Imaging Features of Intraosseous Schwannoma: A Case Series and Review of the Literature
Abstract
1. Introduction
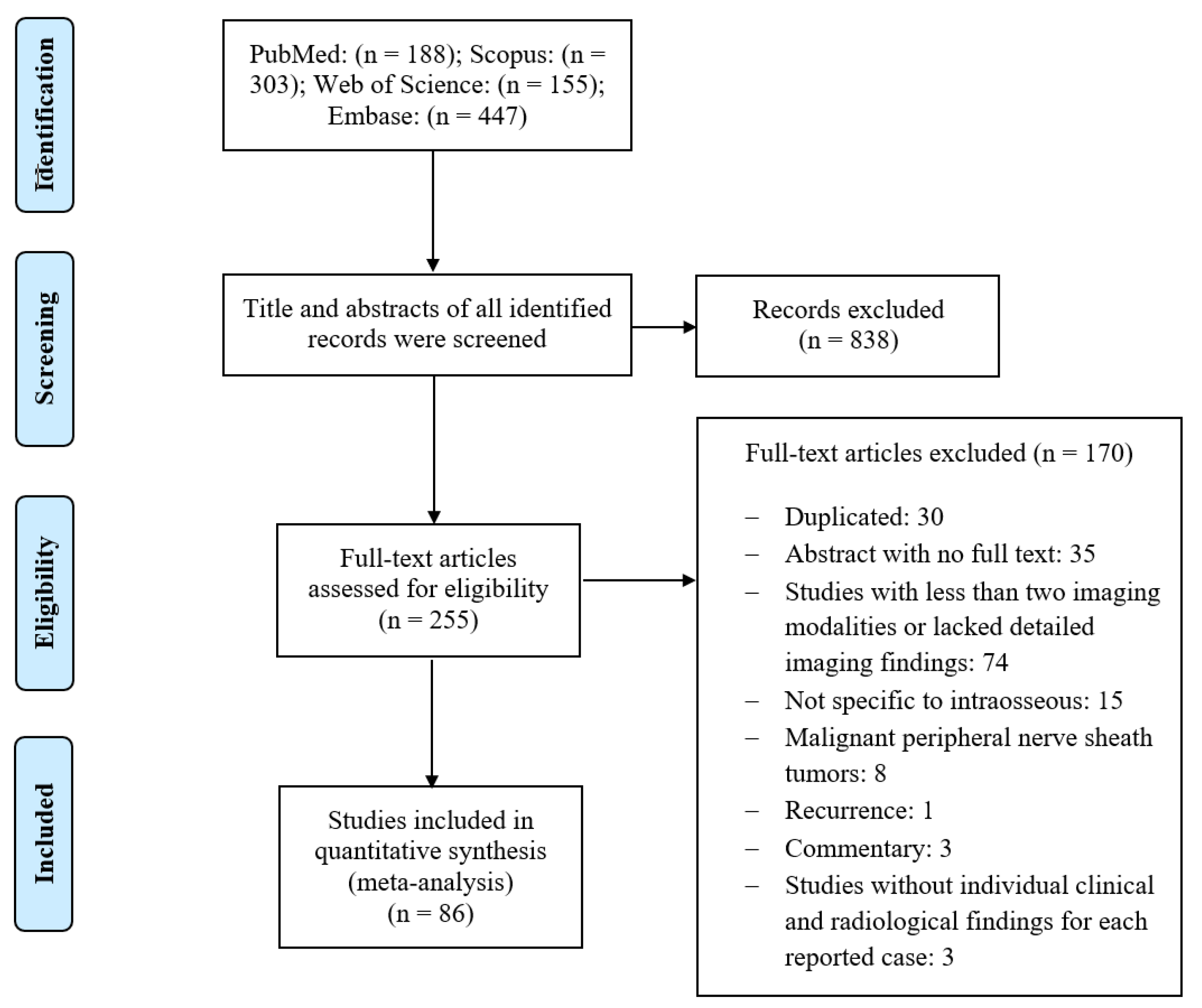
2. Materials and Methods
3. Results
3.1. Case Series
3.1.1. Demographic Characteristics
3.1.2. Radiographic Imaging
3.1.3. Computed Tomography
3.1.4. Magnetic Resonance Imaging
3.1.5. Histologic Findings
3.2. Review of the Literature
3.2.1. Study Selection
3.2.2. Study Characteristics
3.2.3. Imaging Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mutema, G.K.; Sorger, J. Intraosseous schwannoma of the humerus. Skelet. Radiol. 2002, 31, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Haberal, B.; Simsek, D.T.; Simsek, E.K. Intraosseous Schwannoma of the Calcaneus: A Rare Tumor of the Bone. Case Rep. Orthop. 2018, 2018, 9824025. [Google Scholar] [CrossRef]
- Hill, D.A.; Linet, M.S.; Black, P.M.; Fine, H.A.; Selker, R.G.; Shapiro, W.R.; Inskip, P.D. Meningioma and schwannoma risk in adults in relation to family history of cancer. Neuro-Oncol. 2004, 6, 274–280. [Google Scholar] [CrossRef]
- Reyniers, P.; Wafa, H.; Sinnaeve, F.; Debeer, P.; Sciot, R. Intraosseous schwannoma of the glenoid: Case report and literature review. SICOT-J 2021, 7, 2. [Google Scholar] [CrossRef]
- Summers, S.; Jose, J.; Barrera, C.M.; Pretell-Mazzini, J.; Subhawong, T.; Nguyen, N.V.; Kerr, D.; Nielsen, G.P.; E Rosenberg, A. Intraosseous schwannomas involving the sacrum: Characteristic imaging findings and review of the literature. Neuroradiol. J. 2018, 31, 531–540. [Google Scholar] [CrossRef]
- Samter, T.G.; Vellios, F.; Shafer, W.G. Neurilemmona of bone. Report of 3 cases with a review of the literature. Radiology 1960, 75, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Ida, C.M.; Scheithauer, B.W.; Yapicier, O.; Carney, J.A.; Wenger, D.E.; Inwards, C.Y.; Bertoni, F.; Spinner, R.J.; Unni, K.K. Primary schwannoma of the bone: A clinicopathologic and radiologic study of 17 cases. Am. J. Surg. Pathol. 2011, 35, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, K.J.; Dahlin, D.C. Neurilemmoma of bone. Am. J. Clin. Pathol. 1967, 47, 759–766. [Google Scholar] [CrossRef]
- Chi, A.C.; Carey, J.; Muller, S. Intraosseous schwannoma of the mandible: A case report and review of the literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2003, 96, 54–65. [Google Scholar] [CrossRef]
- Wang, X.J.; Hartley, K.; Holt, G.E.; Fadare, O.; Cates, J.M. Intracortical schwannoma of the femur. Skelet. Radiol. 2013, 43, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Rozman, P.A.; Benjamin, C.; Gordon, D.; Sen, C.; Roland, J.T.; Kondziolka, D. Intraosseous Petrous Apex Schwannoma: Case Report. J. Neurol. Surg. Part B Skull Base 2019, 80, P214. [Google Scholar] [CrossRef]
- Sato, M.; Fujio, S.; Takajo, T.; Kamimura, K.; Hiraki, T.; Yamahata, H.; Arita, K.; Yoshimoto, K. Large Intraosseous Schwannoma in Petrous Apex Presenting with Intratumoral Hemorrhage. World Neurosurg. 2019, 131, 53–57. [Google Scholar] [CrossRef]
- Takahashi, S.; Kohno, M.; Kameyama, K.; Fujiwara, H.; Yoshida, K.; Tamura, R. Intraosseous Schwannoma of the Petrous Apex. J. Neurol. Surg. Rep. 2015, 76, e135–e139. [Google Scholar] [CrossRef] [PubMed]
- Goiney, C.; Bhatia, R.; Auerbach, K.; Norenberg, M.; Morcos, J. Intraosseous Schwannoma of the Petrous Apex. J. Radiol. Case Rep. 2011, 5, 8–16. [Google Scholar] [CrossRef]
- Mathieu, F.; Abel, T.J.; Hazrati, L.-N.; Rutka, J.T. Intraosseous schwannoma of the occipital bone: A case report. Child’s Nerv. Syst. 2018, 34, 1803–1805. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Saikia, U.N.; Vashishta, R.K.; Gulati, G.; Sharma, R.K. Intraosseous Schwannoma of the Frontal Bone. Orthopedics 2008, 31, 281. [Google Scholar] [CrossRef] [PubMed]
- El-Bahy, K. Intra-osseous sphenoorbital schwannoma. Acta Neurochir. 2004, 146, 1277–1278. [Google Scholar] [CrossRef]
- Celli, P.; Cervoni, L.; Colonnese, C. Intraosseous schwannoma of the vault of the skull. Neurosurg. Rev. 1998, 21, 158–160. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Iwamoto, A.; Yoshida, R.; Kojima, T.; Hirayama, M.; Takahashi, N.; Nakamoto, M.; Nakayama, H. A rare intraosseous schwannoma in the maxillary left incisor region: A case report. J. Oral Maxillofac. Surg. Med. Pathol. 2020, 32, 114–119. [Google Scholar] [CrossRef]
- Avinash, T.; Sandhya, T.; Dodal, S.; Chande, M.; Pereira, T. Recurrent Ancient Intraosseous Neurilemmoma of Maxilla: A Rare Case Report. Iran. J. Pathol. 2016, 11, 176–180. [Google Scholar]
- Oliveira Alves, M.G.; Rocha, A.C.; Carvalho, Y.R.; Almeida, J.D. Intraosseous neurilemoma of the mandible with unusual multilocular presentation: A case report. Gen. Dent. 2021, 69, 28–32. [Google Scholar]
- Kardouni Khoozestani, N.; Motiee-Langroudi, M.; Salehi, A.; Ranji, P. Intraosseous ancient Schwannoma: A rare case in the mandible and a literature review. Rare Tumors 2021, 13, 20363613211026480. [Google Scholar] [CrossRef] [PubMed]
- Perkins, D.; Stiharu, T.I.; Swift, J.Q.; Dao, T.V.; Mainville, G.N. Intraosseous Schwannoma of the Jaws: An Updated Review of the Literature and Report of 2 New Cases Affecting the Mandible. J. Oral Maxillofac. Surg. 2018, 76, 1226–1247. [Google Scholar] [CrossRef] [PubMed]
- Kargahi, N.; Razavi, S.M.; Hasheminia, D.; Keshani, F.; Safaei, M.; Hashemzadeh, Z. Mandibular intraosseous schwannoma in a child: Report of a rare case. Dent. Res. J. 2012, 9, S119–S122. [Google Scholar]
- Suga, K.; Ogane, S.; Muramatsu, K.; Ohata, H.; Uchiyama, T.; Takano, N.; Shibahara, T.; Eguchi, J.; Murakami, S.; Matsuzaka, K. Intraosseous Schwannoma Originating in Inferior Alveolar Nerve: A Case Report. Bull. Tokyo Dent. Coll. 2013, 54, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xia, B.-Q.; Sun, H.; Wang, L.-Z.; Zhao, Z.-L.; Li, B.; Wang, X.-D. Intraosseous schwannomas of the jaws: 2 case reports and review of the literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, e13–e17. [Google Scholar] [CrossRef]
- Agarwal, K.; Umarji, H.R.; Tupkari, J.V.; Chaudhary, S.; Avadhani, A.; Agrawal, N. Slowly growing swelling on body of the mandible with paresthesia on lower lip. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 677–682. [Google Scholar] [CrossRef]
- Jahanshahi, G.; Haghighat, A.; Azmoodeh, F. Intraosseous Neurilemmoma of the Mandible: Report of a Rare Ancient Type. Dent. Res. J. 2011, 8, 150–153. [Google Scholar]
- Jiang, W.H.; Brillo, G.V.; Cheng, A.H.-A.; Shen, G.F.; Wang, X.D. Endoscope-Assisted Removal of Intraosseous Schwannoma With Preservation of Inferior Alveolar Nerve. J. Craniofac. Surg. 2011, 22, 617–619. [Google Scholar] [CrossRef]
- Jang, K.Y.; Moon, W.S.; Park, H.S. Intraosseous Neurilemmoma of the Mandible—A Case Report. Korean J. Pathol. 2009, 43, 88–91. [Google Scholar] [CrossRef]
- Gallego, L.; Junquera, L.; Rodríguez-Recio, C.; Fresno, M.F. Intraosseous mandibular schwannoma mimicking an odontogenic keratocyst, with a postsurgical pathological fracture. J. Laryngol. Otol. 2009, 123, 560–562. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.C.; McGuff, H.S.; Grant, G. Oral and maxillofacial pathology case of the month. Intraosseous schwannoma. Tex. Dent. J. 2008, 125, 88–89, 94–95. [Google Scholar] [PubMed]
- Minowa, K.; Sakakibara, N.; Yoshikawa, K.; Ohmori, K.; Kitagawa, Y.; Inoue, N.; Totsuka, Y.; Nakamura, M. CT and MRI findings of intraosseous schwannoma of the mandible: A case report. Dentomaxillofac. Radiol. 2007, 36, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Kodani, I.; Ueyama, Y.; Mori, T.; Doi, R.; Osaki, M.; Kataoka, S.; Onda, M.; Sakamoto, H.; Shibata, M.; Ito, H.; et al. Intraosseous schwannoma of the mandible. Asian J. Oral Maxillofac. Surg. 2003, 15, 64–67. [Google Scholar] [CrossRef]
- Nakasato, T.; Katoh, K.; Ehara, S.; Tamakawa, Y.; Hoshino, M.; Izumizawa, M.; Sakamaki, K.; Fukuta, Y.; Kudoh, K. Intraosseous Neurilemmoma of the Mandible. Am. J. Neuroradiol. 2000, 21, 1945–1947. [Google Scholar]
- Park, Y.-K.; Kim, Y.-W.; Yang, M.-H.; Kim, E.J.; Ryu, D.-M. Neurilemmoma of the mandible. Skelet. Radiol. 1999, 28, 536–539. [Google Scholar] [CrossRef]
- Belli, E.; Becelli, R.; Matteini, C.; Iannetti, G. Schwannoma of the mandible. J. Craniofac. Surg. 1997, 8, 413–416. [Google Scholar] [CrossRef]
- Xu, Z.-Q.; Zhang, P.; Zhong, Z.-H.; Zhou, W.; Yu, H.-T. Spinal intraosseous schwannoma without spinal canal and neuroforamina involvement: A case report. World J. Clin. Cases 2020, 8, 1271–1277. [Google Scholar] [CrossRef]
- Mohanty, C.B.; Rao, K.V.L.N.; Sampath, S. Pediatric Cervical Intraosseous Schwannoma. Pediatr. Neurosurg. 2013, 48, 364–370. [Google Scholar] [CrossRef]
- Peng, X.; Chen, L.; Du, H.; Lai, Y.; Li, F.; Zou, X. Malignant Transformation of Benign Intraosseous Schwannoma in the Cervical Spine: A Case Report with an Immunohistochemical Study. Int. Surg. 2011, 96, 337–344. [Google Scholar] [CrossRef]
- Mizutani, A.; Yokota, N.; Kawaji, H.; Yamaguchi-Okada, M.; Miyagawa, T.; Namba, H. Intraosseous schwannoma of the cervical vertebral body: A case report and review of the literature. Br. J. Neurosurg. 2010, 24, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Nannapaneni, R.; Sinar, E.J. Intraosseous schwannoma of the cervical spine. Br. J. Neurosurg. 2005, 19, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Schreuder, H.W.B.; Veth, R.P.H.; Pruszczynski, M.; Lemmens, J.A.M.; Van Laarhoven, E.W. Intraosseous Schwannoma (Neurilemmoma) of the Cervical Spine. Sarcoma 2001, 5, 101–103. [Google Scholar] [CrossRef]
- Mizuno, T.; Usami, N.; Taniguchi, T.; Kawaguchi, K.; Okagawa, T.; Yokoi, K. Schwannoma of the Sternum. Ann. Thorac. Surg. 2010, 89, 1280–1281. [Google Scholar] [CrossRef] [PubMed]
- Takata, K.; Okuda, K.; Ochi, M. Intraosseous neurilemoma of the sternum. Ann. Thorac. Surg. 1999, 67, 1474–1476. [Google Scholar] [CrossRef]
- Lee, K.B.; Kwack, K.S.; Haam, S.J. Intraosseous schwannoma of the rib with cortical breakdown; resembling chondrosarcoma radiologically. Virchows. Archiv. 2019, 475, S407. [Google Scholar]
- Nguyen, K.; Nguyen, B. Multi-imaging modalities of intraosseous schwannoma of the scapula. Jt. Bone Spine 2017, 84, 493. [Google Scholar] [CrossRef]
- Tian, Y.W.; Zhang, L.Y.; Liu, Z.Q. Giant intraosseous schwannoma of scapula: A rare case report and review of the literature. Diagn. Pathol. 2014, 9, 31. [Google Scholar] [CrossRef]
- Zaidman, N.; Merve, A.; Russo, V. Intraosseous Thoracic Schwannoma: Case Report and Review of the Literature. World Neurosurg. 2019, 130, 313–316. [Google Scholar] [CrossRef]
- Kojima, M.; Seichi, A.; Yamamuro, K.; Inoue, H.; Kimura, A.; Hoshino, Y. Intraosseous schwannoma originating from the posterior column of the thoracic spine. Eur. Spine J. 2011, 20, 153–156. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, F.; Jiang, J.; Wang, H. Two Case Reports and an Updated Review of Spinal Intraosseous Schwannoma. J. Korean Neurosurg. Soc. 2015, 57, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Choudry, Q.; Younis, F.; Smith, R.B. Intraosseous schwannoma of D12 thoracic vertebra: Diagnosis and surgical management with 5-year follow-up. Eur. Spine J. 2007, 16, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Nooraie, H.; Taghipour, M.; Arasteh, M.M.; Daneshbod, K.; Erfanie, M.A. Intraosseous schwannoma of T12 with burst fracture of L1. Arch. Orthop. Trauma Surg. 1997, 116, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-C.; Chung, S.-K.; Choe, G.; Kim, H.-J. Spinal Intraosseous Schwannoma: A Case Report and Review. J. Korean Neurosurg. Soc. 2009, 46, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-J.; Huang, J.-S.; Wang, Y.-C.; Huang, S.-H. Intraosseous Schwannoma of the Fourth Lumbar Vertebra: Case Report. Neurosurgery 1998, 43, 1219–1222. [Google Scholar] [CrossRef]
- Song, D.; Chen, Z.; Song, D.; Li, Z. Lumbar intraosseous schwannoma: Case report and review of the literature. Turk. Neurosurg. 2014, 24, 982–986. [Google Scholar] [CrossRef]
- Youn, B.; Lee, S.-H.; Kim, E.-S.; Eoh, W. Intraosseous schwannoma with ancient change on the lumbar spine. Br. J. Neurosurg. 2012, 26, 561–563. [Google Scholar] [CrossRef]
- Aaron, A.D.; Nelson, M.C.; Layug, J.M.; Lage, J.M. Intraforaminal schwannoma of the sacrum. Skelet. Radiol. 1995, 24, 458–461. [Google Scholar] [CrossRef]
- Takeyama, M.; Koshino, T.; Nakazawa, A.; Nitto, H.; Nakamura, J.-I.; Saito, T. Giant Intrasacral Cellular Schwannoma Treated With High Sacral Amputation. Spine 2001, 26, E216–E219. [Google Scholar] [CrossRef]
- Silva, C.D.; Mateus, J.E.; Silva, J.O.; Vaio, T. Sacral schwannoma with intraosseous extension. BMJ Case Rep. 2019, 12, e227095. [Google Scholar] [CrossRef]
- Mutlu, A.; Tutar, S.; Ozturk, E.; Ulusoy, O.L.; Sirvanci, M. Intraosseous schwannoma of the sacrum. Spine J. 2016, 16, e407–e408. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Kanematsu, M.; Ohno, T.; Oshima, K.; Nagano, A.; Hatano, Y.; Nishibori, H. Intraosseous schwannoma of the ilium. Clin. Imaging 2015, 39, 161–164. [Google Scholar] [CrossRef]
- Benazzo, F.; Marullo, M.; Rossi, S.M.P.; Viola, E. Giant Intraosseous Schwannoma of the Ileopubic Ramus. Orthopedics 2013, 36, e982–e985. [Google Scholar] [CrossRef] [PubMed]
- Kamath, J.; Shetty, H.B.; Madegowda, A.; Bhatt, A.S. Intraosseous schwannoma of the humerus: A rarity yet warrants consideration. BMJ Case Rep. 2021, 14, e240007. [Google Scholar] [CrossRef]
- Huajun, J.; Wei, Q.; Yuxuan, W.; Jingjing, Y. Intraosseous schwannoma of the proximal humerus with pathologic fracture. Eur. J. Med. Res. 2021, 26, 72. [Google Scholar] [CrossRef] [PubMed]
- Baǧci, P.; Dervişoǧlu, S.; Hiz, M.; Kanberoǧlu, K. Intraosseous schwannoma of the radius. Turk Patoloji Derg./Turk. J. Pathol. 2010, 26, 173–176. [Google Scholar] [CrossRef]
- Gin, J.; Calmet, J.; Sirvent, J.; Domènech, S. Intraosseous neurilemmoma of the radius: A case report. J. Hand Surg. 2000, 25, 365–369. [Google Scholar] [CrossRef]
- Lim, K.X.; Wu, K. First-ever intraosseous ancient schwannoma of the proximal ulna successfully treated using the cement technique. J. Int. Med Res. 2021, 49, 300060520987732. [Google Scholar] [CrossRef]
- Suzuki, K.; Yasuda, T.; Watanabe, K.; Kanamori, M.; Kimura, T. Association between intraosseous schwannoma occurrence and the position of the intraosseous nutrient vessel: A case report. Oncol. Lett. 2016, 11, 3185–3188. [Google Scholar] [CrossRef]
- Kito, M.; Yoshimura, Y.; Isobe, K.; Aoki, K.; Momose, T.; Kato, H. Intraosseous neurilemmoma of the proximal ulna. Int. J. Surg. Case Rep. 2014, 5, 914–918. [Google Scholar] [CrossRef][Green Version]
- Gurkan, V.; Sonmez, C.; Aralasmak, A.; Yildiz, F.; Erdogan, O. An Unusual Localization of Intraosseous Schwannoma: The Hamate Bone. Clin. Pract. 2017, 7, 920. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vora, R.A.; Mintz, U.N.; Athanasian, E.A. Intraosseous schwannoma of the metacarpal. Skelet. Radiol. 2000, 29, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Afshar, A.; Afaghi, F. Intraosseous Schwannoma of the Second Metacarpal: Case Report. J. Hand Surg. 2010, 35, 776–779. [Google Scholar] [CrossRef]
- Verma, R.R.; Khan, M.T.; Davies, A.M.; Mangham, D.C.; Grimer, R.J. Subperiosteal schwannomas of the femur. Skelet. Radiol. 2002, 31, 422–425. [Google Scholar]
- Hoshi, M.; Takada, J.; Oebisu, N.; Nakamura, H. Intraosseous schwannoma of the proximal femur. Asia-Pac. J. Clin. Oncol. 2012, 8, e29–e33. [Google Scholar] [CrossRef]
- Al-Lhedan, F. Schwannoma of the femur: A rare case report. J. Bone Oncol. 2017, 8, 1–3. [Google Scholar] [CrossRef] [PubMed]
- McAleese, T.; Clesham, K.; Moloney, D.; Hughes, A.; Faheem, N.; Merghani, K. Intraosseous schwannoma of the femur in a patient with monoclonal gammopathy of undetermined significance. Int. J. Surg. Case Rep. 2020, 72, 494–498. [Google Scholar] [CrossRef]
- Mardi, K.; Vijayamohanan, L.; Aggarwal, V.; Kore, V. Intraosseous schwannoma of tibia: Report of a rare case with review of literature. Int. J. Orthop. Surg. 2021, 29, 64. [Google Scholar] [CrossRef]
- Kashima, T.G.; Gibbons, M.R.J.P.; Whitwell, D.; Gibbons, C.L.M.H.; Bradley, K.M.; Ostlere, S.J.; Athanasou, N.A. Intraosseous schwannoma in schwannomatosis. Skelet. Radiol. 2013, 42, 1665–1671. [Google Scholar] [CrossRef]
- Wang, T.; Giugale, J.M.; Ding, M.; Goodman, M.A.; Schoedel, K.; Rao, U.N. Primary intraosseous schwannoma in tibial epiphysis with unique immunohistochemical phenotype: A case report. Int. J. Surg. Pathol. 2014, 22, 574–578. [Google Scholar] [CrossRef]
- Meyer, A.; Sailhan, F.; Coulomb, A.; Thevenin-Lemoine, C.; Mary, P.; Ducou-Lepointe, H.; Damsin, J.P. Proximal tibial epiphyseal intraosseous schwannoma: A rare entity. J. Pediatr. Orthop. 2008, 28, 786–790. [Google Scholar] [CrossRef]
- Ilgenfritz, R.M.; Jones, K.B.; Lueck, N.; Buckwalter, J.A. Intraosseous neurilemmoma involving the distal tibia and fibula: A case report. Iowa Orthop. J. 2006, 26, 138–143. [Google Scholar]
- Palocaren, T.; Walter, N.M.; Madhuri, V.; Gibikote, S. Schwannoma of the fibula. J. Bone Jt. Surg. Br. Vol. 2008, 90, 803–805. [Google Scholar] [CrossRef] [PubMed]
- Aoki, J.; Tanikawa, H.; Fujioka, F.; Ishii, K.; Seo, G.S.; Karakida, O.; Sone, S. Intraosseous neurilemmoma of the fibula. Skelet. Radiol. 1997, 26, 60–63. [Google Scholar] [CrossRef]
- Drumond, G.C.; Nakagawa, S.A.; Costa, F.D.; de Souza, M.Y.T.; Comunello, J.; Chung, W.T. Intraosseous Schwannoma: Case Report and Review of the Literature. Rev. Bras. Ortop. 2020, 55, 258–262. [Google Scholar]
- Sochart, D. Intraosseous Schwannoma of the Calcaneum. Foot 1995, 5, 38–40. [Google Scholar] [CrossRef]
- Pyati, P.S.; Sanzone, A.G. Intraosseous neurilemoma of the calcaneus. Orthopedics 1996, 19, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Flores Santos, F.; Pinheiro, M.; Felicíssimo, P. Large foot schwannoma with bone invasion—A case report. Foot Ankle Surg. Off. J. Eur. Soc. Foot Ankle Surg. 2014, 20, e23–e26. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wen, X.; Qu, L.; Qi, X.; Yang, C. Intraosseous Schwannoma Involving Multiple Bones of the Foot: A Case Report. The J. Foot Ankle Surg. Off. Publ. Am. Coll. Foot Ankle Surg. 2016, 55, 201–206. [Google Scholar] [CrossRef]
- Ansari, M.T.; Rastogi, S.; Alam Khan, S.; Yadav, C.; Rijal, L. Giant Schwannoma of the First Metatarsal: A Rare Entity. J. Foot Ankle Surg. 2014, 53, 335–339. [Google Scholar] [CrossRef]
- Meek, R.; Sharma, H.; Jane, M.; Raby, N.; MacDuff, E.; Reid, R. Solitary Intraosseous Schwannoma of the Metatarsal Bone: A Case Report. Foot Ankle Int. 2007, 28, 845–848. [Google Scholar] [CrossRef]
- Stull, M.A.; Moser, R.P., Jr.; Kransdorf, M.J.; Bogumill, G.P.; Nelson, M.C. Magnetic resonance appearance of peripheral nerve sheath tumors. Skelet. Radiol. 1991, 20, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Jee, W.-H.; Oh, S.-N.; McCauley, T.; Ryu, K.-N.; Suh, J.-S.; Lee, J.-H.; Park, J.-M.; Chun, K.-A.; Sung, M.-S.; Kim, K.; et al. Extraaxial Neurofibromas Versus Neurilemmomas: Discrimination with MRI. Am. J. Roentgenol. 2004, 183, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, S.; Rubin, B.; Djang, D.; Conrad, E.; Turcotte, E.; Eary, J.F. Positron Emission Tomography of Schwannomas: Emphasizing Its Potential in Preoperative Planning. Am. J. Roentgenol. 2004, 182, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Aoki, J.; Watanabe, H.; Shinozaki, T.; Takagishi, K.; Tokunaga, M.; Koyama, Y.; Sato, N.; Endo, K. FDG-PET for preoperative differential diagnosis between benign and malignant soft tissue masses. Skelet. Radiol. 2003, 32, 133–138. [Google Scholar] [CrossRef]
- Yao, T.; Otsuka, H.; Koezuka, S.; Makino, T.; Hata, Y.; Ishiwatari, T.; Shibuya, K.; Iyoda, A. Intraosseous Schwannoma of Rib With Severe Back Pain and Characteristic Pathological Findings. Ann. Thorac. Surg. 2016, 102, e155–e157. [Google Scholar] [CrossRef][Green Version]
- Sestan, B.; Miletic, D.; Jonjic, N.; Gulan, G. Intraosseous Epithelioid Malignant Schwannoma. Orthopedics 2007, 30, 67–69. [Google Scholar] [CrossRef]
- Kaux, J.F.; Crielaard, J.M. Current evidence and indications for prolotherapy with platelet rich plasma in chronic musculoskeletal conditions. Reg. Anesth. Pain Med. 2012, 37, E104–E106. [Google Scholar]
- Terry, D.G.; Sauser, D.D.; Gordon, M.D. Intraosseous malignant peripheral nerve sheath tumor in a patient with neurofibromatosis. Skelet. Radiol. 1998, 27, 346–349. [Google Scholar] [CrossRef]
- Sham, M.E.; Ghorpade; Shetty, A.; Hari, S. Malignant peripheral nerve cell tumour. J. Maxillofac. Oral Surg. 2010, 9, 68–71. [Google Scholar] [CrossRef]
- King, A.T.; A Rutherford, S.; Hammerbeck-Ward, C.; Lloyd, S.K.; Freeman, S.R.; Pathmanaban, O.N.; Kellett, M.; Obholzer, R.; Afridi, S.; Axon, P.; et al. Malignant Peripheral Nerve Sheath Tumors are not a Feature of Neurofibromatosis Type 2 in the Unirradiated Patient. Neurosurgery 2018, 83, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, S.; Conway, S.A.; Pitcher, J.D.; Temple, H.T. Primary Intraosseous Malignant Peripheral Nerve Sheath Tumor of the Medial Cuneiform: A Case Report and Review of the Literature. J. Foot Ankle Surg. 2017, 56, 129–134. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Hu, J.X.; Yang, S.M.; Jiang, L.; Liu, X.G.; Yuan, H.S.; Wei, F.; Liu, Z.J. Intraosseous schwannoma of the mobile spine: A report of twenty cases. Eur. Spine J. 2018, 27, 3092–3104. [Google Scholar] [CrossRef] [PubMed]
- Wirth, W.A.; Bray, C.B. Intra-osseous neurilemoma. Case report and review of thirty-one cases from the literature. J. Bone Jt. Surg. 1977, 59, 252–255. [Google Scholar] [CrossRef]


| Patient | Sex/Age | Lesion Location | Tumor Size (cm) | Clinical Findings | Radiographic Findings | CT Findings | MRI Findings |
|---|---|---|---|---|---|---|---|
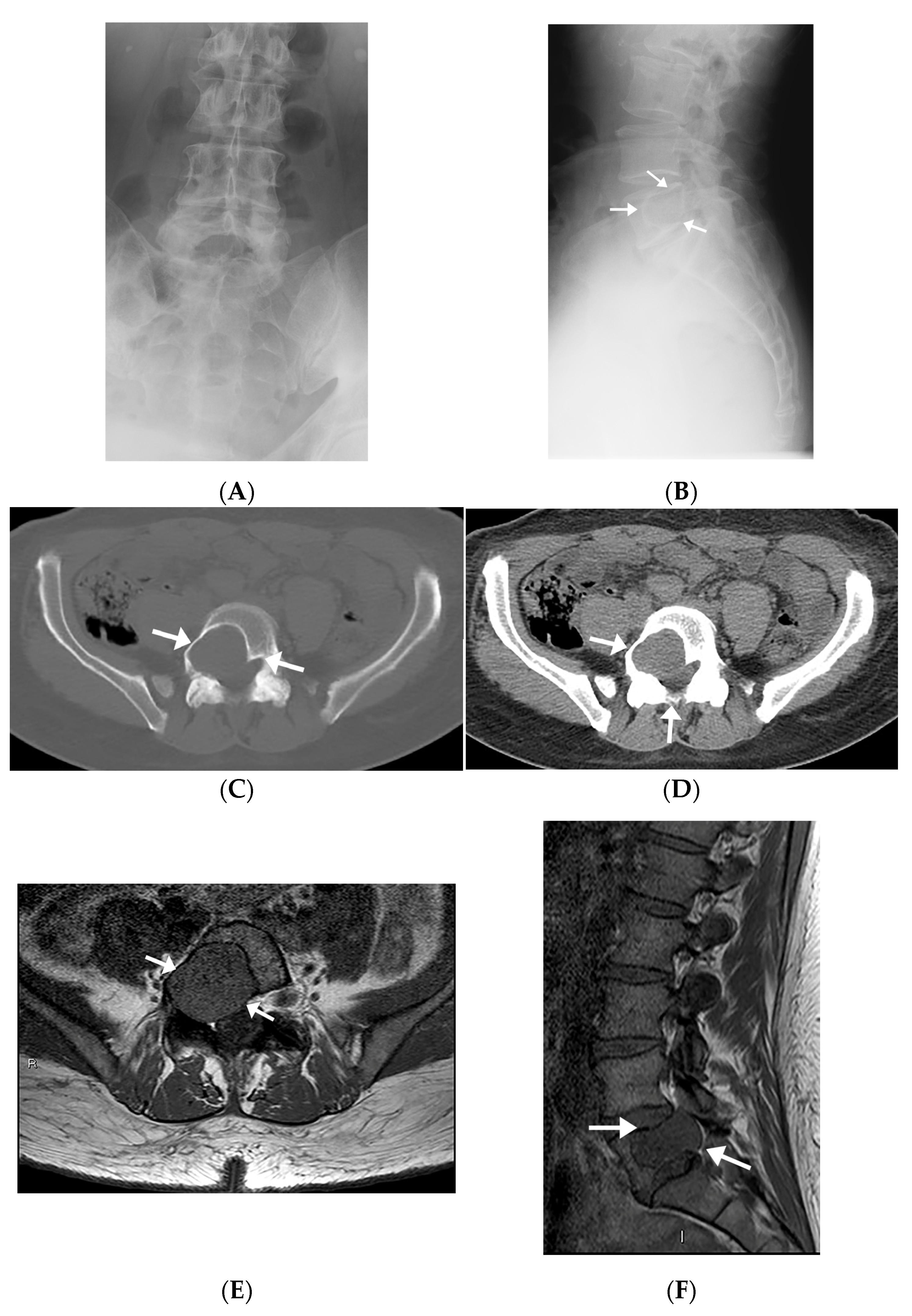
| 1 | M/60 | Left sacral ala | 2.5 × 3.5 × 3.2 | Right foot drop | N/A | Well-defined lytic lesion with sclerotic lobulated margins. The mass encroaches the left S1 neural foramen and abuts the nerve root with cortical destruction at the anterior margin of the vertebral body. | Avidly enhancing lesion invading S1 neural foramen and abuts exiting S1 spinal nerve root with mild mass effect. Isointense on T1WI, increased SI on STIR sequence. |
| 2 | M/51 | Right sacral ala | 2.1 × 1.9 × 2.8 | Lower back/right lower extremity numbness, difficulty walking, and pain | Occult lesion on postoperative radiograph | Intraosseous mass extending into paraspinal soft tissues posteromedial to the right psoas muscle. Mass also extends through the cortex of right sacral wing. | Avidly enhancing lobulated lesion involving the anterior aspect of S1 vertebral body. T1WI low SI, T2WI isointense SI, and STIR high SI. Cortical breakthrough in the anterior aspect. No definite neuroforaminal involvement. |
| 3 | F/40 | Sacrum midline | 5.5 × 2.6 × 4.3 | Severe lower back pain | Ill-defined lucent lesion, difficult to delineate from adjacent bowel gas | Lobulated lytic lesion extending into the paraspinal soft tissues posteromedial to the right psoas, separate from the exiting right L5 and S1 nerve roots. | High SI on T2WI, homogeneously low SI on T1WI, and nearly uniform enhancement. Well-defined expansile lesion involving S2 and S3 levels displacing adjacent nerve roots. |
| 4 | M/64 | S1 and S2 vertebral bodies | 12 × 11 × 7 | Left leg pain | N/A | Heterogenous intraosseous mass with soft tissue extension, hypodense areas centrally, and calcifications in the periphery. | Large destructive mass extending into the pelvis anterior to the sacrum as well as posteriorly into the epidural space and the region of the spinous processes. Low SI on T1WI and heterogeneous SI on T2WI with areas of both high and low signal. |
| 5 | M/57 | L5 vertebral body | 3.8 × 3.2 × 3.5 | Sudden onset right foot drop | Well-defined lytic lesion with anterior sclerotic border and cortical breakthrough posteriorly | Expansile benign appearing lytic lesion extending into the right anterior epidural space and right neural foramen with mass-effect in the thecal sac. | Heterogenous intermediate intensity on T2WI, hypointense on T1WI mass extending into the left pedicle, encroaching upon the right neural foramen and right lateral recess. |
| 6 | M/28 | Left sacral ala | 6.7 × 4.9 × 7 | Severe low back pain | Large expansile lytic lesion with irregular sclerotic borders | Expansile lytic lesion with scalloped bony erosion involving the spinal canal from L5-S1 to S2 and extension to the left posterior pelvis. | Intensely enhancing mass with areas of central necrosis extending through the left S1 neural foramen into the pelvis. Heterogeneous SI on T2WI, isointense to muscle on T1WI. |
| Case | Authors, Year of Publication | Age | Sex | Clinical Findings | Lesion Location | Radiographic Findings | CT Findings | MRI Findings |
|---|---|---|---|---|---|---|---|---|
| 1 | Rozman et al., 2019, cited by [11] | 68 | F | Intermittent vertigo, tinnitus, facial numbness, hearing loss, diplopia, and ataxia | Petrous apex | N/A | Expansile lytic lesion extending through the petrous ridge to the apex. | Destructive lesion with displacement of the petrous and cavernous segments of the internal carotid artery. Low SI on T1-WI and high SI on T2-WI with some enhancement. |
| 2 | Sato et al., 2019, cited by [12] | 35 | F | Hearing disturbance and fullness | Petrous apex | N/A | Isodense lesion destructed bony structure with preserved cortex. | Isointense to brainstem on T1-WI, iso to high SI on T2-WI, with heterogeneous enhancement. |
| 3 | Tamura et al., 2015, cited by [13] | 47 | M | Double vision | Petrous apex | N/A | Lytic expansive lesion with enhancement, scalloped margin, and thin intact rim. | Low SI on T1-WI and slightly high SI on T2-WI with heterogenous enhancement. |
| 4 | Goiney et al., 2011, cited by [14] | 48 | F | New headache and decreased sensation | Petrous apex | N/A | Well-defined, lytic lesion with no extraosseous component. | Isointense to brain on T1-WI, high SI on T2-WI with enhancement. |
| 5 | Mathieu et al., 2018, cited by [15] | 7 | M | Painless protruding mass | Occipital bone | N/A | Well-defined, deforming intraosseous lytic lesion with sclerotic rim. | Well-defined expansile lesion. Low SI on T1-WI, high SI on T2-WI with no enhancement. |
| 6 | Goyal et al., 2008, cited by [16] | 11 | M | Painless swelling | Frontal bone | Lytic lesion with sclerotic rim and trabeculation. | Hypodense expansile lesion with focal cortical destruction and soft-tissue swelling. No periosteal reaction. | N/A |
| 7 | El-Bahy et al., 2004, cited by [17] | 40 | M | Painless proptosis and lateral gaze impairment | Sphenoorbital | N/A | Lytic lesion with sclerotic margins. | Isointense on T1-WI and hyperintense on T2-WI, and homogenous enhancement. |
| 8 | Celli et al., 1998, cited by [18] | 3 | M | Painless swelling | Occipital bone | Lytic lesion with sclerotic borders. | Soft tissue mass with bone erosion. | N/A |
| 9 | 14 | M | Painless swelling | Frontal bone | Well-defined lytic lesion with sclerotic borders. | Soft tissue mass with bone erosion. | Extradural lesion with mix SI on T1-WI, high SI on T2-WI, and peripheral intense enhancement. | |
| 10 | Matsuoka et al., 2020, cited by [19] | 61 | M | Painless swelling | Maxilla | Well-defined lytic lesion. | Bone resorption and thinning in the left incisor region. | Well-defined, homogeneously isointense on T1-WI, and high SI on T2-WI. |
| 11 | Avinash et al., 2016, cited by [20] | 38 | M | Swelling | Maxilla | Partly well-defined lytic lesion that disrupted the floor of maxillary sinus. | Isodense expansile lesion with cortical perforation. | N/A |
| 12 | Oliveira et al., 2021, cited by [21] | 12 | F | Pain and swelling | Mandible | Lytic lesion with sclerotic border. | Hypodense expansile lesion with cortical thinning and destruction. | N/A |
| 13 | Kardouni et al., 2021, cited by [22] | 24 | M | Painless swelling | Mandible | Well-defined lytic lesion with a sclerotic border and root resorption. | Expansile lesion with cortical destruction and homogenous density. | N/A |
| 14 | Perkins et al., 2018, cited by [23] | 22 | M | Swelling | Mandible | Well-defined expansile lytic lesion with sclerotic margin and cortical erosion | Multiloculated lytic lesion with teeth root erosion and scalloping border. | N/A |
| 15 | Kargahi et al., 2012, cited by [24] | 9 | M | Painless swelling | Mandible | Lytic lesion with sclerotic border. | Well-defined expansile lesion with cortical thinning. | N/A |
| 16 | Suga et al., 2013, cited by [25] | 33 | M | Throbbing pain | Mandible | Expansive lytic lesion with sclerotic margin. | Expansive lytic fusiform lesion. | Well-defined lesion with low SI on T1-WI, high SI on T2-WI and enhancement. |
| 17 | Zhang et al., 2012, cited by [26] | 35 | M | Swelling and paresthesia | Mandible | Well-defined bilocular lytic lesion with sclerotic rim and dental root resorption. | Expansive lesion with bilocular destruction of the medial and lingual cortical plates. | N/A |
| 18 | 39 | F | Swelling | Mandible | Well-defined lytic lesion with sclerotic rim and nerve extension. | Inferior alveolar nerve involvement. | N/A | |
| 19 | Agarwal et al., 2012, cited by [27] | 23 | F | Swelling and paresthesia | Mandible | Well-defined expansile lytic lesion with sclerotic and scalloped margin. | Unilocular, expansile, isodense lesion with marked cortical thinning and destruction. | Isointense on T1-WI, mix high SI on T2-WI and homogeneous contrast enhancement. |
| 20 | Jahanshahi et al., 2011, cited by [28] | 11 | F | Swelling | Mandible | Well-defined, unilocular lytic lesion with thin sclerotic borders. | Lingual cortex destruction. | N/A |
| 21 | Jiang et al., 2011, cited by [29] | 39 | F | Painless swelling | Mandible | Well-defined lytic lesion with sclerotic borders. | Unilocular lesion with the inferior alveolar nerve involvement. | N/A |
| 22 | Jang et al., 2009, cited by [30] | 77 | F | Painful swelling | Mandible | Well-defined lytic lesion with sclerotic margin. | Expansile lesion with buccal and lingual cortical thinning. | N/A |
| 23 | Gallego et al., 2009, cited by [31] | 60 | M | Incidental finding | Mandible | Well-defined lytic lesion with sclerotic margin. | Unilocular lesion along the inferior alveolar nerve canal. | N/A |
| 24 | Jones et al., 2008, cited by [32] | 23 | F | Incidental finding | Mandible | Multilocular expansile lytic lesion. | Expansible lesion to coronoid process. | N/A |
| 25 | Minowa et al., 2007, cited by [33] | 67 | F | Incidental finding | Mandible | Well-defined, expansive, lytic lesion with sclerotic margin. | Cortical destruction with no periosteal reaction. | Isointense SI on T1-WI and high SI on T2-WI with enhancement. |
| 26 | Kodani et al., 2003, cited by [34] | 45 | F | Slight diffuse swelling | Mandible | Lytic lesion with sclerotic margin. | Well-demarcated expanding mass with a clear border. | High SI on T2-WI. |
| 27 | Nakasato et al., 2000, cited by [35] | 19 | F | Crepitation in TM joint | Mandible | Expansive lytic lesion with sclerotic margin. No septation. | Peripheral scalloping and cortical erosion with a defect in cortex. | Isointense on T1-WI, high SI on T2-WI with peripheral enhancement. |
| 28 | Park et al., 1999, cited by [36] | 29 | F | Incidental finding | Mandible | Well-defined lytic lesion with sclerotic margin. | Cortical thinning but no destruction. | N/A |
| 29 | Belli et al., 1997, cited by [37] | 8 | M | Painless swelling | Mandible | Well-defined lytic lesion with thin septa. | Enhanced lesion with cortical reabsorption but no destruction or periosteal reaction. | N/A |
| 30 | Xu et al., 2020, cited by [38] | 56 | M | Chronic and persistent neck pain | C7 vertebra | Lytic lesion with sclerotic border and C7 vertebral body height loss. | Lytic bony lesion with well-preserved vertebral cortex. | Well-defined low SI on T1-WI, high SI on T2-WI. Anterior cortex destruction. No spinal cord extension. |
| 31 | Mohanty et al., 2012, cited by [39] | 10 | M | Painless swelling | C4 vertebra | Lytic with sclerotic border and anterior cortical destruction | Lytic lesion with soft tissue component and no intraspinal extension. | Low SI on T1WI, mix SI on T2WI, and uniform enhancement. |
| 32 | Peng et al., 2011 cited by [40] | 44 | M | Progressive dizziness and mild weakness in the right upper limb | C3 vertebra | C3 vertebral collapse, loss of the right C3 pedicle and C3 to C4 disc space, enlargement of intervertebral foramen, and displacement of C2 and C3 vertebrae anteriorly | Expansile, lytic, and invasive lesion destroying the right pedicle, laminar, and spinal process. A soft tissue mass extending from the C3 vertebral body to the right spinal canal and paravertebral. | T2-WI showed an inhomogeneously hyperintense mass involving in the C3 and partial C2 vertebral body, displacing the spinal cord, and extending the spinal cord posteriorly |
| 33 | Mizutani et al., 2010, cited by [41] | 44 | F | Incidental finding | C4 vertebra | N/A | Bony defect with preserved cortex. | Well-defined isointense lesion on T1-WI, high SI on T2-WI, and homogeneous enhancement. |
| 34 | Nannapaneni et al., 2005, cited by [42] | 42 | M | Incidental finding | C5 vertebra | Lytic lesion with C4-5-disc space and C5 vertebral body height loss with soft tissue shadow. | Expansile lytic lesion with sclerotic margin, faintly calcified, and poorly enhanced with cortical destruction. | Well-defined isointense mass on T1-WI, high SI on T2-WI with marginal enhancement and exophytic component displacing carotid sheath. |
| 35 | Schreuder et al., 2001, cited by [43] | 38 | F | Neck pain and dysphagia | C6 vertebra | Lytic with sclerotic borders and anterior cortical destruction. | N/A | Soft tissue displacement with no invasion. High SI on T1 and T2-WI with fat and high fluid content. |
| Case | Authors, Year of Publication | Age | Sex | Clinical Findings | Lesion Location | Radiographic Findings | CT Findings | MRI Findings |
|---|---|---|---|---|---|---|---|---|
| 1 | Mizuno et al., 2010, cited by [44] | 38 | F | Incidental finding | Sternum | N/A | Well-defined low-density nodule expanded into the thoracic cavity. | Isointense on T1-WI and a high SI on T2-WI with no enhancement. |
| 2 | Takata et al., 1999, cited by [45] | 10 | F | N/A | Sternum | Lytic lesion with sclerotic margin | Mass with cortical destruction and soft tissue extension. | Isointense SI on T1-WI and high SI on T2-WI with no invasion to thoracic cavity. |
| 3 | Lee et al., 2019, cited by [46] | 58 | M | Painful mass | Seventh rib | Expansile lytic lesion | A mass with peripherally curved calcification and cortical destruction resulting in soft tissue mass formation. | N/A |
| 4 | Nguyen et al., 2017, cited by [47] | 48 | M | Incidental finding | Scapula | Expansile lytic lesion with sclerotic borders. | Lytic lesion with thin-rimmed cortical bone remodeling and lack of internal calcifications. | Contrast-enhancing, lobulated lesion on T1-WI with no adjacent soft tissue involvement. |
| 5 | Tian et al., 2014, cited by [48] | 42 | F | Shoulder pain | Scapula | Lytic lesion with sclerotic margin | Destructive mass extending into surrounding soft tissues. | Low to intermediate SI on T1-WI, high SI on T2-WI and destruction of left scapula and glenoid. |
| 6 | Reyniers et al., 2021, cited by [4] | 49 | F | Shoulder pain and paresthesia | Glenoid | Well-defined lytic lesion with thin sclerotic rim and trabeculation. | N/A | Expansile lesion with cortical thinning and focal breach. Isointense on T1-WI, high SI on T2-WI, and heterogenous enhancement. |
| 7 | Zaidman et al., 2019, cited by [49] | 56 | F | Incidental finding | T1 vertebra | N/A | Lytic lesion with no sclerosis. | High enhancement and high SI on T2-WI, without extension to surrounding soft tissue. |
| 8 | Kojima et al., 2011, cited by [50] | 60 | M | Pain, gait disturbance, numbness, weakness | T9 vertebra | N/A | Large lytic lesion with erosion of the lamina, spinous process, and cortical destruction. | Isointense on T1-WI, high mixed SI on T2-WI and irregular enhancement with paravertebral muscles extension. |
| 9 | Zhang et al., 2015, cited by [51] | 54 | F | Gait disturbance, paresthesia, | T9 vertebra | N/A | Bone erosion and destruction. | Isointense on T1-WI, mixed SI on T2-WI. Extending to spinal canal and paravertebral areas. |
| 10 | 71 | M | Pain, gait disturbance, paresthesia, | L4 vertebra | N/A | Lytic lesion with sclerotic rim and severe vertebral destruction. | Isointense on T1-WI, heterogeneous on T2-WI, irregular enhancement. | |
| 11 | Choudry et al., 2007, cited by [52] | 18 | M | Back pain, and weakness | T12 vertebra | Gross cystic changes with partial collapse of vertebral body. | N/A | Perivertebral protrusion compressing the thecal sac and neural foramina. Low SI on T1-WI, heterogenous SI on T2-WI and no enhancement. |
| 12 | Nooraie et al., 1997, cited by [53] | 46 | M | Burst fracture following car accident | T12 vertebra | Lytic lesion with a sclerotic rim in the T12 vertebra. | Large lytic lesion, involving all three spinal columns, with sclerotic rim, pedicle erosion, and cortical destruction. | N/A |
| 13 | Park et al., 2009, cited by [54] | 48 | F | Back pain and weakness | L4 vertebra | N/A | Expansile lytic tumor with sclerotic rim. | Peripheral enhancement of the degenerative portion with vertebral body pathologic fracture. High SI on T2-WI and isointense on T1-WI. |
| 14 | Chang et al., 1998, cited by [55] | 58 | M | Numbness, pain, and weakness | L4 vertebra | Expansile lytic lesion with smooth and sclerotic border. | Hypervascular mass with cephalad and caudal extension causing posterior compression of the thecal sac. | L4 and L5 vertebral body mass with thecal sac and bilateral neuroforamina compression. |
| 15 | Song et al., 2014, cited by [56] | 44 | M | Low back pain with radiation | L5 vertebra | N/A | Irregular lytic lesion with marginal sclerosis caused isthmic spondylolysis. Posterior protrusion with cortical destruction, and thecal sac compression. | Low SI on T1-WI and high SI on T2-WI. Mild heterogeneous enhancement. |
| 16 | Youn et al., 2012, cited by [57] | 65 | M | Progressive lower back pain | L2 vertebra | Expansile lytic lesion with sclerotic margin. | Lytic lesion with a sclerotic margin. | High mixed SI on T2-WI corresponding to cystic degeneration, isointense on T1-WI, and irregular enhancement. |
| 17–29 | Summers et al., 2018, cited by [5] | 25–80 | 7 M 6 F | Low back pain (6/13), Radiculopathy (2/13), incidental (4/13), not specified (7/13) | Sacrum | N/A | Solid, expansile lytic lesions with sclerotic margins, pathologic fracture in 1/13. | T1-WI: Hypointense (5/8) Isointense (3/8), T2-WI: Heterogenous (8/8) solid (4/8) or cystic lesions with fluid-fluid levels (4/8) that may exhibit target sign. Post-contrast images: Heterogeneous enhancing solid component (8/8), non-enhancing cysts (4/8), and no necrosis. |
| 30 | Aaron et al., 1995, cited by [58] | 53 | M | Back pain and paresthesia | Midline of sacrum | Lytic with thin sclerotic border. | Lucent homogenous lesion without calcification abutting the paraspinal musculature. | Solitary isointense mass on T1-WI, moderately high SI on T2-WI and marked enhancement. |
| 31 | Takeyama et al., 2001, cited by [59] | 45 | M | Pain and claudication | Sacrum | Lytic lesion with cortical destruction. | Gigantic retroperitoneal mass displacing the bladder ventrally. | Sacral lesion with cranial expansion of tumor. High SI on T2-WI. |
| 32 | Silva et al., 2019, cited by [60] | 22 | M | Lumbar pain and difficulty walking | Sacrum | N/A | Lytic lesion with soft tissue component and sacral wing scalloping. | Contrast-enhancing lesion. |
| 33 | Mutlu et al., 2015, cited by [61] | 38 | M | Progressive pain and claudication | Sacrum | N/A | A soft tissue mass with scalloping of the surrounding bone. | Obliteration of sacral canal. Isointense on T1-WI, and hyperintense on T2-WI. |
| 34 | Kato et al., 2015, cited by [62] | 27 | M | Incidental finding | Ilium | N/A | Well-demarcated lytic lesion with punctate calcification, sclerotic rim and cortical erosion. Heterogenous enhancement. | Heterogeneously high SI on T2-WI with peripheral hypointense rim. Low SI on T1-WI and heterogenous enhancement. |
| 35 | Benazzo et al., 2013, cited by [63] | 63 | F | Paroxysmal pain | Iliopubic ramus | Well-defined expansile lytic lesion with cortical destruction | N/A | Expansile lesion with extraosseous extension. Low SI on T1-WI, and high SI on T2-WI with heterogenous enhancement. |
| Case | Authors, Year of Publication | Age | Sex | Clinical Findings | Lesion Location | Radiographic Findings | CT Findings | MRI Findings |
|---|---|---|---|---|---|---|---|---|
| 1 | Kamath et al., 2021, cited by [64] | 45 | F | Fracture following low impact fall | Humerus | Lytic lesion with pathological fracture | N/A | Low SI on T1-WI and high SI on T2-WI/STIR with few septations and cortical thinning. No cortical erosions. |
| 2 | Huajun et al., 2021, cited by [65] | 55 | F | Fracture following low impact fall | Humerus | Well-defined lytic with sclerotic margin, endosteal scalloping. No calcifications | Periosteal elevation and pathologic fracture. | Cortical invasion with associated soft tissue edema. Isointense on T1-WI, and high SI on T2-WIs. |
| 3 | Mutema and Sorger, 2002, cited by [1] | 33 | M | Pain, swelling and reduced ROM | Humerus | Expansile, lytic lesion with sclerotic margin, endosteal scalloping and no cortical destruction | N/A | Low SI on T1-WI and high SI on T2-WI associated with soft tissue edema. |
| 4 | Baǧci et al., 2010, cited by [66] | 19 | F | Pathologic fracture | Radius | Expansile lytic lesion with sclerotic rim | N/A | Intramedullary mass with soft tissue component and no homogeneous pattern. |
| 5 | Giné et al., 1999, cited by [67] | 45 | M | Painless swelling | Radius | Lytic lesion with sclerotic borders, trabeculation and cortical destruction | Destruction of the dorsal cortex with associated soft tissue component. | Hyperintense mass on T1-WI and hypointense on T2-WI. |
| 6 | Lim et al., 2021, cited by [68] | 77 | F | Fracture following low impact fall | Ulna | Well-defined lytic lesion with a thin sclerotic rim, pathological fracture | N/A | Expansile intraosseous mass invading the adjacent muscle and subcutaneous fat tissue. |
| 7 | Suzuki et al., 2016, cited by [69] | 87 | F | Rapidly-growing mass with tenderness | Ulna | Well-defined, lytic lesion with sclerotic margin, pathological fracture, and cortical thinning | Cortical expansion and thinning with no calcification. | Isointense on T1-WI, and heterogenous high SI on T2-WI with soft tissue extension. |
| 8 | Kito et al., 2014, cited by [70] | 21 | F | Pain | Ulna | Well-defined, lytic expansile with marginal sclerosis and trabeculation | Cortical destruction with associated soft tissue mass. No periosteal reaction. | Isointense mass on T1-WI, heterogeneously hyperintense on T2-WI, and uniform enhancement. |
| 9 | Gurkan et al., 2017, cited by [71] | 34 | F | Pain and mild swelling | Hamate | Well-defined lytic lesion with sclerotic borders and cortical destruction. No calcifications | Expansile lytic lesion with dorsal cortical breakthrough. No periosteal reaction. | Cortical disruption with soft tissue extension. Hyperintense on PD-fat sat and T2-WI sequences and homogenous enhancement. |
| 10 | Vora et al., 2000, cited by [72] | 45 | M | Pain with no swelling | Metacarpal | Expansile lytic with pathologic fracture and periosteal reaction. No sclerosis or calcification | N/A | Homogeneously low SI on T1-WI and high SI on T2-WI with endosteal scalloping and periosteal reaction. |
| 11 | Afshar and Afaghi, 2010, cited by [73] | 12 | M | Painless swelling | Metacarpal | Well-defined, lytic expansile lesion with sclerotic margin and cortical disruption | N/A | Cortical disruption and marrow replacement with heterogeneous SI on T1-WI and high SI on T2-WI. |
| 12 | Verma et al., 2002, cited by [74] | 38 | M | Pain and insidious weakness | Femur | Smooth scalloping of the outer aspect of the medial cortex with no matrix mineralization | N/A | Well-defined isointense mass on T1-WI, hyperintense on both T2-WI and STIR, with enhancement and no cortical breaching. |
| 13 | Hoshi et al., 2012, cited by [75] | 44 | F | Pain | Femur | Well-defined lytic with sclerotic margin at the lesser trochanter. | Osteolytic with cortical ballooning and thinning, and periosteal reaction. | Expansile low- to iso-intensity on T1-WI and heterogeneously high SI on T2-WI with marked enhancement. |
| 14 | Wang et al., 2014, cited by [10] | 42 | M | Pain | Femur | Well-defined expansile lytic with sclerotic margins and cortical destruction | N/A | Heterogeneously isointense on T1-WI, and hyperintense on FSE-T2-WI, with homogenous enhancement. |
| 15 | Al-Lhedan, 2017, cited by [76] | 18 | F | Painless swelling | Femur | Expansile lytic lesion with sclerotic margins | N/A | Low SI on T1-WI and high SI on fluid sensitive sequences with avid enhancement and soft tissue component. |
| 16 | McAleese et al., 2020, cited by [77] | 55 | F | Pain | Femur | N/A | Lytic lesion with a thin sclerotic margin causing mild cortical scalloping. | Well marginated expansile lesion with a homogeneous consistency. Low SI on T1-WI, high SI on T2-WI. |
| 17 | Mardi et al., 2021, cited by [78] | 46 | F | Pain and swelling | Tibia | Expansile lytic lesion with sclerotic margin and trabeculation | N/A | Well-defined lobulated eroding mass with thin septa. |
| 18 | Kashima et al., 2013, cited by [79] | 55 | F | Leg pain | Tibia | Well-defined lytic lesion with a thin sclerotic rim | N/A | Isointense on T1-WI and high SI on T2-W fat-suppressed sequence. |
| 19 | 62 | F | Fracture following low impact fall | Tibia | Pathological fracture through well-defined lytic lesion with thin sclerotic rim | N/A | Dumbbell-shaped intramedullary lesion with the waist of the dumbbell centered on the cortex. | |
| 20 | Wang et al., 2014, cited by [80] | 75 | F | Painful small lump | Tibia | Well-defined lytic lesion with a sclerotic rim. No calcification or periosteal reaction | Lobulated intraosseous lesion with cortical breakthrough. | N/A |
| 21 | Meyer et al., 2008, cited by [81] | 13 | M | Increasing pain | Tibia | Well-defined lytic lesion with sclerotic rim and intact growth plate | Lytic lesion cortical thinning and destruction. No calcification or periosteal reaction | Intra-articulaire expansion. Isointense on T1-WI, heterogeneous high SI on T2-WI and intensive enhancement. |
| 22 | Ilgenfritz et al., 2006, cited by [82] | 34 | F | Pain and asymmetry | Tibia and fibula | Lytic lesions with well-defined sclerotic margins and periosteal reaction | Cortical destruction and with eroding soft tissue mass. | Isointense on T1-WI and heterogenous hyperintense on T2-WI. |
| 23 | Palocaren et al., 2008, cited by [83] | 14 | M | Pain and swelling of the right leg | Fibula | Well-defined lytic expansile lesion with sclerotic rim, trabeculations, and cortical destruction | Cortical expansion and destruction with soft tissue extension. | Isointense on T1-WI and homogeneous high SI on T2-WI with surrounding soft-tissue edema. No fluid levels. |
| 24 | Aoki et al., 1997, cited by [84] | 56 | F | Right knee pain | Fibula | Well-defined lytic lesion with marginal sclerosis and calcifications | Cortical expansion and destruction with partial discontinuity, no soft tissue extension. | Isointense SI on T1-WI and higher SI than subcutaneous fat on T2-WI. Uneven enhancement with avidly enhancing nodules. |
| 25 | Drumond et al., 2020, cited by [85] | 49 | M | Pain and swelling | Calcaneus | Well-delimited lytic lesion with sclerotic rim and cortical rupture. No calcification | N/A | Low SI on T1-WI, heterogeneous SI on T2-WI, and intense enhancement. Cortical rupture with extensive soft tissue component. |
| 26 | Haberal et al., 2018, cited by [2] | 35 | F | Right heel pain | Calcaneus | Cystic lytic with sclerotic rims | N/A | Cystic hypointense lesion on T1-WI and hyperintense on T2-WI with cortical involvement and well-defined borders. |
| 27 | Sochart et al., 1995, cited by [86] | 23 | F | Intermittent pain and swelling | Calcaneum | Cystic lesion with sclerotic margins and no calcification | Attenuation and thinning of the lateral cortex. | N/A |
| 28 | Pyati et al., 1996, cited by [87] | 35 | F | Painful heel caused limping | Calcaneus | Multiloculated, expansile lytic lesion | Large lytic lesion with medial wall erosion. | N/A |
| 29 | Flores Santos et al., 2014, cited by [88] | 70 | F | Visible mass with pain and swelling | Cuboid | Lytic lesion with sclerotic margins and cortical disruption | Large soft tissue mass with extensive bone involvement. | Isointense mass on T1-WI along the posterior tibial nerve. Hyperintense signal on T2-WI. |
| 30 | Wang et al., 2016, cited by [89] | 50 | F | Swelling with dull pain | Foot | Well-defined lytic lesion with sclerotic margin and trabeculation | Bone destruction with extension to subcutaneous tissue. No calcifications. | Isointense to skeletal muscle on T1-WI and hyperintense to subcutaneous fat on T2-WI. |
| 31 | Ansari et al., 2014, cited by [90] | 48 | F | Swelling | First metatarsal | Well-defined expansible lytic lesion with sclerotic margins | N/A | Isointense on T1-WI, high SI on T2-WI, with cortical break. |
| 32 | Meek et al., 2007, cited by [91] | 27 | F | Pathologic fracture | Metatarsal | Lytic lesion with sclerotic margins | N/A | Amorphous mass in medullary canal with cortical destruction. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shomal Zadeh, F.; Azhideh, A.; Mantilla, J.G.; Kosaraju, V.; Venugopal, N.; Gaskin, C.M.; Pooyan, A.; Alipour, E.; Chalian, M. Imaging Features of Intraosseous Schwannoma: A Case Series and Review of the Literature. Diagnostics 2023, 13, 1610. https://doi.org/10.3390/diagnostics13091610
Shomal Zadeh F, Azhideh A, Mantilla JG, Kosaraju V, Venugopal N, Gaskin CM, Pooyan A, Alipour E, Chalian M. Imaging Features of Intraosseous Schwannoma: A Case Series and Review of the Literature. Diagnostics. 2023; 13(9):1610. https://doi.org/10.3390/diagnostics13091610
Chicago/Turabian StyleShomal Zadeh, Firoozeh, Arash Azhideh, Jose G. Mantilla, Vijaya Kosaraju, Nitin Venugopal, Cree M. Gaskin, Atefe Pooyan, Ehsan Alipour, and Majid Chalian. 2023. "Imaging Features of Intraosseous Schwannoma: A Case Series and Review of the Literature" Diagnostics 13, no. 9: 1610. https://doi.org/10.3390/diagnostics13091610
APA StyleShomal Zadeh, F., Azhideh, A., Mantilla, J. G., Kosaraju, V., Venugopal, N., Gaskin, C. M., Pooyan, A., Alipour, E., & Chalian, M. (2023). Imaging Features of Intraosseous Schwannoma: A Case Series and Review of the Literature. Diagnostics, 13(9), 1610. https://doi.org/10.3390/diagnostics13091610





