Description of Peripheral Blood Perfusion by Laser Speckle Contrast Analysis (LASCA) in ‘Early’ versus ‘Clinically Overt’ Systemic Sclerosis in Routine Clinics
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Vote
2.2. Study Population
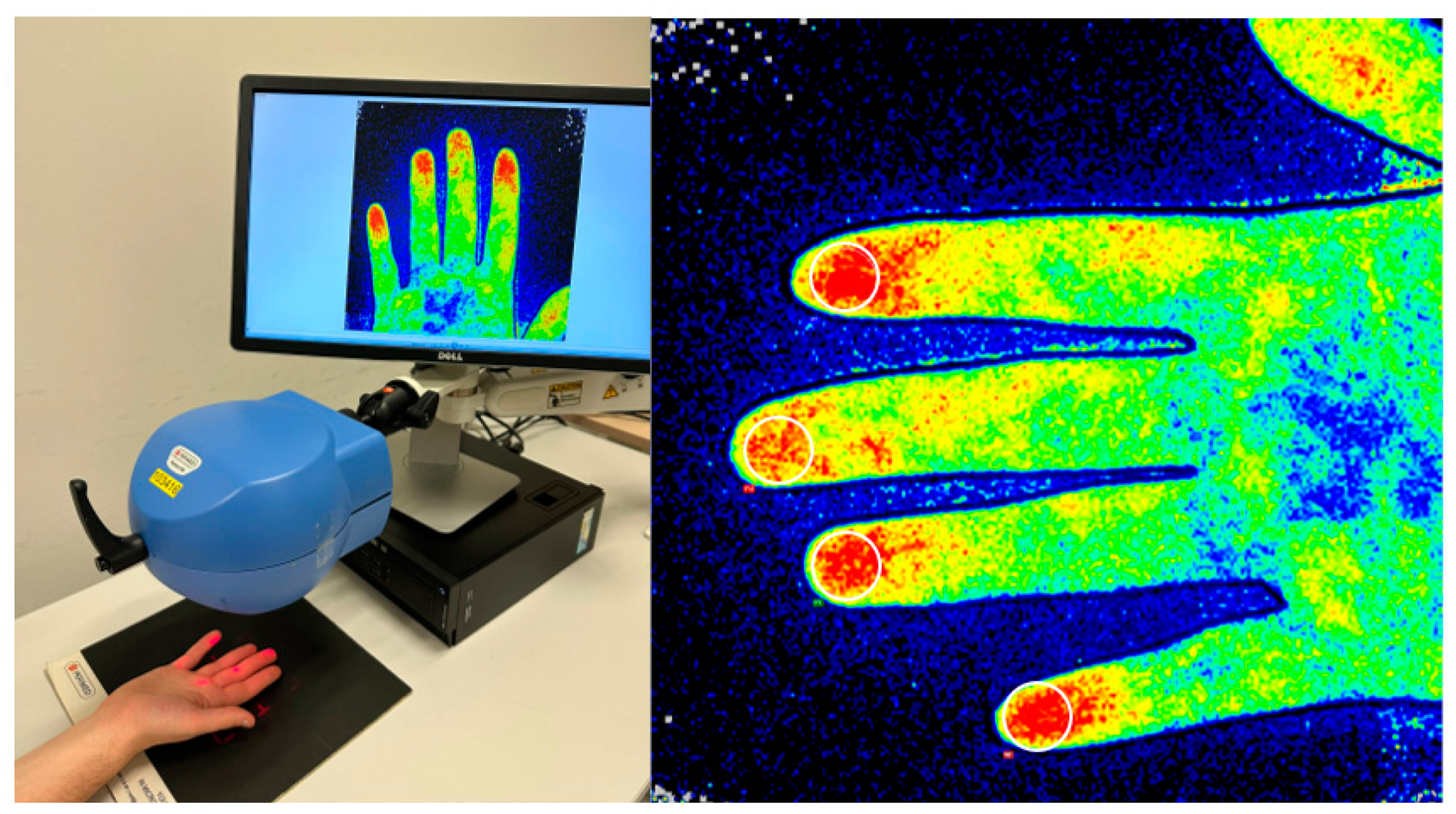
2.3. Data Collection
2.4. Study Design
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. LASCA Examination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leroy, E.C.; Black, C.; Fleischmajer, R.; Jablonska, S.; Krieg, T.; Medsger, T.A., Jr.; Rowell, N.; Wollheim, F. Scleroderma (systemic sclerosis): Classification, subsets and pathogenesis. J. Rheumatol. 1988, 15, 202–205. [Google Scholar]
- Volkmann, E.R.; Andréasson, K.; Smith, V. Systemic sclerosis. Lancet 2023, 401, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Prescott, R.J.; Freemont, A.J.; Jones, C.J.P.; Hoyland, J.; Fielding, P. Sequential dermal microvascular and perivascular changes in the development of scleroderma. J. Pathol. 1992, 166, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Armando Gabrielli Enrico, V. Avvedimento and TK. Scleroderma Aust. J. Politics History 2009, 14, 451–453. [Google Scholar]
- Cutolo, M.; Sulli, A.; Smith, V. Assessing microvascular changes in systemic sclerosis diagnosis and management. Nat. Rev. Rheumatol. 2010, 6, 578–587. [Google Scholar] [CrossRef]
- Matucci-Cerinic, M.; Kahaleh, B.; Wigley, F.M. Systemic Sclerosis (Scleroderma, SSc) is a Vascular Disease. Arthritis Rheum. 2013, 65, 1953–1962. [Google Scholar] [CrossRef]
- Vanhaecke, A.; Cutolo, M.; Distler, O.; Riccieri, V.; Allanore, Y.; Denton, C.P.; Hachulla, E.; Ingegnoli, F.; Deschepper, E.; Avouac, J.; et al. Nailfold capillaroscopy in SSc: Innocent bystander or promising biomarker for novel severe organ involvement/progression? Rheumatology 2022, 61, 4384–4396. [Google Scholar] [CrossRef]
- LeRoy, E.C.; Medsger, J. Criteria for the classification of early systemic sclerosis. J. Rheumatology 2001, 28, 1573–1576. [Google Scholar]
- van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An american college of rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum. 2013, 65, 2737–2747. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.; Decuman, S.; Sulli, A.; Bonroy, C.; Piettte, Y.; Deschepper, E.; de Keyser, F.; Cutolo, M. Do worsening scleroderma capillaroscopic patterns, predict future severe organ involvement? A pilot study. Ann. Rheum. Dis. 2012, 71, 1636–1639. [Google Scholar] [CrossRef]
- Cutolo, M.; Smith, V. Detection of microvascular changes in systemic sclerosis and other rheumatic diseases. Nat. Rev. Rheumatol. 2021, 17, 665–677. [Google Scholar] [CrossRef]
- Soulaidopoulos, S.; Triantafyllidou, E.; Garyfallos, A.; Kitas, G.D.; Dimitroulas, T. The role of nailfold capillaroscopy in the assessment of internal organ involvement in systemic sclerosis: A critical review. Autoimmun. Rev. 2017, 16, 787–795. [Google Scholar] [CrossRef]
- Smith, V.; Herrick, A.L.; Ingegnoli, F.; Damjanov, N.; de Angelis, R.; Denton, C.P.; Distler, O.; Espejo, K.; Foeldvari, I.; Frech, T.; et al. Standardisation of nailfold capillaroscopy for the assessment of patients with Raynaud’s phenomenon and systemic sclerosis. Autoimmun. Rev. 2020, 19, 102458. [Google Scholar] [CrossRef] [PubMed]
- Repa, A.; Avgoustidis, N.; Kougkas, N.; Bertsias, G.; Zafiriou, M.; Sidiropoulos, P. Nailfold Videocapillaroscopy as a Candidate Biomarker for Organ Involvement and Prognosis in Patients with Systemic Sclerosis. Mediterr. J. Rheumatol. 2019, 30, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Briers, J.D.; Webster, S. Laser speckle contrast analysis (LASCA): A nonscanning, fullfield technique for monitoring capillary blood flow. J. Biomed. Opt. 1996, 1, 174–179. [Google Scholar] [CrossRef]
- Ruaro, B.; Sulli, A.; Alessandri, E.; Pizzorni, C.; Ferrari, G.; Cutolo, M. Laser speckle contrast analysis: A new method to evaluate peripheral blood perfusion in systemic sclerosis patients. Ann. Rheum. Dis. 2014, 73, 1181–1185. [Google Scholar] [CrossRef]
- Cutolo, M.; Vanhaecke, A.; Ruaro, B.; Deschepper, E.; Ickinger, C.; Melsens, K.; Piette, Y.; Trombetta, A.C.; De Keyser, F.; Smith, V. Is laser speckle contrast analysis (LASCA) the new kid on the block in systemic sclerosis? A systematic literature review and pilot study to evaluate reliability of LASCA to measure peripheral blood perfusion in scleroderma patients. Autoimmun. Rev. 2018, 17, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, V.; Cutolo, M.; de Keyser, F.; Decuman, S.; Ruaro, B.; Sulli, A.; Deschepper, E.; Smith, V. Reliability of the quantitative assessment of peripheral blood perfusion by laser speckle contrast analysis in a systemic sclerosis cohort. Ann. Rheum. Dis. 2016, 75, 1263–1264. [Google Scholar] [CrossRef] [PubMed]
- Ruaro, B.; Sulli, A.; Pizzorni, C.; Paolino, S.; Smith, V.; Alessandri, E.; Trombetta, A.; Alsheyyab, J.; Cutolo, M. Correlations between blood perfusion and dermal thickness in different skin areas of systemic sclerosis patients. Microvasc Res. 2018, 115, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Ickinger, C.; Lambrecht, V.; Tikly, M.; Vanhaecke, A.; Cutolo, M.; Smith, V. Laser speckle contrast analysis is a reliable measure of digital blood perfusion in Black Africans with systemic sclerosis. Clin. Exp. Rheumatol. 2021, 39, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Ruaro, B.; Sulli, A.; Pizzorni, C.; Paolino, S.; Smith, V.; Cutolo, M. Correlations between skin blood perfusion values and nailfold capillaroscopy scores in systemic sclerosis patients. Microvasc. Res. 2016, 105, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, A.C.; Pizzorni, C.; Ruaro, B.; Paolino, S.; Sulli, A.; Smith, V.; Cutolo, M. Effects of longterm treatment with bosentan and iloprost on nailfold absolute capillary number, fingertip blood perfusion, and clinical status in systemic sclerosis. J. Rheumatol. 2016, 43, 2033–2041. [Google Scholar] [CrossRef] [PubMed]
- Ruaro, B.; Pizzorni, C.; Paolino, S.; Alessandri, E.; Sulli, A. Aminaphtone efficacy in primary and secondary Raynaud’s phenomenon: A feasibility study. Front. Pharmacol. 2019, 10, 293. [Google Scholar] [CrossRef]
- Di Battista, M.; Da Rio, M.; Logiacco, A.; Barsotti, S.; Della Rossa, A.; Mosca, M. Kinetics of response to iloprost evaluated by laser speckle contrast analysis in systemic sclerosis. Scand. J. Rheumatol. 2022, 52, 302–305. [Google Scholar] [CrossRef]
- Della Rossa, A.; Cazzato, M.; D’Ascanio, A.; Tavoni, A.; Bencivelli, W.; Pepe, P.; Mosca, M.; Baldini, C.; Rossi, M.; Bombardieri, S. Alteration of microcirculation is a hallmark of very early systemic sclerosis patients: A laser speckle contrast analysis. Clin. Exp. Reumatol. 2013, 31, 109–114. [Google Scholar]
- della Rossa, A.; D’Ascanio, A.; Barsotti, S.; Stagnaro, C.; Mosca, M. Post-occlusive reactive hyperaemia (POHR) in systemic sclerosis: Very early disease (VEDOSS) represents a separate entity compared to established disease. Scand. J. Rheumatol. 2016, 45, 408–411. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 14 April 2023).
- Pinheiro, J.; Bates, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models, R Package Version 3.1-160. 2022. Available online: https://CRAN.R-project.org/package=nlme (accessed on 14 April 2023).
- Barsotti, S.; d’Ascanio, A.; Valentina, V.; Chiara, S.; Silvia, B.; Laura, A.; Mosca, M.; Della Rossa, A. Is there a role for laser speckle contrast analysis (LASCA) in predicting the outcome of digital ulcers in patients with systemic sclerosis? Clin. Rheumatol. 2020, 39, 69–75. [Google Scholar] [CrossRef]
- Gigante, A.; Villa, A.; Rosato, E. Laser speckle contrast analysis predicts major vascular complications and mortality of patients with systemic sclerosis. Rheumatology 2021, 60, 1850–1857. [Google Scholar] [CrossRef]
- Vanhaecke, A.; Debusschere, C.; Cutolo, M.; Smith, V.; EULAR Study Group on Microcirculation in systemic sclerosis. Predictive value of laser speckle contrast analysis in systemic sclerosis. A Syst. Rev. Pilot Study Eur. J. Clin. Investig. 2022, 52, e13672. [Google Scholar]


| Baseline Characteristics General | ||||
|---|---|---|---|---|
| Age (Years), Mean ± SD | 53 ± 12.6 | |||
| Gender (♂/♀), n (%) | 15 (25)/45 (75) | |||
| Disease duration (months), mean ± SD | 73.1 ± 89 | |||
| Raynaud’s phenomenon, n (%) | 60 (100) | |||
| Smoking, n (%) | 32 (53.3) | |||
| Past smoking, n (%) | 22 (36.6) | |||
| Active smoking, n (%) | 10 (16.6) | |||
| mRSS, mean ± SD | 12 (9.7) | |||
| LeRoy subset, ‘Early’ SSc/LcSSc/DcSSc, n (%) | 20 (33.3)/20 (33.3)/20 (33.3) | |||
| Baseline characteristics per subset | ||||
| Subset (n) | Total (n = 60) | ‘Early’ SSc (n = 20) | LcSSc (n = 20) | DcSSc (n = 20) |
| SSc-specific Ab, n (%) | 29 (48.3) | 7 (35.0) | 11 (55.0) | 11 (55.0) |
| NVC scleroderma pattern, n (%) | 54 a (90) | 17 (85) | 20 (100) | 17 a (85) |
| Anticentromere Ab, n (%) | 13 (21.7) | 4 (20.0) | 8 (40.0) | 1 (5.0) |
| Anti-topoisomerase-I Ab, n (%) | 14 (23.3) | 2 (10.0) | 3 (15.0) | 9 (45.0) |
| Anti-RNA-polymerase III Ab, n (%) | 2 (3.3) | 1 (5.0) | 0 (0.0) | 1 (5.0) |
| Vasoactive medication, n (%) | 20 (33.3) | 3 (15.0) | 6 (30.0) | 11 (55.0) |
| CCB, n (%) | 11 (18.3) | 3 (15.0) | 4 (20.0) | 4 (20.0) |
| PDE5-i, n (%) | 1 (1.7) | 0.0 (0.0) | 0.0 (0.0) | 1 (5.0) |
| CCB + PDE5-i, n (%) | 3 (5.0) | 0.0 (0.0) | 0.0 (0.0) | 3 (15.0) |
| CCB + PGE1, n (%) | 2 (3.3) | 0.0 (0.0) | 1 (5.0) | 1 (5.0) |
| PDE5-i + PGE1, n (%) | 2 (3.3) | 0.0 (0.0) | 1 (5.0) | 1 (5.0) |
| CCB + PDE5-i + PGE1, n (%) | 1 (1.7) | 0.0 (0.0) | 0.0 (0.0) | 1 (5.0) |
| History of DTL, n (%) | 16 (26.7) | 0.0 (0.0) | 5 (0.25) | 11 (55.0) |
| History of pitting scars, n (%) | 15 (25.0) | 0.0 (0.0) | 4 (20.0) | 11 (55.0) |
| History of DU, n (%) | 12 (20.0) | 0.0 (0.0) | 3 (15.0) | 9 (45.0) |
| Characteristics | ‘Early’ SSc (n = 20) | ‘Clinically Overt’ SSc (n = 40) | p |
|---|---|---|---|
| Females, n (%) | 17 (85.0) | 28 (70.0) | 0.34 |
| Age (years), mean (SD) | 48.8 (13.9) | 55.1 (11.5) | 0.07 |
| Active smoking | 1 (5.0%) | 9 (22.5%) | 0.14 a |
| SSc-specific Ab, n (%) | 7 (35.0) | 22 (55.0) | 0.24 |
| mRSS, mean (SD) | 0 (0.0) | 12 (9.7) | |
| Vasoactive medication, n (%) | 3 (15.3) | 17 (42.5) | 0.04 a |
| History of DTL, n (%) | 0 (0.0) | 16 (40.0) |
| Statistical Significance | ||||||||
|---|---|---|---|---|---|---|---|---|
| ‘Early’ (n = 20) | ‘Overt’ (n = 40) | LcSSc (n = 20) | DcSSc (n = 20) | ‘Early’ vs. ‘Overt’ | ‘Early’ vs. LcSSc | ‘Early’ vs. DcSSc | LcSSc vs. DcSSc | |
| PU, mean | 144 | 150 | 141 | 157 | p = 0.77 | p = 0.89 | p = 0.62 | p = 0.53 |
| 95% CI | 107–182 | 124–175 | 102–179 | 122–192 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willems, S.; Smith, V.; Wallaert, S.; Gotelli, E.; Du Four, T.; Wyckstandt, K.; Cere, A.; Cutolo, M. Description of Peripheral Blood Perfusion by Laser Speckle Contrast Analysis (LASCA) in ‘Early’ versus ‘Clinically Overt’ Systemic Sclerosis in Routine Clinics. Diagnostics 2023, 13, 1566. https://doi.org/10.3390/diagnostics13091566
Willems S, Smith V, Wallaert S, Gotelli E, Du Four T, Wyckstandt K, Cere A, Cutolo M. Description of Peripheral Blood Perfusion by Laser Speckle Contrast Analysis (LASCA) in ‘Early’ versus ‘Clinically Overt’ Systemic Sclerosis in Routine Clinics. Diagnostics. 2023; 13(9):1566. https://doi.org/10.3390/diagnostics13091566
Chicago/Turabian StyleWillems, Seppe, Vanessa Smith, Steven Wallaert, Emanuele Gotelli, Tessa Du Four, Kaat Wyckstandt, Andrea Cere, and Maurizio Cutolo. 2023. "Description of Peripheral Blood Perfusion by Laser Speckle Contrast Analysis (LASCA) in ‘Early’ versus ‘Clinically Overt’ Systemic Sclerosis in Routine Clinics" Diagnostics 13, no. 9: 1566. https://doi.org/10.3390/diagnostics13091566
APA StyleWillems, S., Smith, V., Wallaert, S., Gotelli, E., Du Four, T., Wyckstandt, K., Cere, A., & Cutolo, M. (2023). Description of Peripheral Blood Perfusion by Laser Speckle Contrast Analysis (LASCA) in ‘Early’ versus ‘Clinically Overt’ Systemic Sclerosis in Routine Clinics. Diagnostics, 13(9), 1566. https://doi.org/10.3390/diagnostics13091566






