Histopathological Image Diagnosis for Breast Cancer Diagnosis Based on Deep Mutual Learning
Abstract
:1. Introduction
2. Literature Review
3. Research Methodology
3.1. Training Based on DML
3.2. Label Propagation for Image Segmentation
3.3. Proposed Methodology
| Algorithm 1 Deep Mutual Learning for Breast Cancer Histopathology Image Diagnosis |
| Require: Image datasets (BreakHis, BACH, PUIH) |
| Ensure: Trained model for breast cancer histopathology image diagnosis |
| 1: Initialize two identical neural network models θ1; and θ2 |
| 2: for each batch of input images do |
| 3: Preprocess images (revision, normalization) |
| 4: Perform image segmentation |
| 5: Calculate segment difference score using label propagation |
| 6: Compute multi-class cross-entropy loss LC for each model |
| 7: LC = −(yC · log(ΦC) + (1 − yC) · log(1 − ΦC)) |
| 8: Calculate KL divergence DkL, between the two models |
| 9: DKL = P20 · log(P20/P10) + P21 · log(P21/P11) |
| 10: Update total loss functions for each model |
| 11: Lθ1 = LC1 + DKL(P2 || P1) |
| 12: Lθ2 = LC2 + DKL(P1 || P2) |
| 13: Train both models using the computed losses |
| 14: end for |
| 15: Repeat steps 2–11 until convergence |
| 16: Evaluate the trained model on test datasets |
4. Results
5. Conclusions and Future Scope
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fedewa, S.A.; Ahnen, D.J.; Meester, R.G.; Barzi, A.; Jemal, A. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Spanhol, F.A.; Oliveira, L.S.; Cavalin, P.R.; Petitjean, C.; Heutte, L. Deep features for breast cancer histopathological image classification. In Proceedings of the 2017 IEEE International Conference on Systems, Man and Cybernetics (SMC), Banff, AB, Canada, 5–8 October 2017; pp. 1868–1873. [Google Scholar]
- Desai, M.; Shah, M. An anatomization on breast cancer detection and diagnosis employing multi-layer perceptron neural network (MLP) and Convolutional neural network (CNN). Clin. eHealth 2021, 4, 1–11. [Google Scholar] [CrossRef]
- Alanazi, S.A.; Kamruzzaman, M.M.; Sarker, N.I.; Alruwaili, M.; Alhwaiti, Y.; Alshammari, N.; Siddiqi, M.H. Boosting Breast Cancer Detection Using Convolutional Neural Network. J. Health Eng. 2021, 2021, 5528622. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, S.R.; Ellis, I.O.; Schnitt, S.; Tan, P.; van de Vijver, M. WHO Classification of Tumors of the Breast, 4th ed.; WHO Press: Geneva, Switzerland, 2012; Volume 4.
- Das, A.; Devarampati, V.K.; Nair, M.S. NAS-SGAN: A Semi-Supervised Generative Adversarial Network Model for Atypia Scoring of Breast Cancer Histopathological Images. IEEE J. Biomed. Health Inform. 2021, 26, 2276–2287. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, A.; Liu, J.; Meng, G.; Wang, M. TSDLPP: A Novel Two-Stage Deep Learning Framework for Prognosis Prediction Based on Whole Slide Histopathological Images. IEEE/ACM Trans. Comput. Biol. Bioinform. 2021, 19, 2523–2532. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cancer. 2019. Available online: https://www.who.int/health-topics/cancer (accessed on 15 June 2022).
- Bejnordi, B.E.; Zuidhof, G.; Balkenhol, M.; Hermsen, M.; Bult, P.; van Ginneken, B.; Karssemeijer, N.; Litjens, G.; van der Laak, J. Context-aware stacked convolutional neural networks for classification of breast carcinomas in whole-slide histopathology images. J. Med. Imaging 2017, 4, 044504. [Google Scholar] [CrossRef] [PubMed]
- Houssein, E.H.; Emam, M.M.; Ali, A.A.; Suganthan, P.N. Deep and machine learning techniques for medical imaging-based breast cancer: A comprehensive review. Expert Syst. Appl. 2021, 167, 114161. [Google Scholar] [CrossRef]
- Houssein, E.H.; Emam, M.M.; Ali, A.A. An efficient multilevel thresholding segmentation method for thermography breast cancer imaging based on improved chimp optimization algorithm. Expert Syst. Appl. 2021, 185, 115651. [Google Scholar] [CrossRef]
- Zebari, D.A.; Ibrahim, D.A.; Zeebaree, D.Q.; Haron, H.; Salih, M.S.; Damaševičius, R.; Mohammed, M.A. Systematic Review of Computing Approaches for Breast Cancer Detection Based Computer Aided Diagnosis Using Mammogram Images. Appl. Artif. Intell. 2021, 35, 2157–2203. [Google Scholar] [CrossRef]
- Gupta, I.; Gupta, S.; Singh, S. Different CNN-based Architectures for Detection of Invasive Ductal Carcinoma in Breast Using Histopathology Images. Int. J. Image Graph. 2021, 21, 2140003. [Google Scholar] [CrossRef]
- Ogundokun, R.O.; Misra, S.; Douglas, M.; Damaševičius, R.; Maskeliūnas, R. Medical Internet-of-Things Based Breast Cancer Diagnosis Using Hyperparameter-Optimized Neural Networks. Futur. Internet 2022, 14, 153. [Google Scholar] [CrossRef]
- Motlagh, M.H.; Jannesari, M.; Aboulkheyr, H.; Khosravi, P.; Elemento, O.; Totonchi, M.; Hajirasouliha, I. Breast cancer histopathological image classification: A deep learning approach. bioRxiv 2018. [Google Scholar] [CrossRef]
- Sharma, S.; Mehra, R. Conventional Machine Learning and Deep Learning Approach for Multi-Classification of Breast Cancer Histopathology Images—A Comparative Insight. J. Digit. Imaging 2020, 33, 632–654. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Han, Z.; He, X.; Yin, Y. Deep learning model-based breast cancer histopathological image classification. In Proceedings of the 2017 IEEE 2nd International Conference on Cloud Computing and Big Data Analysis (ICCCBDA), Chengdu, China, 28–30 April 2017; pp. 348–353. [Google Scholar]
- Oyewola, D.O.; Dada, E.G.; Misra, S.; Damaševičius, R. A Novel Data Augmentation Convolutional Neural Network for Detecting Malaria Parasite in Blood Smear Images. Appl. Artif. Intell. 2022, 36, 2033473. [Google Scholar] [CrossRef]
- Diwakaran, M.; Surendran, D. Breast Cancer Prognosis Based on Transfer Learning Techniques in Deep Neural Networks. Inf. Technol. Control. 2023, 52, 381–396. [Google Scholar] [CrossRef]
- Li, L.; Pan, X.; Yang, H.; Liu, Z.; He, Y.; Li, Z.; Fan, Y.; Cao, Z.; Zhang, L. Multi-task deep learning for fine-grained classification and grading in breast cancer histopathological images. Multimed. Tools Appl. 2020, 79, 14509–14528. [Google Scholar] [CrossRef]
- El Agouri, H.; Azizi, M.; El Attar, H.; El Khannoussi, M.; Ibrahimi, A.; Kabbaj, R.; Kadiri, H.; BekarSabein, S.; EchCharif, S.; El Khannoussi, B. Assessment of deep learning algorithms to predict histopathological diagnosis of breast cancer: First Moroccan prospective study on a private dataset. BMC Res. Notes 2022, 15, 66. [Google Scholar] [CrossRef]
- Said, B.; Liu, X.; Wan, Y.; Zheng, Z.; Ferkous, C.; Ma, X.; Li, Z.; Bardou, D. Conventional machine learning versus deep learning for magnification dependent histopathological breast cancer image classification: A comparative study with a visual explanation. Diagnostics 2021, 11, 528. [Google Scholar]
- Sohail, A.; Khan, A.; Nisar, H.; Tabassum, S.; Zameer, A. Mitotic nuclei analysis in breast cancer histopathology images using deep ensemble classifier. Med. Image Anal. 2021, 72, 102121. [Google Scholar] [CrossRef]
- Zeiser, F.A.; da Costa, C.A.; Ramos, G.d.O.; Bohn, H.C.; Santos, I.; Roehe, A.V. DeepBatch: A hybrid deep learning model for interpretable diagnosis of breast cancer in whole-slide images. Expert Syst. Appl. 2021, 185, 115586. [Google Scholar] [CrossRef]
- Altaf, M.M. A hybrid deep learning model for breast cancer diagnosis based on transfer learning and pulse-coupled neural networks. Math. Biosci. Eng. 2021, 18, 5029–5046. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.; Azizpour, H.; Smith, K.; Hartman, J. Digital image analysis in breast pathology—From image processing techniques to artificial intelligence. Transl. Res. 2017, 1931–5244. [Google Scholar] [CrossRef] [PubMed]
- Ching, T.; Himmelstein, D.S.; Beaulieu-Jones, B.K.; Kalinin, A.A.; Do, B.T.; Way, G.P.; Ferrero, E.; Agapow, P.-M.; Xie, W.; Rosen, G.L.; et al. Opportunities and Obstacles for Deep Learning in Biology and Medicine. bioRxiv 2017. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, R. Histopathological image analysis for breast cancer detection using cubic SVM. In Proceedings of the 2020 7th International Conference on Signal Processing and Integrated Networks (SPIN), Noida, India, 27–28 February 2020; pp. 498–503. [Google Scholar]
- Li, G.; Li, C.; Wu, G.; Ji, D.; Zhang, H. Multi-View Attention-Guided Multiple Instance Detection Network for Interpretable Breast Cancer Histopathological Image Diagnosis. IEEE Access 2021, 9, 79671–79684. [Google Scholar] [CrossRef]
- Spanhol, F.A.; Oliveira, L.S.; Petitjean, C.; Heutte, L. A Dataset for Breast Cancer Histopathological Image Classification. IEEE Trans. Biomed. Eng. 2016, 63, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Breve, F. Interactive image segmentation using label propagation through complex networks. Expert Syst. Appl. 2019, 123, 18–33. [Google Scholar] [CrossRef]
- Kadry, S.; Rajinikanth, V.; Taniar, D.; Damaševičius, R.; Valencia, X.P.B. Automated segmentation of leukocyte from hematological images—a study using various CNN schemes. J. Supercomput. 2022, 78, 6974–6994. [Google Scholar] [CrossRef]
- Wan, T.; Cao, J.; Chen, J.; Qin, Z. Automated grading of breast cancer histopathology using cascaded ensemble with combination of multi-level image features. Neurocomputing 2017, 229, 34–44. [Google Scholar] [CrossRef]
- Gour, M.; Jain, S.; Kumar, T.S. Residual learning based CNN for breast cancer histopathological image classification. Int. J. Imaging Syst. Technol. 2020, 30, 621–635. [Google Scholar] [CrossRef]
- Budak, Ü.; Cömert, Z.; Rashid, Z.N.; Şengür, A.; Çıbuk, M. Computer-aided diagnosis system combining FCN and bi-LSTM model for efficient breast cancer detection from histopathological images. Appl. Soft Comput. 2019, 85, 105765. [Google Scholar] [CrossRef]
- Sudharshan, P.J.; Petitjean, C.; Spanhol, F.; Oliveira, L.E.; Heutte, L.; Honeine, P. Multiple instances learning for histopathological breast cancer image classification. Expert Syst. Appl. 2019, 117, 103–111. [Google Scholar] [CrossRef]
- Rony, J.; Belharbi, S.; Dolz, J.; Ayed, I.B.; McCaffrey, L.; Granger, E. Deep weakly-supervised learning methods for classification and localization in histology images: A survey. arXiv 2019, arXiv:1909.03354. [Google Scholar] [CrossRef]
- Ahmad, H.M.; Ghaffar, S.; Khurshid, K. Classification of breast cancer histology images using transfer learning. In Proceedings of the 16th International Bhurban Conference on Applied Sciences and Technology (IBCAST), Islamabad, Pakistan, 8–12 January 2019; pp. 812–819. [Google Scholar]
- Yang, H.; Kim, J.-Y.; Kim, H.; Adhikari, S.P. Guided Soft Attention Network for Classification of Breast Cancer Histopathology Images. IEEE Trans. Med. Imaging 2019, 39, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Ren, F.; Wang, Z.; Wang, L.; Zhang, T.; Liu, Y.; Rao, X.; Zheng, C.; Zhang, F. Breast cancer histopathological image classification using a hybrid deep neural network. Methods 2020, 173, 52–60. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Y.; Tang, P.; Bai, X.; Liu, W. Revisiting multiple instance neural networks. Pattern Recognit. 2018, 74, 15–24. [Google Scholar] [CrossRef]

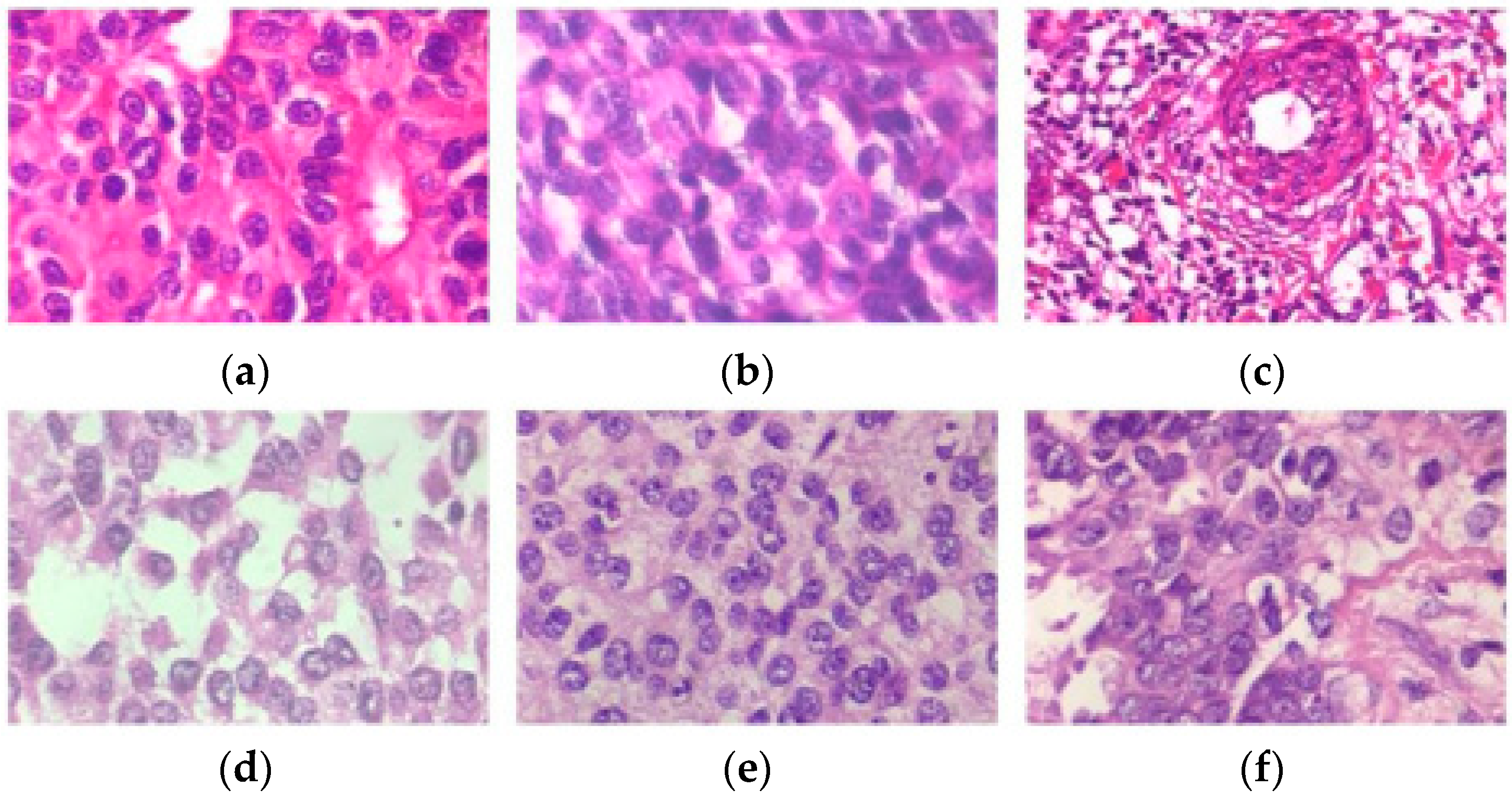












| Dataset | Image Size | Magnification Factor | Benign | Malignant | Total |
|---|---|---|---|---|---|
| BreakHis | 700 × 460 | 40× | 625 | 1370 | 1995 |
| 100× | 644 | 1437 | 2081 | ||
| 200× | 623 | 1390 | 2013 | ||
| 400× | 588 | 1232 | 1820 | ||
| BACH | 2048 × 1536 | _ | 200 | 200 | 400 |
| PUIH | 2048 × 1536 | _ | 1529 | 2491 | 4020 |
| Training Methods | BreakHis-200× | BACH | PUIH |
|---|---|---|---|
| MA-MIDN-DML [33] | 94.90 | 92.67 | 91.33 |
| MA-MIDN-Ind [33] | 96.87 | 94.54 | 93.65 |
| Proposed DML | 98.97 | 96.78 | 96.34 |
| Methods | 40× | 100× | 200× | 400× | Mean |
|---|---|---|---|---|---|
| Res Hist-Aug [34] | 90.42 | 90.87 | 93.88 | 89.54 | 90.89 |
| FCN + Bi-LSTM [35] | 95.78 | 94.51 | 97.23 | 94.30 | 95.89 |
| MI-SVM [36] | 86.44 | 82.90 | 81.75 | 82.78 | 83.45 |
| Deep MIL [37] | 91.92 | 89.66 | 91.78 | 85.99 | 89.84 |
| MA-MIDN [33] | 96.56 | 96.99 | 97.88 | 95.66 | 89.83 |
| DML | 97.87 | 98.56 | 98.34 | 96.54 | 93.37 |
| Methods | Magnification Factor | AUC | Precision | Recall |
|---|---|---|---|---|
| Res Hist-Aug [34] | 40× | 94.67 | 93.77 | 87.21 |
| 100× | 93.22 | 90.44 | 89.44 | |
| 200× | 94.89 | 94.26 | 92.69 | |
| 400× | 95.34 | 91.45 | 86.89 | |
| MA-MIDN [33] | 40× | 95.56 | 95.78 | 88.87 |
| 100× | 94.43 | 92.19 | 90.63 | |
| 200× | 95.67 | 94.89 | 94.78 | |
| 400× | 96.33 | 93.67 | 91.56 | |
| DML | 40× | 97.89 | 97.56 | 92.56 |
| 100× | 98.34 | 95.38 | 95.45 | |
| 200× | 97.89 | 98.65 | 98.31 | |
| 400× | 99.44 | 99.71 | 96.44 |
| Datasets | Methods | Accuracy | AUC | Precision | Recall |
|---|---|---|---|---|---|
| BACH | Patch Vote [38] | 86.22 | 92.29 | 86.98 | 81.97 |
| B + FA + GuSA [39] | 91.35 | 96.67 | 95.78 | 86.67 | |
| MA-MIDN [33] | 94.67 | 97.98 | 96.45 | 95.26 | |
| DML | 97.46 | 98.67 | 97.89 | 96.36 | |
| PUIH | Hybrid-DNN [40] | 92.25 | - | - | - |
| MA-MIDN [33] | 93.76 | 97.26 | 95.07 | 95.19 | |
| DML | 95.56 | 98.67 | 96.83 | 97.17 |
| Attention Mechanisms | BreakHis-200× | BACH | PUIH |
|---|---|---|---|
| No Attention (MI-Net) [41] | 76.47 | 71.67 | 70.43 |
| Attention over instances (AOIs) | 74.78 | 69.34 | 67.73 |
| Attention over classes (AOCs) | 88.28 | 83.88 | 81.71 |
| Datasets | Average Test Time of Each Image in Each Batch | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Mean | |
| BreakHis-200× | 0.04 | 0.03 | 0.03 | 0.03 | 0.04 | 0.03 |
| BACH | 0.09 | 0.09 | 0.11 | 0.09 | 0.10 | 0.09 |
| PUIH | 0.10 | 0.11 | 0.10 | 0.10 | 0.09 | 0.10 |
| Datasets | Average Test Time Each Image in Each Batch | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Mean | |
| BreakHis-200× | 0.82 | 0.67 | 0.70 | 0.67 | 0.67 | 0.71 |
| BACH | 1.44 | 1.66 | 1.66 | 1.36 | 1.61 | 1.55 |
| PUIH | 1.43 | 1.56 | 1.46 | 1.62 | 1.55 | 1.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, A.; Kaushal, C.; Sandhu, J.K.; Damaševičius, R.; Thakur, N. Histopathological Image Diagnosis for Breast Cancer Diagnosis Based on Deep Mutual Learning. Diagnostics 2024, 14, 95. https://doi.org/10.3390/diagnostics14010095
Kaur A, Kaushal C, Sandhu JK, Damaševičius R, Thakur N. Histopathological Image Diagnosis for Breast Cancer Diagnosis Based on Deep Mutual Learning. Diagnostics. 2024; 14(1):95. https://doi.org/10.3390/diagnostics14010095
Chicago/Turabian StyleKaur, Amandeep, Chetna Kaushal, Jasjeet Kaur Sandhu, Robertas Damaševičius, and Neetika Thakur. 2024. "Histopathological Image Diagnosis for Breast Cancer Diagnosis Based on Deep Mutual Learning" Diagnostics 14, no. 1: 95. https://doi.org/10.3390/diagnostics14010095
APA StyleKaur, A., Kaushal, C., Sandhu, J. K., Damaševičius, R., & Thakur, N. (2024). Histopathological Image Diagnosis for Breast Cancer Diagnosis Based on Deep Mutual Learning. Diagnostics, 14(1), 95. https://doi.org/10.3390/diagnostics14010095






