Comparative Evaluation of Chest Ultrasonography and Computed Tomography as Predictors of Malignant Pleural Effusion: A Prospective Study
Abstract
1. Introduction
2. Methods
2.1. Ethical Statement
2.2. Study Design and Eligibility Criteria
2.3. Chest US Protocol
2.4. Chest CT Protocol
2.5. CT Image Analysis
2.6. Reference Standard
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics and Final Diagnoses
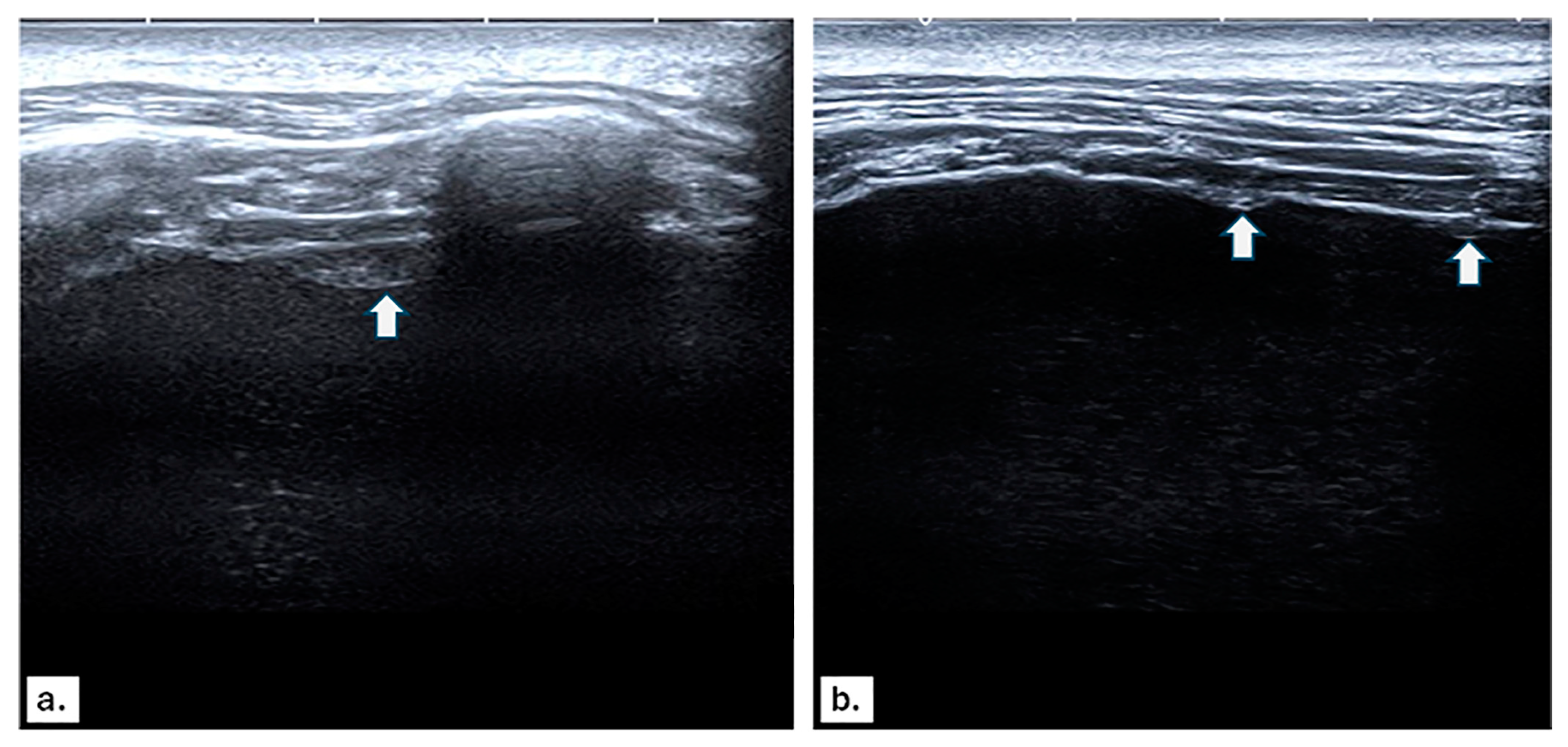
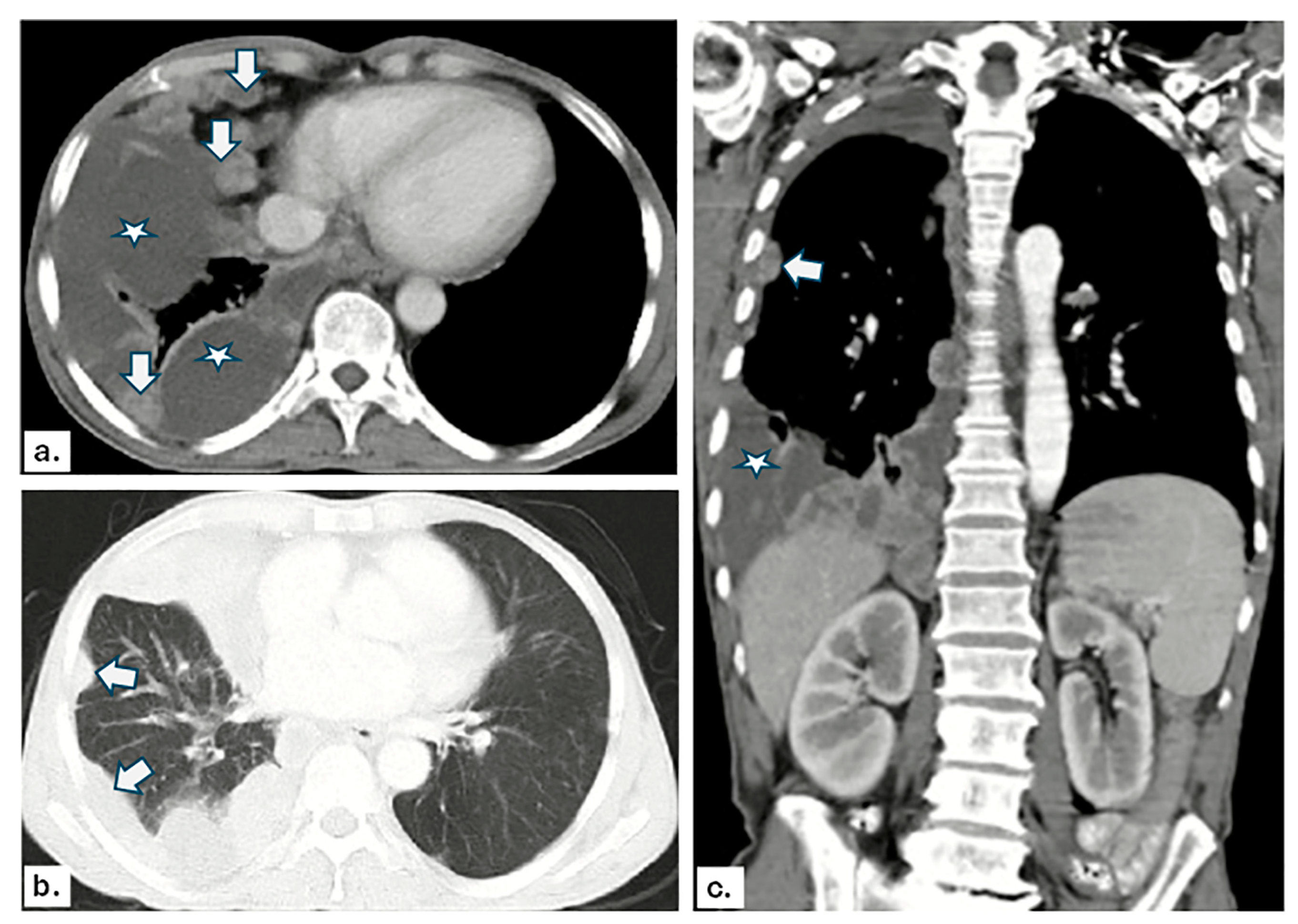
3.2. US and CT Findings in MPE
3.3. Imaging Findings in Benign Pleural Effusion
3.4. Validity of US and CT Findings in Detecting MPE
3.5. Logistic Regression Analysis for MPE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonnelli, F.; Hassan, W.; Bonifazi, M.; Pinelli, V.; Bedawi, E.O.; Porcel, J.M.; Rahman, N.M.; Mei, F. Malignant pleural effusion: Current understanding and therapeutic approach. Respir. Res. 2024, 25, 47. [Google Scholar] [CrossRef] [PubMed]
- Fenton, K.N.; Richardson, J.D. Diagnosis and management of malignant pleural effusions. Am. J. Surg. 1995, 170, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.R.; Lee, H.J. Diagnosis and management of malignant pleural effusions: State of the art in 2017. J. Thorac. Dis. 2017, 9 (Suppl. S10), S1111–S1122. [Google Scholar] [CrossRef] [PubMed]
- Dixit, R.; Agarwal, K.C.; Gokhroo, A.; Patil, C.B.; Meena, M.; Shah, N.S.; Arora, P. Diagnosis and management options in malignant pleural effusions. Lung India 2017, 34, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.A.; Louw, E.H.; Koegelenberg, C.F.N. A practical approach to the diagnosis and management of malignant pleural effusions in resource-constrained settings. Breathe 2023, 19, 230140. [Google Scholar] [CrossRef]
- Grosu, H.B.; Kazzaz, F.; Vakil, E.; Molina, S.; Ost, D. Sensitivity of Initial Thoracentesis for Malignant Pleural Effusion Stratified by Tumor Type in Patients with Strong Evidence of Metastatic Disease. Respiration 2018, 96, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Addala, D.N.; Kanellakis, N.I.; Bedawi, E.O.; Dong, T.; Rahman, N.M. Malignant pleural effusion: Updates in diagnosis, management and current challenges. Front. Oncol. 2022, 12, 1053574. [Google Scholar] [CrossRef] [PubMed]
- Ozcelik, N.; Ozcelik, A.E.; Zirih, N.M.G.; Selimoglu, I.; Gumus, A. Deep learning for diagnosis of malign pleural effusion on computed tomography images. Clinics 2023, 78, 100210. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Touman, A.A.; Grabczak, E.M.; Skaarup, S.H.; Faber, K.; Blyth, K.G.; Pochepnia, S. Imaging of pleural disease. Breathe 2024, 20, 230172. [Google Scholar] [CrossRef]
- Soni, N.J.; Franco, R.; Velez, M.I.; Schnobrich, D.; Dancel, R.; Restrepo, M.I.; Mayo, P.H. Ultrasound in the diagnosis and management of pleural effusions. J. Hosp. Med. 2015, 10, 811–816. [Google Scholar] [CrossRef]
- Pathak, T.; Parmar, M.S. (F)utility of computed tomography of the chest in the presence of pleural effusion. Pleura Peritoneum. 2017, 2, 181–186. [Google Scholar] [CrossRef]
- Buda, N.; Kosiak, W.; Wełnicki, M.; Skoczylas, A.; Olszewski, R.; Piotrkowski, J.; Skoczyński, S.; Radzikowska, E.; Jassem, E.; Grabczak, E.M.; et al. Recommendations for Lung Ultrasound in Internal Medicine. Diagnostics 2020, 10, 597. [Google Scholar] [CrossRef]
- Xirouchaki, N.; Magkanas, E.; Vaporidi, K.; Kondili, E.; Plataki, M.; Patrianakos, A.; Akoumianaki, E.; Georgopoulos, D. Lung ultrasound in critically ill patients: Comparison with bedside chest radiography. Intensive Care Med. 2011, 37, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Brogi, E.; Gargani, L.; Bignami, E.; Barbariol, F.; Marra, A.; Forfori, F.; Vetrugno, L. Thoracic ultrasound for pleural effusion in the intensive care unit: A narrative review from diagnosis to treatment. Crit. Care 2017, 21, 325. [Google Scholar] [CrossRef]
- Na, M.J. Diagnostic tools of pleural effusion. Tuberc. Respir. Dis. 2014, 76, 199. [Google Scholar] [CrossRef] [PubMed]
- Abramowitz, Y.; Simanovsky, N.; Goldstein, M.S.; Hiller, N. Pleural effusion: Characterization with CT attenuation values and CT appearance. Am. J. Roentgenol. 2009, 192, 618–623. [Google Scholar] [CrossRef]
- Karkhanis, V.S.; Joshi, J.M. Pleural effusion: Diagnosis, treatment, and management. Open Access Emerg. Med. 2012, 4, 31–52. [Google Scholar] [CrossRef] [PubMed]
- Bellolio, M.F.; Bellew, S.D.; Sangaralingham, L.R.; Campbell, R.L.; Cabrera, D.; Jeffery, M.M.; Shah, N.D.; Hess, E.P. Access to primary care and computed tomography use in the emergency department. BMC Health Serv. Res. 2018, 18, 154. [Google Scholar] [CrossRef]
- Jacobs, B.; Sheikh, G.; Youness, H.A.; Keddissi, J.I.; Abdo, T. Diagnosis and Management of Malignant Pleural Effusion: A Decade in Review. Diagnostics 2022, 12, 1016. [Google Scholar] [CrossRef]
- Shkolnik, B.; Judson, M.A.; Austin, A.; Hu, K.; D’Souza, M.; Zumbrunn, A.; Huggins, J.T.; Yucel, R.; Chopra, A. Diagnostic accuracy of thoracic ultrasonography to differentiate transudative from exudative pleural effusion. Chest 2020, 158, 692–697. [Google Scholar] [CrossRef]
- Shiroshita, A.; Nozaki, S.; Tanaka, Y.; Luo, Y.; Kataoka, Y. Thoracic ultrasound for malignant pleural effusion: A systematic review and meta-analysis. ERJ Open Res. 2020, 6, 00464–02020. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, N.R.; Rahman, N.M.; Gleeson, F.V. Thoracic ultrasound in the diagnosis of malignant pleural effusion. Thorax 2009, 64, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Messina, G.; Bove, M.; Natale, G.; Di Filippo, V.; Opromolla, G.; Rainone, A.; Leonardi, B.; Martone, M.; Fiorelli, A.; Vicidomini, G.; et al. Diagnosis of malignant pleural disease: Ultrasound as “a detective probe”. Thorac. Cancer 2023, 14, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, W.; Feng, X.; Guo, J.; Chen, B.; Zhang, F.; Wang, H.; Fan, M.; Zhu, Y.; Sun, Y.; et al. Diagnostic accuracy of thoracic CT to differentiate transudative from exudative pleural effusion prior to thoracentesis. Respir. Res. 2024, 25, 53. [Google Scholar] [CrossRef] [PubMed]
- Bugalho, A.; Semedo, J.; Alpendre, J.; Cepeda Ribeiro, J.; Carreiro, L. Ecografia na patologia torácica [Ultrasound in chest disease]. Rev. Port. Pneumol. 2010, 16, 589–606. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.N.; Müller, N.L.; Miller, R.R. CT in differential diagnosis of diffuse pleural disease. AJR Am. J. Roentgenol. 1990, 154, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Koenig, S.J.; Narasimhan, M.; Mayo, P.H. Thoracic ultrasonography for the pulmonary specialist. Chest 2011, 140, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Bediwy, A.S.; Badawy, M.E.; Salama, A.A.; Zayed, H.A. The use of multi-detector computed tomography and ultrasonography for evaluation of pleural lesions. Egypt. J. Chest Dis. Tuberc. 2015, 64, 161–168. [Google Scholar] [CrossRef]
- Drawish, A.A.; ElWahsh, R.A.; Elgaml, H.A.; Eldahdouh, S.S. Chest ultrasonography versus computed tomography in the diagnosis of pleural effusion (Menoufia experience). Egypt. J. Chest Dis. Tuberc. 2021, 70, 395–402. [Google Scholar]
- Hassan, M.; Mercer, R.M.; Rahman, N.M. Thoracic ultrasound in the modern management of pleural disease. Eur. Respir. Rev. 2020, 29, 190136. [Google Scholar] [CrossRef]
- Hussein, S.A.; Kamel, K.M.; Elkorashy, R.I.; Abdelnasser, H.G.; Elboghdady, S.M. Role of transthoracic ultrasonography in evaluation of pleural thickening observed during medical thoracoscopy. Egypt. J. Chest Dis. Tuberc. 2020, 69, 708–715. [Google Scholar] [CrossRef]
- Bugalho, A.; Ferreira, D.; Dias, S.S.; Schuhmann, M.; Branco, J.C.; Marques Gomes, M.J.; Eberhardt, R. The diagnostic value of transthoracic ultrasonographic features in predicting malignancy in undiagnosed pleural effusions: A prospective observational study. Respiration 2014, 87, 270–278. [Google Scholar] [CrossRef]
- Pandey, S.; Jaipal, U.; Mannan, N.; Yadav, R. Diagnostic accuracy of multidetector computed tomography scan in mediastinal masses assuming histopathological findings as gold standard. Pol. J. Radiol. 2018, 83, e234–e242. [Google Scholar] [CrossRef]
- Cardinale, L.; Ardissone, F.; Gned, D.; Sverzellati, N.; Piacibello, E.; Veltri, A. Diagnostic Imaging and workup of Malignant Pleural Mesothelioma. Acta Biomed 2017, 88, 134–142. [Google Scholar] [PubMed]
- Sconfienza, L.M.; Mauri, G.; Grossi, F.; Truini, M.; Serafini, G.; Sardanelli, F.; Murolo, C. Pleural and peripheral lung lesions: Comparison of US- and CT-guided biopsy. Radiology 2013, 266, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.M.; Ullah, K.; Patail, H.; Ahmad, S. Ultrasound for Pleural Disease. Beyond A Pocket Pleural Fluid. Ann. Am. Thorac. Soc. 2021, 18, 749–756. [Google Scholar] [CrossRef]
- Gonfiotti, A.; Salvicchi, A.; Voltolini, L. Role of total-body computed tomography and 18-fluorine-fluorodeoxyglucose positron emission tomography/computed tomography scan in malignant pleural effusion patients with unknown primary tumor site. J. Xiangya Med. 2020, 5, 29. [Google Scholar] [CrossRef]
- Kaul, V.; McCracken, D.J.; Rahman, N.M.; Epelbaum, O. Contemporary Approach to the Diagnosis of Malignant Pleural Effusion. Ann. Am. Thorac. Soc. 2019, 16, 1099–1106. [Google Scholar] [CrossRef]
- Piskac Zivkovic, N.; Cikara, I.; Novak, N.P.; Brkljacic, B.; Tudoric, N. A Retrospective Study of Ultrasound Characteristics and Macroscopic Findings in Confirmed Malignant Pleural Effusion. Pulm. Med. 2019, 2019, 5628267. [Google Scholar] [CrossRef]
- Safai Zadeh, E.; Weide, J.; Dietrich, C.F.; Trenker, C.; Koczulla, A.R.; Görg, C. Diagnostic Accuracy of B-Mode- and Contrast-Enhanced Ultrasound in Differentiating Malignant from Benign Pleural Effusions. Diagnostics 2021, 11, 1293. [Google Scholar] [CrossRef]
- Keskin, Z.; Yeşildağ, M.; Alkan, E.; Kayhan, A.; Tolu, İ.; Keskin, S. Differentiation between transudative and exudative pleural effusions by diffusion-weighted magnetic resonance imaging. Iran. J. Radiol. 2019, 16, e78775. [Google Scholar] [CrossRef]
- Qureshi, N.R.; Gleeson, F.V. Imaging of pleural disease. Clin. Chest Med. 2006, 27, 193–213. [Google Scholar] [CrossRef] [PubMed]
| Variable | Value |
|---|---|
| Gender | |
| Male | 34 (63) |
| Female | 20 (37) |
| Age (years), Mean ± SD | 50.8 ± 8.5 |
| Pathological type of pleural effusions | |
| Malignant | 33 (61.1) |
| Benign | 21 (38.9) |
| Final diagnosis | |
| Benign pleural effusions | |
| Nonspecific inflammatory lesion | 3 (5.6) |
| Tuberculosis | 11 (20.4) |
| Pneumonia | 7 (13) |
| Malignant pleural effusions | |
| Metastatic | 21 (38.8) |
| Lung cancer | 16 (29.6) |
| Adenocarcinoma | 11 (20.4) |
| Small cell lung cancer | 5 (9.2) |
| Breast cancer | 4 (7.4) |
| Gastro-intestinal cancer | 1 (1.9) |
| Mesothelioma | 12 (22.2) |
| Findings | US | CT | p-Value |
|---|---|---|---|
| Parietal pleural thickening | 0.125 | ||
| Absent | 21 (63.6) | 17 (51.5) | |
| Present | 12 (36.4) | 16 (48.5) | |
| Parietal pleural thickening | 0.016 | ||
| Absent | 21 (63.6) | 17 (51.5) | |
| <10 mm | 3 (9.1) | 3 (9.1) | |
| ≥10 mm | 9 (27.3) | 13 (39.4) | |
| Parietal pleural nodularity | 0.500 | ||
| Absent | 23 (69.7) | 25 (57.8) | |
| Present | 10 (30.3) | 8 (24.2) | |
| Diaphragmatic pleural thickening | 0.001 | ||
| Absent | 19 (57.6) | 30 (90.9) | |
| Present | 14 (42.4) | 3 (9.1) | |
| Diaphragmatic pleural thickening | <0.001 | ||
| Absent | 19 (57.6) | 30 (90.9) | |
| <10 mm | 3 (9.1) | 1 (3) | |
| ≥10 mm | 11 (33.3) | 2 (6.1) | |
| Diaphragmatic pleural nodularity | <0.001 | ||
| Absent | 18 (54.5) | 32 (97) | |
| Present | 15 (45.5) | 1 (3) | |
| Mediastinal thickening | 0.002 | ||
| Absent | 27 (81.8) | 17 (51.5) | |
| Present | 6 (15.2) | 16 (48.5) | |
| Pleural rind | <0.001 | ||
| Absent | 33 (100) | 24 (72.7) | |
| Present | 0 (0) | 9 (27.3) | |
| Peripheral lung lesion | 0.250 | ||
| Absent | 27 (81.8) | 24 (72.7) | |
| Present | 6 (18.2) | 9 (27.3) | |
| Chest wall invasion | 0.250 | ||
| Absent | 29 (87.9) | 32 (97) | |
| Present | 4 (12.1) | 1 (3) | |
| Liver metastasis | 0.500 | ||
| Absent | 30 (90.9) | 32 (97) | |
| Present | 3 (9.1) | 1 (3) |
| Findings | US | CT | p-Value |
|---|---|---|---|
| Parietal pleural thickening | 1.000 | ||
| Absent | 19 (90.5) | 19 (90.5) | |
| Present | 2 (9.5) | 2 (9.5) | |
| Parietal pleural thickening | 1.000 | ||
| Absent | 19 (90.5) | 19 (90.5) | |
| <10 mm | 2 (9.5) | 2 (9.5) | |
| >10 mm | 0 (0) | 0 (0) | |
| Parietal pleural nodularity | 1.000 | ||
| Absent | 20 (95.2) | 20 (95.2) | |
| Present | 1 (4.8) | 1 (4.8) | |
| Diaphragmatic pleural thickening | ---- | ||
| Absent | 21 (100) | 21 (100) | |
| Present | 0 (0) | 0 (0) | |
| Diaphragmatic pleural thickening | ---- | ||
| Absent | 21 (100) | 21 (100) | |
| <10 mm | 0 (0) | 0 (0) | |
| >10 mm | 0 (0) | 0 (0) | |
| Diaphragmatic pleural nodularity | ---- | ||
| Absent | 21 (100) | 21 (100) | |
| Present | 0 (0) | 0 (0) | |
| Mediastinal thickening | 1.000 | ||
| Absent | 20 (95.2) | 19 (90.5) | |
| Present | 1 (4.8) | 2 (9.5) | |
| Pleural rind | ----- | ||
| Absent | 21(100) | 21(100) | |
| Present | 0 (0) | 0 (0) | |
| Peripheral lung lesion | 1.000 | ||
| Absent | 16 (76.2) | 15 (71.4) | |
| Present | 5 (23.8) | 6 (28.6) |
| Image | TP | FP | TN | FN | SN | SP | PPV | NPV | |
|---|---|---|---|---|---|---|---|---|---|
| Parietal pleural thickening | US | 12 | 2 | 19 | 21 | 36.4% | 90.5% | 85.7% | 47.5% |
| CT | 16 | 2 | 19 | 17 | 48.5% | 90.5% | 88.9% | 52.8% | |
| Parietal pleural nodularity | US | 10 | 1 | 20 | 23 | 30.3% | 95.2% | 90.9% | 46.5% |
| CT | 8 | 1 | 20 | 25 | 24.2% | 95.2% | 88.9% | 44.4% | |
| Diaphragmatic pleural thickening | US | 14 | 0 | 21 | 19 | 42.4% | 100% | 100% | 52.5% |
| CT | 3 | 0 | 21 | 30 | 9.1% | 100% | 100% | 41.2% | |
| Diaphragmatic pleural nodularity | US | 15 | 0 | 21 | 18 | 45.5% | 100% | 100% | 53.8% |
| CT | 1 | 0 | 21 | 32 | 3% | 100% | 100% | 39.6% | |
| Mediastinal thickening | US | 6 | 1 | 20 | 27 | 18.2% | 95.2% | 85.7% | 42.6% |
| CT | 16 | 2 | 19 | 17 | 48.5% | 90.5% | 88.9% | 52.8% | |
| Pleural rind | US | 0 | 0 | 21 | 33 | 0% | 100% | 0% | 38.9% |
| CT | 9 | 0 | 21 | 24 | 27.3% | 100% | 100% | 46.7% | |
| Peripheral lung lesion | US | 6 | 5 | 16 | 27 | 18.2% | 76.2% | 54.5% | 37.2% |
| CT | 9 | 6 | 15 | 24 | 27.3% | 71.4% | 60% | 38.5% | |
| Chest wall invasion | US | 4 | 0 | 21 | 29 | 12.1% | 100% | 100% | 42% |
| CT | 1 | 0 | 21 | 32 | 3% | 100% | 100% | 39.6% | |
| Liver metastasis | US | 3 | 0 | 21 | 30 | 9.1% | 100% | 100% | 41.2% |
| CT | 1 | 0 | 21 | 32 | 3% | 100% | 100% | 39.6% |
| Findings | US OR (95% CI) | p-Value | CT OR (95% CI) | p-Value |
|---|---|---|---|---|
| Parietal pleural thickening ≥ 10 mm | 2.15 (1.02–4.53) | 0.044 | 3.12 (1.28–7.62) | 0.012 |
| Parietal pleural nodularity | 1.67 (0.79–3.55) | 0.182 | 2.41 (0.92–6.31) | 0.073 |
| Diaphragmatic pleural thickening ≥ 10 mm | 5.28 (2.19–12.72) | <0.001 | 2.67 (0.68–11.23) | 0.157 |
| Diaphragmatic pleural nodularity | 9.45 (3.56–25.08) | <0.001 | 1.89 (0.22–16.01) | 0.563 |
| Mediastinal thickening | 1.22 (0.49–3.04) | 0.671 | 4.67 (1.83–11.92) | 0.001 |
| Pleural rind | ---- | ---- | 6.82 (2.47–18.86) | <0.001 |
| Peripheral lung lesion | 0.72 (0.31–1.68) | 0.448 | 1.14 (0.51–2.54) | 0.748 |
| Chest Wall Invasion | 3.25 (0.98–10.76) | 0.054 | 1.62 (0.19–13.73) | 0.658 |
| Liver Metastasis | 3.89 (1.02–14.86) | 0.047 | 1.78 (0.21–15.21) | 0.603 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shehata, S.M.; Almalki, Y.E.; Basha, M.A.A.; Hendy, R.M.; Mahmoud, E.M.; Abd Elhamed, M.E.; Alduraibi, S.K.; Aboualkheir, M.; Almushayti, Z.A.; Alduraibi, A.K.; et al. Comparative Evaluation of Chest Ultrasonography and Computed Tomography as Predictors of Malignant Pleural Effusion: A Prospective Study. Diagnostics 2024, 14, 1041. https://doi.org/10.3390/diagnostics14101041
Shehata SM, Almalki YE, Basha MAA, Hendy RM, Mahmoud EM, Abd Elhamed ME, Alduraibi SK, Aboualkheir M, Almushayti ZA, Alduraibi AK, et al. Comparative Evaluation of Chest Ultrasonography and Computed Tomography as Predictors of Malignant Pleural Effusion: A Prospective Study. Diagnostics. 2024; 14(10):1041. https://doi.org/10.3390/diagnostics14101041
Chicago/Turabian StyleShehata, Samah M., Yassir Edrees Almalki, Mohammad Abd Alkhalik Basha, Rasha Mohamed Hendy, Eman M. Mahmoud, Marwa Elsayed Abd Elhamed, Sharifa Khalid Alduraibi, Mervat Aboualkheir, Ziyad A. Almushayti, Alaa K. Alduraibi, and et al. 2024. "Comparative Evaluation of Chest Ultrasonography and Computed Tomography as Predictors of Malignant Pleural Effusion: A Prospective Study" Diagnostics 14, no. 10: 1041. https://doi.org/10.3390/diagnostics14101041
APA StyleShehata, S. M., Almalki, Y. E., Basha, M. A. A., Hendy, R. M., Mahmoud, E. M., Abd Elhamed, M. E., Alduraibi, S. K., Aboualkheir, M., Almushayti, Z. A., Alduraibi, A. K., Basha, A. M. A., & Alsadik, M. E. (2024). Comparative Evaluation of Chest Ultrasonography and Computed Tomography as Predictors of Malignant Pleural Effusion: A Prospective Study. Diagnostics, 14(10), 1041. https://doi.org/10.3390/diagnostics14101041








