Diffuse Reflectance Spectroscopy of the Oral Mucosa: In Vivo Experimental Validation of the Precancerous Lesions Early Detection Possibility
Abstract
1. Introduction
2. Materials and Methods
- —the average normalized spectrum of the light source.
- —α-quantile of Student’s distribution (α = 0.05) c degrees of freedom.
- —sample variance of the average spectrum value at a given wavelength for the intact and lesioned area, respectively.
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2016, Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.E. The World Oral Health Report 2003: Continuous improvement of oral health in the 21st century—The approach of the WHO Global Oral Health Programe. Community Dent. Oral Epidemiol. 2003, 31, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.E.M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer, Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2018.
- Gazhva, S.I.; Stepanyan, T.B.; Goryacheva, T.P. The prevalence of dental diseases of the oral mucosa and their diagnosis. Int. J. Appl. Fundam. Res. 2014, 5, 41–44. [Google Scholar]
- Kaprina, A.D.; Starinsky, V.V.; Shakhzadova, A.O. Malignant Neoplasms in Russia in 2019 (Morbidity and Mortality); MNIIOI n.a. P.A. Herzen-Branch of the Federal State Budgetary Institution “NMITs Radiology” of the Ministry of Health of Russia: Moscow, Russia, 2020. (In Russian) [Google Scholar]
- Cherkasov, S.M. Analysis of the prevalence of diseases of the dentoalveolar system that form the demand for dental services. Fundam. Res. 2014, 2, 186–189. (In Russian) [Google Scholar]
- Zhidovinov, A.V.; Mikhalchenko, D.V. Retrospective analysis of statistical data on the incidence of malignant neoplasms of maxillofacial localization. Mod. Probl. Sci. Educ. 2016, 6, 151. (In Russian) [Google Scholar]
- Danilevsky, N.F.; Leontiev, V.K.; Nesin, A.F.; Rachniy, Z.I. Diseases of the Oral Mucosa; “Stomatologiya”: Moscow, Russia, 2001. (In Russian) [Google Scholar]
- Volkova, M.N.; Chernyavsky, Y.P.; Sakharuk, N.A.; Elena, Y.R. Diseases of the Oral Mucosa: Study Guide; VSMU: Vitebsk, Belarus, 2016. (In Russian) [Google Scholar]
- Dedova, L.N.; Shebeko, L.V.; Urbanovich, V.I.; Belyasova, L.V. Lesions of the Mucous Membrane of the Oral Cavity of White Color (Leukoplakia, P 59 Lichen Planus): Study Guide; BSMU: Minsk, Belarus, 2010. (In Russian) [Google Scholar]
- Dedova, L.N.; Fedorova, I.N. Systematization of Erosive and Ulcerative Lesions of Mucosa Mouth; BSMU: Minsk, Belarus, 2010. (In Russian) [Google Scholar]
- Kolesnikova, L.L.; Arutyunova, S.D.; Lebedenko, I.Y.; Degtyarev, V.P. Anatomy, Physiology and Biomechanics of the Dental System; GEOTAR-Media: Moscow, Russia, 2009. (In Russian) [Google Scholar]
- Makedonova, Y.A.; Fedotova, Y.M.; Firsova, I.V.; Poroisky, S.V. Modern aspects of the treatment of erosive and ulcerative form of lichen planus of the oral mucosa. Mod. Probl. Sci. Educ. 2016, 2, 108. (In Russian) [Google Scholar]
- Grudyanov, A.I.; Zorina, O.A. Methods for Diagnosing Inflammatory Periodontal Diseases: A Guide for Physicians; LLC Publishing House “Medical Information Agency”: Moscow, Russia, 2009. [Google Scholar]
- Sinyaeva, M.L.; Mamedov, A.A.; Admakin, O.I. The method of fluorescent diagnostics in dentistry. Pediatr. Dent. Prev. 2007, 3, 26–30. (In Russian) [Google Scholar]
- Fattal, R.K.; Ammaev, M.G.; Melekhov, S.V. Evaluation of the effectiveness of infiltration of initial caries with the material “ICON” (DMG, Germany) (clinical and laboratory study). Int. J. Appl. Basic Res. 2014, 2, 189–193. (In Russian) [Google Scholar]
- Bulgakova, N.N.; Volkov, E.A.; Pozdnyakova, T.I. Autofluorescent somatoscope as a method of oncoscience diseases of the oral mucosa. Ross. Stomatol. Zhurnal 2015, 19, 27–30. (In Russian) [Google Scholar]
- Wilder-Smith, P.; Holtzman, J.; Epstein, J.; Le, A. Optical diagnostics in the oral cavity: An overview. Oral Dis. 2010, 16, 717–728. [Google Scholar] [CrossRef]
- Balevi, B. Evidence-based decision making: Should the general dentist adopt the use of the VELscope for routine screening for oral cancer? J. Can. Dent. Assoc. 2007, 73, 603–606. [Google Scholar]
- Kirillova, V.P.; Kaganov, O.I.; Gabrielyan, A.G.; Postnikov, M.A.; Orlov, A.E. Methods of early diagnosis of cancer of the oral mucosa. Aspir. Vestn. Povolzhiya 2019, 5–6, 86–90. (In Russian) [Google Scholar] [CrossRef]
- Tuchin, V.V. Handbook of Optical Biomedical Diagnostics; Fizmatlit: Moscow, Russia, 2007. [Google Scholar]
- Bydlon, T.M.; Nachabé, R.; Ramanujam, N.; Sterenborg, H.J.; Hendriks, B.H. Chromophore based analyses of steady-state diffuse reflectance spectroscopy: Current status and perspectives for clinical adoption. J. Biophotonics 2015, 1–2, 9–24. [Google Scholar] [CrossRef]
- Spliethoff, J.W.; Prevoo, W.; Meier, M.A.; de Jong, J.; Klomp, H.M.; Evers, D.J.; Sterenborg, H.J.; Lucassen, G.W.; Hendriks, B.H.; Ruers, T.J. Real-time In Vivo Tissue Characterization with Diffuse Reflectance Spectroscopy during Transthoracic Lung Biopsy: A Clinical Feasibility Study. Clin. Cancer Res. 2015, 22, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.S.; Barman, I.; Dingari, N.C.; Volynskaya, Z.; Liu, W.; Klein, N.; Plecha, D.; Dasari, R.R.; Fitzmaurice, M. Diagnostic power of diffuse reflectance spectroscopy for targeted detection of breast lesions with microcalcifications. Proc. Natl. Acad. Sci. USA 2013, 110, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Zonios, G.; Perelman, L.T.; Backman, V.; Manoharan, R.; Fitzmaurice, M.; Van Dam, J.; Feld, M.S. Diffuse reflectance spectroscopy of human adenomatous colon polyps in vivo. Appl. Opt. 1999, 38, 6628–6637. [Google Scholar] [CrossRef]
- Safonova, L.P.; Orlova, V.G.; Shkarubo, A.N. Investigation of Neurovascular Structures Using Phase-Modulation Spectrophotometry. Opt. Spectrosc. 2019, 126, 745–757. [Google Scholar] [CrossRef]
- Orlova, V.G.; Safonova, L.P.; Soloveva, P.M. In Vitro Study of Optical Properties of the Central Nervous System Components. In Proceedings of the 2020 ElConRus, St. Petersburg and Moscow, Russia, 27–30 January 2020; pp. 1567–1570. [Google Scholar] [CrossRef]
- Schmitt, J.M. Optical Measurement of Blood Oxygenation by Implantable Telemetry. Technical Report G558-15; Stanford University: Stanford, CA, USA, 1986. [Google Scholar]
- Smetanina, O.A.; Kazarina, L.N.; Gordetsov, A.S.; Krasnikova, O.V. Infrared spectroscopy of the oral fluid as a method of early diagnosis of inflammatory periodontal diseases in children. Mod. Probl. Sci. Educ. 2016, 6. (In Russian) [Google Scholar] [CrossRef]
- Kazarina, L.N.; Gordetsov, A.S.; Krasnikova, O.V.; Belozerov, A.E.; Pursanova, A.E. Diagnosis of precancerous diseases of the oral mucosa by infrared spectroscopy. Health Educ. XXI Century 2019, 12, 77–82. (In Russian) [Google Scholar]
- Jeng, M.J.; Sharma, M.; Sharma, L.; Chao, T.Y.; Huang, S.F.; Chang, L.B.; Wu, S.L.; Chow, L. Raman Spectroscopy Analysis for Optical Diagnosis of Oral Cancer Detection. J. Clin. Med. 2019, 8, 1313. [Google Scholar] [CrossRef]
- Thorlabs. Compact Stabilized Broadband Light Sources. Available online: https://www.thorlabs.com/newgrouppage9.cfm?objectgroup_id=7269 (accessed on 26 January 2023).
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, 37. [Google Scholar] [CrossRef]
- Stark, H. Applications of Optical Fourier Transforms; Academic Press: Cambridge, MA, USA, 1982. [Google Scholar]
- Lariviere, B.; Garman, K.S.; Ferguson, N.L.; Fisher, D.A.; Jokerst, N.M. Spatially resolved diffuse reflectance spectroscopy endoscopic sensing with custom Si photodetectors. Biomed. Opt. Exp. 2018, 9, 1164–1176. [Google Scholar] [CrossRef]
- Greening, G.J.; James, H.M.; Dierks, M.K.; Vongkittiargorn, N.; Osterholm, S.M.; Rajaram, N.; Muldoon, T.J. Towards monitoring dysplastic progression in the oral cavity using a hybrid fiber-bundle imaging and spectroscopy probe. Sci. Rep. 2016, 6, 26734. [Google Scholar] [CrossRef]
- Kistenev, Y.; Borisov, A.; Titarenko, M.; Baydik, O.; Shapovalov, A. Diagnosis of oral lichen planus from analysis of saliva samples using terahertz time-domain spectroscopy and chemometrics. J. Biomed. Opt. 2018, 23, 045001. [Google Scholar] [CrossRef]
- Mezhevikina, G.S.; Glukhova, E.A. Modern diagnostic methods precancerous and cancerous changes of the oral mucosa. Sci. Young 2018, 6, 600–606. (In Russian) [Google Scholar] [CrossRef]
- Gorban, A.N.; Kegl, B.; Wunsch, D.; Zinovyev, A.Y. (Eds.) Principal Manifolds for Data Visualisation and Dimension Reduction; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2007. [Google Scholar]
- Dahlstrand, U.; Sheikh, R.; Dybelius Ansson, C.; Memarzadeh, K.; Reistad, N.; Malmsjo, M. Extended-wavelength diffuse reflectance spectroscopy with a machine-learning method for in vivo tissue classification. PLoS ONE 2019, 14, e0223682. [Google Scholar] [CrossRef]
- Lalla, Y.; Matias, M.A.; Farah, C.S. Assessment of oral mucosal lesions with autofluorescence imaging and reflectance spectroscopy. J. Am. Dent. Assoc. 2016, 24, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Tuchina, D.K. Investigation of the Diffusion of Chemical Agents in Biological Tissues by Optical Methods in Normal and Model Diabetes. Ph.D. Thesis, Saratov State University, Saratov, Russia, 2016. (In Russian). [Google Scholar]
- Mendes, S.F.; Ramos, G.D.O.; Rivero, E.R.C.; Modolo, F.; Grando, L.J.; Meurer, M.I. Techniques for precancerous lesion diagnosis. J. Oncol. 2011, 2011, 326094. [Google Scholar] [CrossRef] [PubMed]
- Abati, S.; Bramati, C.; Bondi, S.; Lissoni, A.; Trimarchi, M. Oral Cancer and Precancer: A Narrative Review on the Relevance of Early Diagnosis. Int. J. Environ. Res. Public Health 2020, 17, 9160. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Shah, A.; Nagarajan, V.K.; Ferris, D.G. Diffuse reflectance spectroscopy of epithelial tissue with a smart fiber-optic probe. Biomed. Opt. Express 2014, 5, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Idrees, B.S.; Wang, Q.; Khan, M.N.; Teng, G.; Cui, X.; Xiangli, W.; Wei, K. In-vitro study on the identification of gastrointestinal stromal tumor tissues using laser-induced breakdown spectroscopy with chemometric methods. Biomed. Opt. Express 2022, 13, 26–38. [Google Scholar] [CrossRef]
- Verdel, N.; Marin, A.; Milanič, M.; Majaron, B. Physiological and structural characterization of human skin in vivo using combined photothermal radiometry and diffuse reflectance spectroscopy. Biomed. Opt. Express 2019, 10, 944–960. [Google Scholar] [CrossRef]
- Zhelnov, V.; Zaytsev, K.; Kucheryavenko, A.; Katyba, G.; Dolganova, I.; Ponomarev, D.; Kurlov, V.; Skorobogatiy, M.; Chernomyrdin, N. Object-dependent spatial resolution of the reflection-mode terahertz solid immersion microscopy. Opt. Express 2021, 29, 3553–3566. [Google Scholar] [CrossRef]
- Tetè, G.; D’orto, B.; Ferrante, L.; Polizzi, E.; Cattoni, F. Role of mast cells in oral inflammation. J. Biol. Regul. Homeost. Agents 2021, 35 (Suppl. 1), 65–70. [Google Scholar] [CrossRef] [PubMed]
- Laishram, D.; Rao, K.; Devi, H.S.U.; Priya, N.S.; Smitha, T.; Sheethal, H.S. Mast cells and angiogenesis in malignant and premalignant oral lesions: An immunohistochemical study. J. Oral Maxillofac. Pathol. 2017, 21, 229–238. [Google Scholar] [CrossRef]
- Brouwer de Koning, S.G.; Baltussen, E.J.; Karakullukcu, M.B.; Dashtbozorg, B.; Smit, L.A.; Dirven, R.; Hendriks, B.H.; Sterenborg, H.J.; Ruers, T.J. Toward complete oral cavity cancer resection using a handheld diffuse reflectance spectroscopy probe. J. Biomed. Opt. 2018, 23, 121611. [Google Scholar] [CrossRef]
- Gkouzionis, I.; Nazarian, S.; Kawka, M.; Darzi, A.W.; Patel, N.; Peters, C.J.; Elson, D.S. Real-time tracking of a diffuse reflectance spectroscopy probe used to aid histological validation of margin assessment in upper gastrointestinal cancer resection surgery. J. Biomed. Opt. 2022, 27, 025001. [Google Scholar] [CrossRef]
- Nogueira, M.S.; Maryam, S.; Amissah, M.; Lu, H.; Lynch, N.; Killeen, S.; O’Riordain, M.; Andersson-Engels, S. Evaluation of wavelength ranges and tissue depth probed by diffuse reflectance spectroscopy for colorectal cancer detection. Sci. Rep. 2021, 11, 798. [Google Scholar] [CrossRef] [PubMed]
- Reistad, N.; Sturesson, C. Distinguishing tumor from healthy tissue in human liver ex vivo using machine learning and multivariate analysis of diffuse reflectance spectra. J. Biophotonics 2022, 15, e202200140. [Google Scholar] [CrossRef] [PubMed]
- Grigoroiu, A.; Yoon, J.; Bohndiek, S.E. Deep learning applied to hyperspectral endoscopy for online spectral classification. Sci. Rep. 2020, 10, 3947. [Google Scholar] [CrossRef]
- Wilson, R.H.; Chandra, M.; Scheiman, J.M.; Lee, S.Y.; Lee, O.E.; McKenna, B.J.; Simeone, D.M.; Taylor, J.M.; Mycek, M.A. Tissue Classification Using Optical Spectroscopy Accurately Differentiates Cancer and Chronic Pancreatitis. Pancreas 2017, 46, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Mirabal, Y.N.; Chang, S.K.; Atkinson, E.N.; Malpica, A.; Follen, M.; Richards-Kortum, R. Reflectance spectroscopy for in vivo detection of cervical precancer. J. Biomed. Opt. 2002, 7, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Brouwer de Koning, S.G.; Weijtmans, P.; Karakullukcu, M.B.; Shan, C.; Baltussen, E.J.M.; Smit, L.A.; van Veen, R.L.P.; Hendriks, B.H.W.; Sterenborg, H.J.C.M.; Ruers, T.J.M. Toward assessment of resection margins using hyperspectral diffuse reflection imaging (400–1700 nm) during tongue cancer surgery. Lasers Surg. Med. 2020, 52, 496–502. [Google Scholar] [CrossRef]
- Kamath, S.D.; Mahato, K.K. Optical pathology using oral tissue fluorescence spectra: Classification by principal component analysis and k-means nearest neighbor analysis. J. Biomed. Opt. 2007, 12, 014028. [Google Scholar] [CrossRef]
- Yan, H.; Yu, M.; Xia, J.; Zhu, L.; Zhang, T.; Zhu, Z.; Sun, G. Diverse Region-Based CNN for Tongue Squamous Cell Carcinoma Classification With Raman Spectroscopy. IEEE Access 2020, 8, 127313–127328. [Google Scholar] [CrossRef]
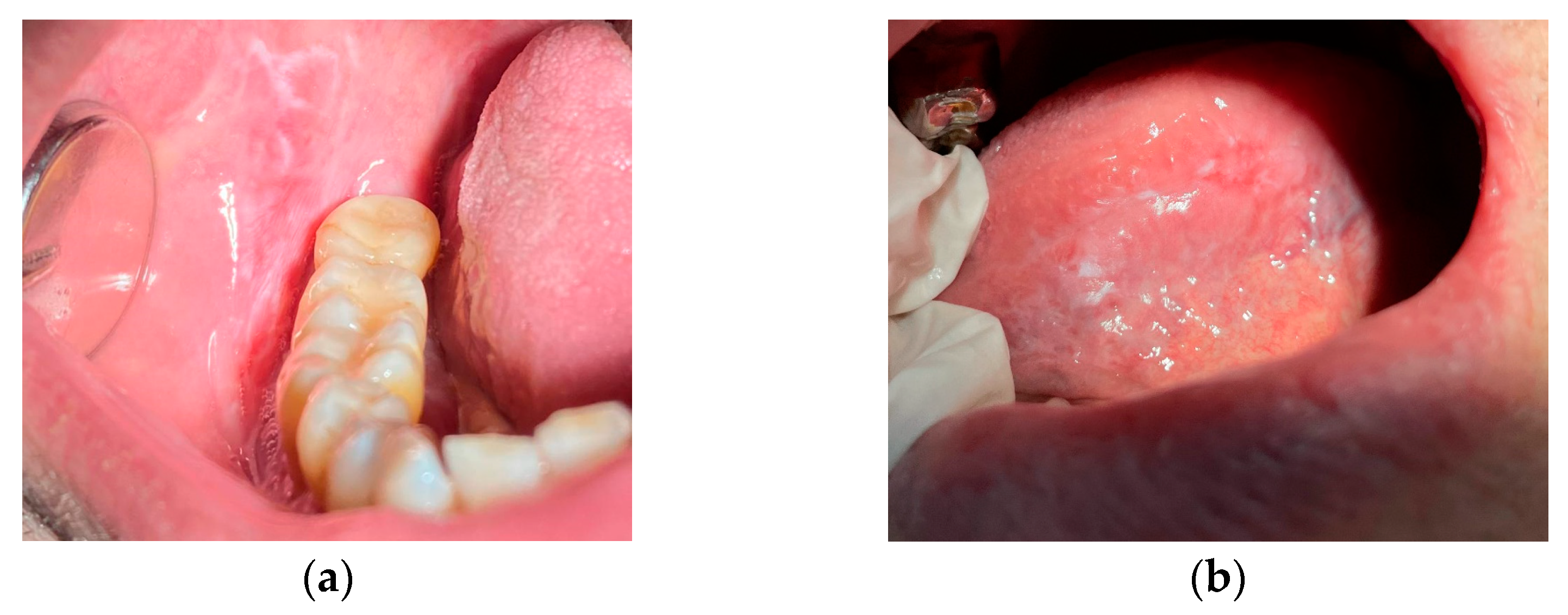

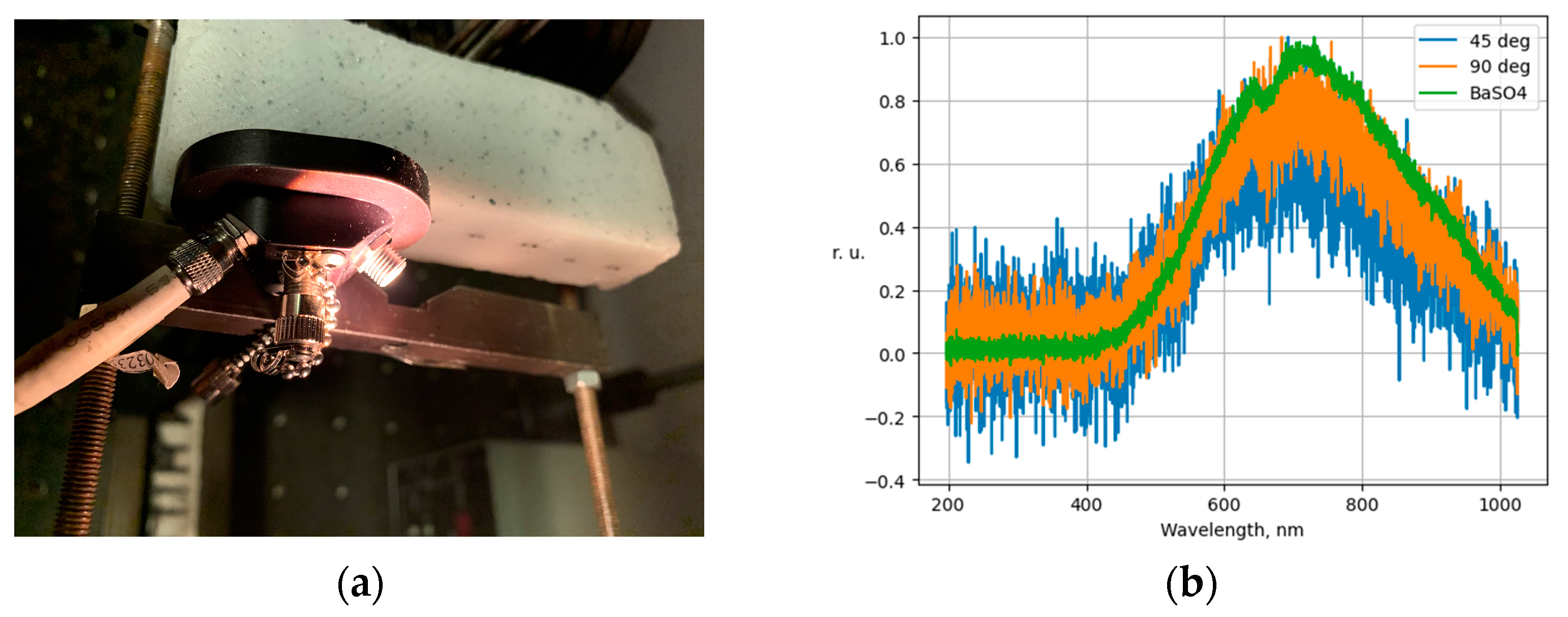
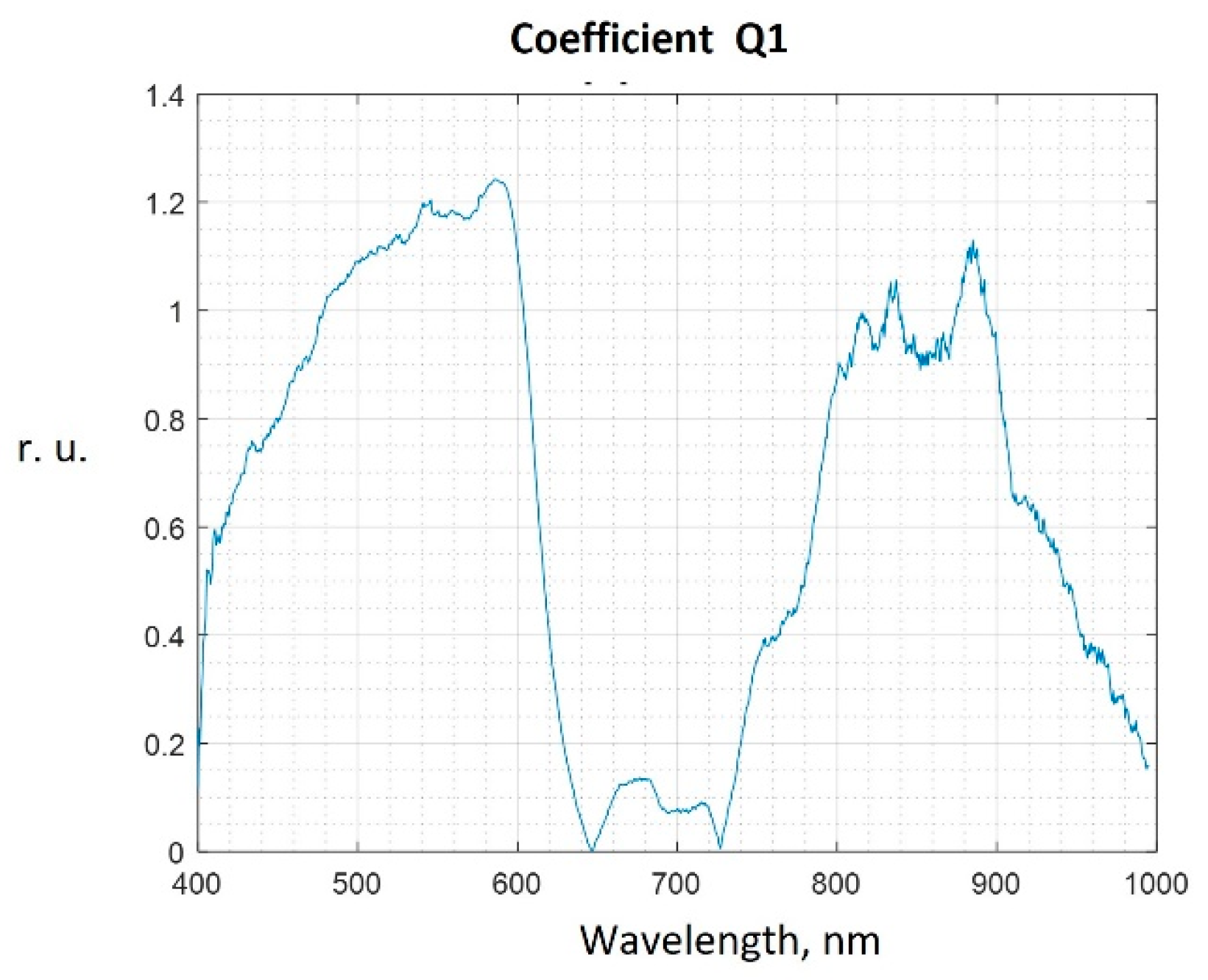
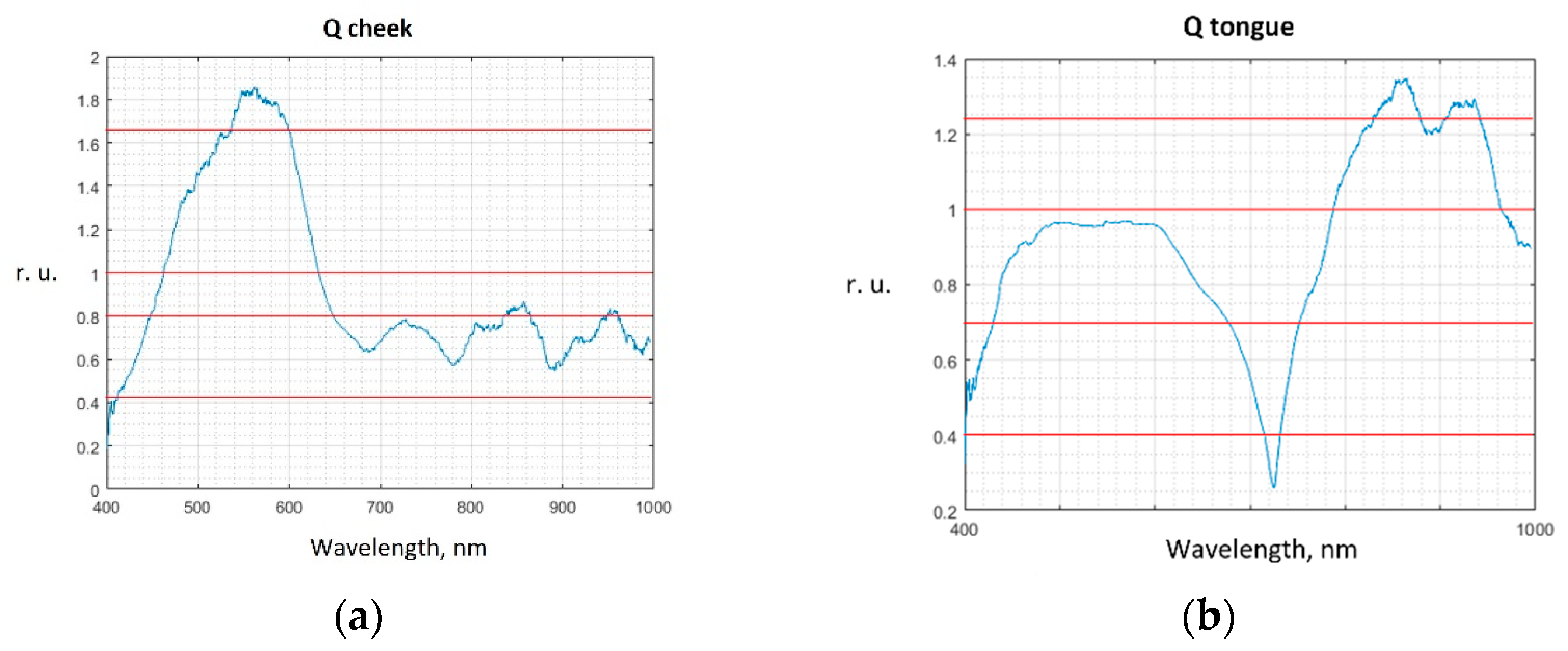




| Classification Result | Relative Frequency | Interpretation |
|---|---|---|
| True positive | NTP | The spectrum of the “hyperkeratosis” class is correctly classified as the spectrum of The “hyperkeratosis” class |
| False positive | NFP | “intact area” class spectrum is falsely classified as “hyperkeratosis” class spectrum |
| True negative | NTN | The spectrum of the “intact area” class is correctly classified as the spectrum of the “intact area” class |
| False negative | NFN | “hyperkeratosis” class spectrum is falsely classified as “intact area” class spectrum |
| No. | Lesion Type | Number of Patients |
|---|---|---|
| 1 | Lichen planus | 25 |
| 2 | Leukoplakia | 9 |
| 3 | Traumatic erosion | 16 |
| 4 | Glossitis | 6 |
| 5 | Fibroma | 4 |
| 6 | Unmarked lesions | 41 |
| Method | Relative Frequencies of Hyperkeratosis Areas Detection on the Mucous Membrane of the Tongue | |||||||
|---|---|---|---|---|---|---|---|---|
| NTP | NFN | NFP | NTP | |||||
| w/o PCA | PCA | w/o PCA | PCA | w/o PCA | PCA | w/o PCA | PCA | |
| Qth = 1.2 | ||||||||
| DT | 0.69 ± 0.06 | 0.73 ± 0.03 | 0.40 ± 0.05 | 0.38 ± 0.08 | 0.31 ± 0.06 | 0.27 ± 0.03 | 0.60 ± 0.05 | 0.63 ± 0.08 |
| DA | 0.81 ± 0.03 | 0.81 ± 0.03 | 0.33 ± 0.06 | 0.40 ± 0.05 | 0.19 ± 0.03 | 0.19 ± 0.03 | 0.68 ± 0.06 | 0.60 ± 0.05 |
| LR | 0.80 ± 0.05 | 0.79 ± 0.01 | 0.38 ± 0.01 | 0.40 ± 0.05 | 0.20 ± 0.05 | 0.21 ± 0.01 | 0.63 ± 0.01 | 0.60 ± 0.05 |
| NKB | 0.79 ± 0.01 | 0.84 ± 0.05 | 0.33 ± 0.06 | 0.48 ± 0.15 | 0.21 ± 0.01 | 0.16 ± 0.05 | 0.68 ± 0.06 | 0.53 ± 0.15 |
| SVM | 0.87 ± 0.05 | 0.86 ± 0.05 | 0.38 ± 0.06 | 0.43 ± 0.06 | 0.13 ± 0.05 | 0.14 ± 0.05 | 0.63 ± 0.11 | 0.58 ± 0.06 |
| kNN | 0.90 ± 0.07 | 0.96 ± 0.09 | 0.45 ± 0.06 | 0.90 ± 0.20 | 0.10 ± 0.07 | 0.04 ± 0.09 | 0.55 ± 0.13 | 0.10 ± 0.09 |
| Qth = 1.0 | ||||||||
| DT | 0.79 ± 0.01 | 0.71 ± 0.01 | 0.25 ± 0.01 | 0.28 ± 0.05 | 0.21 ± 0.01 | 0.29 ± 0.01 | 0.75 ± 0.01 | 0.73 ± 0.05 |
| DA | 0.79 ± 0.01 | 0.80 ± 0.03 | 0.28 ± 0.09 | 0.45 ± 0.06 | 0.21 ± 0.01 | 0.20 ± 0.03 | 0.73 ± 0.09 | 0.55 ± 0.06 |
| LR | 0.79 ± 0.05 | 0.79 ± 0.01 | 0.55 ± 0.06 | 0.45 ± 0.06 | 0.21 ± 0.05 | 0.21 ± 0.01 | 0.45 ± 0.06 | 0.55 ± 0.06 |
| NKB | 0.79 ± 0.01 | 0.81 ± 0.06 | 0.25 ± 0.08 | 0.45 ± 0.10 | 0.21 ± 0.01 | 0.19 ± 0.06 | 0.75 ± 0.08 | 0.55 ± 0.10 |
| SVM | 0.81 ± 0.07 | 0.84 ± 0.07 | 0.40 ± 0.09 | 0.48 ± 0.15 | 0.19 ± 0.07 | 0.16 ± 0.07 | 0.60 ± 0.09 | 0.53 ± 0.15 |
| kNN | 0.89 ± 0.10 | 0.89 ± 0.10 | 0.65 ± 0.22 | 0.65 ± 0.24 | 0.11 ± 0.10 | 0.11 ± 0.10 | 0.35 ± 0.22 | 0.35 ± 0.24 |
| Qth = 0.7 | ||||||||
| DT | 0.84 ± 0.06 | 0.90 ± 0.06 | 0.23 ± 0.09 | 0.88 ± 0.14 | 0.16 ± 0.03 | 0.10 ± 0.06 | 0.78 ± 0.09 | 0.13 ± 0.10 |
| DA | 0.80 ± 0.05 | 0.99 ± 0.03 | 0.18 ± 0.06 | 0.98 ± 0.05 | 0.20 ± 0.05 | 0.01 ± 0.03 | 0.83 ± 0.06 | 0.03 ± 0.03 |
| LR | 0.74 ± 0.09 | 0.97 ± 0.03 | 0.63 ± 0.14 | 0.98 ± 0.05 | 0.26 ± 0.09 | 0.03 ± 0.03 | 0.38 ± 0.14 | 0.03 ± 0.03 |
| NKB | 0.81 ± 0.06 | 0.91 ± 0.05 | 0.23 ± 0.15 | 0.90 ± 0.09 | 0.19 ± 0.06 | 0.09 ± 0.05 | 0.78 ± 0.15 | 0.10 ± 0.09 |
| SVM | 0.93 ± 0.06 | 1.00 ± 0.01 | 0.38 ± 0.11 | 0.98 ± 0.05 | 0.07 ± 0.06 | 0.00 | 0.63 ± 0.11 | 0.03 ± 0.03 |
| kNN | 0.90 ± 0.07 | 1.00 ± 0.01 | 0.38 ± 0.26 | 1.00 ± 0.01 | 0.10 ± 0.07 | 0.00 | 0.63 ± 0.26 | 0.00 |
| Qth = 0.4 | ||||||||
| DT | 0.84 ± 0.03 | 0.81 ± 0.03 | 0.28 ± 0.05 | 0.85 ± 0.15 | 0.16 ± 0.03 | 0.19 ± 0.03 | 0.73 ± 0.05 | 0.15 ± 0.09 |
| DA | 0.74 ± 0.06 | 0.89 ± 0.06 | 0.13 ± 0.01 | 1.00 ± 0.01 | 0.26 ± 0.06 | 0.11 ± 0.06 | 0.88 ± 0.01 | 0.00 |
| LR | 0.64 ± 0.08 | 0.87 ± 0.07 | 0.70 ± 0.13 | 1.00 ± 0.01 | 0.36 ± 0.08 | 0.13 ± 0.07 | 0.30 ± 0.13 | 0.00 |
| NKB | 0.81 ± 0.03 | 0.90 ± 0.06 | 0.40 ± 0.05 | 0.88 ± 0.11 | 0.19 ± 0.03 | 0.10 ± 0.06 | 0.60 ± 0.05 | 0.13 ± 0.11 |
| SVM | 0.90 ± 0.07 | 1.00 ± 0.01 | 0.50 ± 0.08 | 1.00 ± 0.01 | 0.10 ± 0.07 | 0.00 | 0.50 ± 0.08 | 0.00 |
| kNN | 0.94 ± 0.07 | 1.00 ± 0.01 | 0.50 ± 0.11 | 1.00 ± 0.01 | 0.06 ± 0.07 | 0.00 | 0.50 ± 0.11 | 0.00 |
| Method | Relative Frequencies of Hyperkeratosis Areas Detection on the Mucous Membrane of the Tongue | |||||||
|---|---|---|---|---|---|---|---|---|
| NTP | NFN | NFP | NTP | |||||
| w/o PCA | PCA | w/o PCA | PCA | w/o PCA | PCA | w/o PCA | PCA | |
| Qth = 1.0 | ||||||||
| DT | 0.37 ± 0.07 | 0.50 ± 0.01 | 0.11 ± 0.06 | 0.00 | 0.63 ± 0.07 | 0.50 ± 0.01 | 0.89 ± 0.06 | 1.00 ± 0.01 |
| DA | 0.50 ± 0.01 | 0.50 ± 0.01 | 0.14 ± 0.01 | 0.17 ± 0.06 | 0.50 ± 0.01 | 0.50 ± 0.01 | 0.86 ± 0.01 | 0.83 ± 0.06 |
| LR | 0.57 ± 0.08 | 0.50 ± 0.01 | 0.34 ± 0.07 | 0.23 ± 0.07 | 0.43 ± 0.08 | 0.50 ± 0.01 | 0.66 ± 0.07 | 0.77 ± 0.07 |
| NKB | 0.50 ± 0.01 | 0.47 ± 0.07 | 0.23 ± 0.07 | 0.20 ± 0.07 | 0.50 ± 0.01 | 0.53 ± 0.07 | 0.77 ± 0.07 | 0.80 ± 0.07 |
| SVM | 0.70 ± 0.12 | 0.47 ± 0.07 | 0.06 ± 0.11 | 0.03 ± 0.06 | 0.30 ± 0.12 | 0.53 ± 0.07 | 0.94 ± 0.11 | 0.97 ± 0.06 |
| kNN | 0.77 ± 0.23 | 0.87 ± 0.12 | 0.20 ± 0.19 | 0.46 ± 0.14 | 0.23 ± 0.21 | 0.13 ± 0.12 | 0.80 ± 0.19 | 0.54 ± 0.14 |
| Qth = 0.8 | ||||||||
| DT | 0.77 ± 0.13 | 0.43 ± 0.13 | 0.14 ± 0.09 | 0.03 ± 0.03 | 0.23 ± 0.13 | 0.57 ± 0.13 | 0.86 ± 0.09 | 0.97 ± 0.06 |
| DA | 0.57 ± 0.17 | 0.43 ± 0.13 | 0.20 ± 0.07 | 0.14 ± 0.01 | 0.43 ± 0.17 | 0.57 ± 0.13 | 0.80 ± 0.07 | 0.86 ± 0.01 |
| LR | 0.43 ± 0.13 | 0.47 ± 0.07 | 0.34 ± 0.15 | 0.23 ± 0.07 | 0.57 ± 0.13 | 0.53 ± 0.07 | 0.66 ± 0.15 | 0.77 ± 0.07 |
| NKB | 0.67 ± 0.11 | 0.47 ± 0.07 | 0.26 ± 0.06 | 0.20 ± 0.07 | 0.33 ± 0.11 | 0.53 ± 0.07 | 0.74 ± 0.06 | 0.80 ± 0.07 |
| SVM | 0.80 ± 0.07 | 0.43 ± 0.08 | 0.00 | 0.06 ± 0.11 | 0.20 ± 0.07 | 0.57 ± 0.08 | 1.00 ± 0.01 | 0.94 ± 0.11 |
| kNN | 0.80 ± 0.16 | 0.63 ± 0.22 | 0.11 ± 0.06 | 0.34 ± 0.11 | 0.20 ± 0.16 | 0.37 ± 0.22 | 0.89 ± 0.06 | 0.66 ± 0.11 |
| Qth = 0.4 | ||||||||
| DT | 0.67 ± 0.11 | 0.27 ± 0.08 | 0.20 ± 0.11 | 0.00 | 0.33 ± 0.11 | 0.73 ± 0.08 | 0.80 ± 0.11 | 1.00 ± 0.01 |
| DA | 0.70 ± 0.12 | 0.97 ± 0.07 | 0.26 ± 0.06 | 0.20 ± 0.15 | 0.30 ± 0.12 | 0.03 ± 0.07 | 0.74 ± 0.06 | 0.80 ± 0.15 |
| LR | 0.67 ± 0.21 | 0.70 ± 0.07 | 0.60 ± 0.11 | 0.14 ± 0.01 | 0.33 ± 0.21 | 0.30 ± 0.07 | 0.40 ± 0.11 | 0.86 ± 0.01 |
| NKB | 0.77 ± 0.08 | 0.97 ± 0.07 | 0.23 ± 0.07 | 0.20 ± 0.15 | 0.23 ± 0.08 | 0.03 ± 0.01 | 0.77 ± 0.07 | 0.80 ± 0.15 |
| SVM | 0.90 ± 0.13 | 0.93 ± 0.08 | 0.00 | 0.00 | 0.10 ± 0.09 | 0.07 ± 0.05 | 1.00 ± 0.01 | 1.00 ± 0.01 |
| kNN | 0.87 ± 0.07 | 0.97 ± 0.07 | 0.06 ± 0.05 | 0.00 | 0.13 ± 0.07 | 0.03 ± 0.01 | 0.94 ± 0.07 | 1.00 ± 0.01 |
| Parameter | The Mucous Membrane of the Ventrolateral Surface of the Tongue | The Mucous Membrane of the Cheek |
|---|---|---|
| Sensitivity | 0.83 ± 0.17 | 1.00 ± 0.00 |
| Specificity | 0.80 ± 0.17 | 0.97 ± 0.20 |
| Wavelength Range, nm | Classification Accuracy, % | Wavelength Range, nm | Classification Accuracy, % |
|---|---|---|---|
| 500–900 | 75.00 | 600–800 | 75.62 |
| 500–850 | 75.50 | 600–750 | 75.64 |
| 500–800 | 75.00 | 600–701 | 75.73 |
| 500–750 | 74.75 | 650–900 | 75.38 |
| 500–701 | 74.80 | 650–850 | 75.29 |
| 550–900 | 75.00 | 650–800 | 75.17 |
| 550–850 | 75.14 | 650–750 | 74.95 |
| 550–800 | 75.12 | 650–701 | 74.55 |
| 550–750 | 75.11 | 700–900 | 74.33 |
| 550–701 | 75.00 | 700–800 | 74.22 |
| 600–900 | 75.27 | 700–750 | 74.12 |
| 600–850 | 75.42 | 700–701 | 73.36 |
| 700–850 | 74.27 |
| Method | Tissue Type | Wavelength Range, nm | Specificity, % | Sensitivity, % | Accuracy, % | Reference |
|---|---|---|---|---|---|---|
| DRS in vivo | Oral mucosa | 500–900 | - | - | 75 | Current study |
| DRS ex vivo | Oral mucosa | 400–1700 | 89 | 82 | 86 | [51] |
| DRS ex vivo | Esophagus mucosa; stomach mucosa | 400–1000 | - | - | 96—for esophagus mucosa; 94—for stomach mucosa | [52] |
| DRS ex vivo | Colorectal mucosa | 400–1000 | 91 | 92 | 91 | [53] |
| DRS ex vivo | Liver tissue | 450–1550 | 99 | 100 | - | [54] |
| HSI ex vivo | Esophagus tissue | 400–750 | - | - | 85–95 | [55] |
| RS ex vivo | Pancreatic tissue | 400–700 | 91 | 82 | - | [56] |
| RS in vivo | Cervical tissue | 355–655 | 79 | 78 | - | [57] |
| DRS ex vivo | Tongue tissue | 400–1000 | 84 | 80 | 82 | [58] |
| FS ex vivo | Oral mucosa | 325 | 100 | 93–97 | 96–98 | [59] |
| RS in vivo | Tongue tissue/Buccal mucosa/Gingiva tissue | 532 | 92–100/79–84/75–92 | 63–72/93/91–100 | 79–88/85–88/87–91 | [31] |
| RS ex vivo | Tongue tissue | 785 | 99 | 99 | - | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolpakov, A.V.; Moshkova, A.A.; Melikhova, E.V.; Sokolova, D.Y.; Muravskaya, N.P.; Samorodov, A.V.; Kopaneva, N.O.; Lukina, G.I.; Abramova, M.Y.; Mamatsashvili, V.G.; et al. Diffuse Reflectance Spectroscopy of the Oral Mucosa: In Vivo Experimental Validation of the Precancerous Lesions Early Detection Possibility. Diagnostics 2023, 13, 1633. https://doi.org/10.3390/diagnostics13091633
Kolpakov AV, Moshkova AA, Melikhova EV, Sokolova DY, Muravskaya NP, Samorodov AV, Kopaneva NO, Lukina GI, Abramova MY, Mamatsashvili VG, et al. Diffuse Reflectance Spectroscopy of the Oral Mucosa: In Vivo Experimental Validation of the Precancerous Lesions Early Detection Possibility. Diagnostics. 2023; 13(9):1633. https://doi.org/10.3390/diagnostics13091633
Chicago/Turabian StyleKolpakov, Alexander V., Anastasia A. Moshkova, Ekaterina V. Melikhova, Diana Yu. Sokolova, Natalia P. Muravskaya, Andrey V. Samorodov, Nina O. Kopaneva, Galina I. Lukina, Marina Ya. Abramova, Veta G. Mamatsashvili, and et al. 2023. "Diffuse Reflectance Spectroscopy of the Oral Mucosa: In Vivo Experimental Validation of the Precancerous Lesions Early Detection Possibility" Diagnostics 13, no. 9: 1633. https://doi.org/10.3390/diagnostics13091633
APA StyleKolpakov, A. V., Moshkova, A. A., Melikhova, E. V., Sokolova, D. Y., Muravskaya, N. P., Samorodov, A. V., Kopaneva, N. O., Lukina, G. I., Abramova, M. Y., Mamatsashvili, V. G., & Parshkov, V. V. (2023). Diffuse Reflectance Spectroscopy of the Oral Mucosa: In Vivo Experimental Validation of the Precancerous Lesions Early Detection Possibility. Diagnostics, 13(9), 1633. https://doi.org/10.3390/diagnostics13091633







