, G.E.; et al. In Utero Exposure to Maternal Hyperglycemia Increases Childhood Cardiometabolic Risk in Offspring. Diabetes Care 2017, 40, 679–686. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, H.D.; Colagiuri, S.; Roglic, G.; Hod, M. Diagnosis of GDM: A suggested consensus. Best Pr. Res. Clin. Obstet. Gynaecol. 2015, 29, 194–205. [Google Scholar] [CrossRef]
- Silverman, B.L.; A Rizzo, T.; Cho, N.H.; E Metzger, B. Long-term effects of the intrauterine environment. The Northwestern University Diabetes in Pregnancy Center. Diabetes Care 1998, 21 (Suppl. S2), B142. [Google Scholar]
- Sert, U.Y.; Ozgu-Erdinc, A.S. Gestational Diabetes Mellitus Screening and Diagnosis. Am. Fam. Physician 2021, 1307, 231–255. [Google Scholar] [CrossRef]
- Corcoran, S.M.; Achamallah, N.; Loughlin, J.O.; Stafford, P.; Dicker, P.; Malone, F.D.; Breathnach, F. First trimester serum biomarkers to predict gestational diabetes in a high-risk cohort: Striving for clinically useful thresholds. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 222, 7–12. [Google Scholar] [CrossRef]
- Thagaard, I.N.; Krebs, L.; Holm, J.-C.; Lange, T.; Larsen, T.; Christiansen, M. Adiponectin and leptin as first trimester markers for gestational diabetes mellitus: A cohort study. Clin. Chem. Lab. Med. 2017, 55, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Thaware, P.K.; Patterson, C.C.; Young, I.; Casey, C.; McCance, D.R. Clinical utility of ultrasonography-measured visceral adipose tissue depth as a tool in early pregnancy screening for gestational diabetes: A proof-of-concept study. Diabet. Med. 2019, 36, 898–901. [Google Scholar] [CrossRef]
- Alves, J.G.; Souza, A.S.R.; Figueiroa, J.N.; de Araújo, C.A.L.; Guimarães, A.; Ray, J.G. Visceral Adipose Tissue Depth in Early Pregnancy and Gestational Diabetes Mellitus—A Cohort Study. Sci. Rep. 2020, 10, 2032. [Google Scholar] [CrossRef] [PubMed]
- D’ambrosi, F.; Rossi, G.; Soldavini, C.M.; Di Maso, M.; Carbone, I.F.; Cetera, G.E.; Colosi, E.; Ferrazzi, E. Ultrasound assessment of maternal adipose tissue during 1st trimester screening for aneuploidies and risk of developing gestational diabetes. Acta Obstet. Gynecol. Scand. 2020, 99, 644–650. [Google Scholar] [CrossRef]
- Lei, A.; You, H.; Hu, J.; Liu, Y.; Luo, B. Risk of type 2 diabetes mellitus after gestational diabetes mellitus: A systematic review & meta-analysis. Indian J. Med. Res. 2021, 154, 62. [Google Scholar] [CrossRef]
- Basevi, V.; Di Mario, S.; Morciano, C.; Nonino, F.; Magrini, N. Comment On: American Diabetes Association. Standards of Medical Care in Diabetes—2011. Diabetes Care 2011, 34 (Suppl. S1), S11–S61. [Google Scholar]
- Yarrington, C.; Zera, C. Health Systems Approaches to Diabetes Screening and Prevention in Women with a History of Gestational Diabetes. Curr. Diabetes Rep. 2015, 15, 114. [Google Scholar] [CrossRef]
- Gunderson, E.P.; Jacobs, D.R., Jr.; Chiang, V.; Lewis, C.E.; Feng, J.; Quesenberry, C.P., Jr.; Sidney, S. Duration of Lactation and Incidence of the Metabolic Syndrome in Women of Reproductive Age According to Gestational Diabetes Mellitus Status: A 20-Year Prospective Study in Cardia (Coronary Artery Risk Development in Young Adults). Diabetes 2010, 59, 495–504. [Google Scholar] [CrossRef]
- Yasuhi, I.; Soda, T.; Yamashita, H.; Urakawa, A.; Izumi, M.; Kugishima, Y.; Umezaki, Y. The effect of high-intensity breastfeeding on postpartum glucose tolerance in women with recent gestational diabetes. Int. Breastfeed. J. 2017, 12, 32. [Google Scholar] [CrossRef]
- Schaefer-Graf, U.M.; Hartmann, R.; Pawliczak, J.; Passow, D.; Abou-Dakn, M.; Vetter, K.; Kordonouri, O. Association of Breast-Feeding and Early Childhood Overweight in Children from Mothers with Gestational Diabetes Mellitus. Diabetes Care 2006, 29, 1105–1107. [Google Scholar] [CrossRef] [PubMed]
- Sotiriadis, A.; Chatzakis, C.; Cavoretto, P. Gestational Diabetes Mellitus Pharmacological Prevention and Treatment. Curr. Pharm. Des. 2021, 27, 3833–3840. [Google Scholar] [CrossRef] [PubMed]
- Ma’ayeh, M.; Costantine, M.M. Prevention of Preeclampsia. Semin. Fetal. Neonatal. Med. 2020, 25, 101123. [Google Scholar] [CrossRef] [PubMed]
- Tornaghi, G.; Raiteri, R.; Pozzato, C.; Rispoli, A.; Bramani, M.; Cipolat, M.; Craveri, A. Anthropometric or ultrasonic measurements in assessment of visceral fat? A comparative study. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 1994, 18, 771–775. [Google Scholar]
- Maack, H.P.; Poromaa, I.S.; Lindström, L.; Mulic-Lutvica, A.; Junus, K.; Wikström, A.-K. Ultrasound estimated subcutaneous and visceral adipose tissue thicknesses and risk of pre-eclampsia. Sci. Rep. 2021, 11, 22740. [Google Scholar] [CrossRef]
- Kuchenbecker, W.K.H.; Groen, H.; Pel, H.; Bolster, J.H.T.; Wolffenbuttel, B.H.R.; Land, J.A.; Hoek, A.; Corpeleijn, E. Validation of the measurement of intra-abdominal fat between ultrasound and CT scan in women with obesity and infertility. Obesity 2013, 22, 537–544. [Google Scholar] [CrossRef]

| Adipokine | Biological Function | References |
|---|---|---|
| Leptin | Levels during pregnancy are associated with adequate fetal growth. Pro-oxidant role. | Walsh J.M. et al., 2014 [25] |
| Adiponectin | High levels in lean women, limits placental nutrient transfer and fetal growth compared to obese women. Antidiabetic, anti-inflammatory and cardioprotective. | Sivan E. et al., 2003 [26] |
| Resistin | It prevents glucose uptake by adipocytes, increasing plasma glucose and leading to insulin resistance. Its levels are increased during pregnancy. | Chen D. et al., 2005 [27] |
| Angiotensin | Its production is increased under conditions of obesity. | Merrill D. et al., 2002 [28] |
| IL-6 | Elevated levels are associated with higher birth weight. It has a pro-inflammatory role and increases insulin resistance. | Aye I. L. et al., 2015 [29] |
| IL-10 | Has anti-inflammatory effects. | Houra M. et al., 2021 [30] |
| TNF-α | It is associated with higher birth weight in newborns. It is a pro-inflammatory cytokine. | Hunt J.S. et al., 1996 [31] |
| MCP-1 | Increased levels in mothers with obesity. Pro-inflammatory effects. | Rull A. et al., 2010 [32] |
| Grelin | Increases appetite by modulating orexigenic peptides in the hypothalamus. Coordinates energy balance and weight regulation. | Kierson J. A. et al., 2006 [33] |
| Apelin | Anti-diabetic and anti-obesogenic properties. | Boucher J. et al., 2005 [34] |
| ASP | Inhibits hormone-sensitive lipase and leads to increased insulin release from beta cells. | Cianflone K. et al., 2003 [35] |
| SAA | Directly measures inflammation associated with obesity. | Ostensen M. G. et al., 1985 [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puche-Juarez, M.; Toledano, J.M.; Ochoa, J.J.; Diaz-Castro, J.; Moreno-Fernandez, J. Influence of Adipose Tissue on Early Metabolic Programming: Conditioning Factors and Early Screening. Diagnostics 2023, 13, 1510. https://doi.org/10.3390/diagnostics13091510
Puche-Juarez M, Toledano JM, Ochoa JJ, Diaz-Castro J, Moreno-Fernandez J. Influence of Adipose Tissue on Early Metabolic Programming: Conditioning Factors and Early Screening. Diagnostics. 2023; 13(9):1510. https://doi.org/10.3390/diagnostics13091510
Chicago/Turabian StylePuche-Juarez, Maria, Juan M. Toledano, Julio J. Ochoa, Javier Diaz-Castro, and Jorge Moreno-Fernandez. 2023. "Influence of Adipose Tissue on Early Metabolic Programming: Conditioning Factors and Early Screening" Diagnostics 13, no. 9: 1510. https://doi.org/10.3390/diagnostics13091510
APA StylePuche-Juarez, M., Toledano, J. M., Ochoa, J. J., Diaz-Castro, J., & Moreno-Fernandez, J. (2023). Influence of Adipose Tissue on Early Metabolic Programming: Conditioning Factors and Early Screening. Diagnostics, 13(9), 1510. https://doi.org/10.3390/diagnostics13091510









