Cross-Cultural Adaptation and Validation of the Portuguese Version of the SARC-F in Community-Dwelling Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Procedure
2.3. Outcome Measures
2.4. Statistical Analysis
3. Results
3.1. Reliability
3.2. Clinical Validity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 8 February 2024).
- Rosenberg, I. Summary comments: Epidemiological and methodological problems in determining nutritional status of older persons. Am. J. Clin. Nutr. 1989, 50, 1231–1233. [Google Scholar] [CrossRef]
- Anker, S.D.; Morley, J.E.; von Haehling, S. Welcome to the ICD-10 code for sarcopenia. J. Cachexia Sarcopenia Muscle 2016, 7, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Van Kan, G.A.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism 2023, 144, 155533. [Google Scholar] [CrossRef]
- Cegielski, J.; Bass, J.J.; Willott, R.; Gordon, A.L.; Wilkinson, D.J.; Smith, K.; Atherton, P.J.; Phillips, B.E. Exploring the variability of sarcopenia prevalence in a research population using different disease definitions. Aging Clin. Exp. Res. 2023, 35, 2271–2275. [Google Scholar] [CrossRef]
- Beaudart, C.; Demonceau, C.; Reginster, J.Y.; Locquet, M.; Cesari, M.; Cruz Jentoft, A.J.; Bruyère, O. Sarcopenia and health-related quality of life: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2023, 14, 1228–1243. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.V.; Hsu, T.H.; Wu, W.T.; Huang, K.C.; Han, D.S. Association Between Sarcopenia and Cognitive Impairment: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2016, 17, 1164.e7–1164.e15. [Google Scholar] [CrossRef] [PubMed]
- Bahat, G.; Ilhan, B. Sarcopenia and the cardiometabolic syndrome: A narrative review. Eur. Geriatr. Med. 2016, 7, 220–223. [Google Scholar] [CrossRef]
- Quan, Y.; Wang, C.; Wang, L.; Li, G. Geriatric sarcopenia is associated with hypertension: A systematic review and meta-analysis. J. Clin. Hypertens. 2023, 25, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Liu, C.; Liu, Y.; Tang, G.; Li, C.; Guo, L. Association between sarcopenia with incident cardio-cerebrovascular disease: A systematic review and meta-analysis. Biosci. Trends 2023, 17, 293–301. [Google Scholar] [CrossRef] [PubMed]
- De Buyser, S.L.; Petrovic, M.; Taes, Y.E.; Toye, K.R.; Kaufman, J.M.; Lapauw, B.; Goemaere, S. Validation of the FNIH sarcopenia criteria and SOF frailty index as predictors of long-term mortality in ambulatory older men. Age Ageing 2016, 45, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Yakti, F.A.Z.; Abusalah, L.; Ganji, V. Sarcopenia and Mortality in Critically Ill COVID-19 Patients. Life 2023, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Parra-Rodríguez, L.; Szlejf, C.; García-González, A.I.; Malmstrom, T.K.; Cruz-Arenas, E.; Rosas-Carrasco, O. Cross-Cultural Adaptation and Validation of the Spanish-Language Version of the SARC-F to Assess Sarcopenia in Mexican Community-Dwelling Older Adults. J. Am. Med. Dir. Assoc. 2016, 17, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, T.K.; Morley, J.E. SARC-F: A simple questionnaire to rapidly diagnose sarcopenia. J. Am. Med. Dir. Assoc. 2013, 14, 531–532. [Google Scholar] [CrossRef]
- Malmstrom, T.K.; Miller, D.K.; Simonsick, E.M.; Ferrucci, L.; Morley, J.E. SARC-F: A symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J. Cachexia Sarcopenia Muscle 2016, 7, 28–36. [Google Scholar] [CrossRef]
- Bahat, G.; Yilmaz, O.; Oren, M.M.; Karan, M.A.; Reginster, J.Y.; Bruyère, O.; Beaudart, C. Cross-cultural adaptation and validation of the SARC-F to assess sarcopenia: Methodological report from European Union Geriatric Medicine Society Sarcopenia Special Interest Group. Eur. Geriatr. Med. 2018, 9, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Kera, T.; Kawai, H.; Hirano, H.; Kojima, M.; Watanabe, Y.; Motokawa, K.; Fujiwara, Y.; Ihara, K.; Kim, H.; Obuchi, S. SARC-F: A validation study with community-dwelling older Japanese adults. Geriatr. Gerontol. Int. 2019, 19, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Ida, S.; Kojima, Y.; Hamaoka, S.; Urawa, N.; Araki, J.; Kaneko, R.; Murata, K. Validity of Japanese version of SARC-F questionnaire in patients with chronic liver disease. J. Gastroenterol. Hepatol. 2019, 34, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Tsekoura, M.; Billis, E.; Tsepis, E.; Lampropoulou, S.; Beaudart, C.; Bruyere, O.; Yilmaz, O.; Bahat, G.; Gliatis, J. Cross-cultural adaptation and validation of the Greek Version of the SARC-F for evaluating sarcopenia in Greek older adults. J. Musculoskelet. Neuronal Interact. 2020, 20, 505–512. [Google Scholar] [PubMed]
- Perna, S.; Gasparri, C.; Ferraris, C.; Barrile, G.C.; Cavioni, A.; Mansueto, F.; Patelli, Z.; Peroni, G.; Tartara, A.; Zese, M.; et al. Validation of the Italian Version of the SARC-F Questionnaire to Assess Sarcopenia in Older Adults. Nutrients 2022, 14, 2533. [Google Scholar] [CrossRef] [PubMed]
- Faria, Â.; Sousa-Santos, A.R.; Mendes, J.; Limas de Sousa, A.S.; Amaral, T.F. Desenvolvimento das versões portuguesas dos questionários FRAIL Scale e SARC-F: Ferramentas de rastreio para a fragilidade física e sarcopenia. Acta Port. Nutr. 2021, 26, 90–94. [Google Scholar] [CrossRef]
- Barbosa-Silva, T.G.; Menezes, A.M.; Bielemann, R.M.; Malmstrom, T.K.; Gonzalez, M.C.; Grupo de Estudos em Composição Corporal e Nutrição (COCONUT). Enhancing SARC-F: Improving Sarcopenia Screening in the Clinical Practice. J. Am. Med. Dir. Assoc. 2016, 17, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, D.; Marco, E.; Dávalos-Yerovi, V.; López-Escobar, J.; Messaggi-Sartor, M.; Barrera, C.; Ronquillo-Moreno, N.; Vázquez-Ibar, O.; Calle, A.; Inzitari, M.; et al. Translation and Validation of the Spanish Version of the SARC-F Questionnaire to Assess Sarcopenia in Older People. J. Nutr. Health Aging 2019, 23, 518–524. [Google Scholar] [CrossRef]
- World Health Organization. Obesity: Preventing and Management of the Global Epidemic; Report of the WHO Consultation: Technical Report Series. No. 894; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Schlüssel, M.M.; dos Anjos, L.A.; de Vasconcellos, M.T.; Kac, G. Reference values of handgrip dynamometry of healthy adults: A population-based study. Clin. Nutr. 2008, 27, 601–607. [Google Scholar] [CrossRef]
- Tanita. Instruction Manual for InnerScan V Segmental Body Composition Monitor, BC-601, Tanita Publication, Japan. May 2024. Available online: https://www.manualslib.com/manual/2723866/Tanita-Innerscan-V-Bc-601.html (accessed on 15 January 2024).
- Cesari, M.; Kritchevsky, S.B.; Penninx, B.W.; Nicklas, B.J.; Simonsick, E.M.; Newman, A.B.; Tylavsky, F.A.; Brach, J.S.; Satterfield, S.; Bauer, D.C.; et al. Prognostic value of usual gait speed in well-functioning older people—Results from the Health, Aging and Body Composition Study. J. Am. Geriatr. Soc. 2005, 53, 1675–1680. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Shrout, P.E.; Fleiss, J.L. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Deyo, R.A.; Diehr, P.; Patrick, D.L. Reproducibility and responsiveness of health status measures. Statistics and strategies for evaluation. Control Clin. Trials 1991, 12, 142S–158S. [Google Scholar] [CrossRef]
- McHorney, C.A.; Tarlov, A.R. Individual-patient monitoring in clinical practice: Are available health status surveys adequate? Qual. Life Res. 1995, 4, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.A. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Linden, A. Measuring Diagnostic and Predictive Accuracy in Disease Management: An Introduction to Receiver Operating Characteristic (ROC) Analysis. J. Eval. Clin. Pract. 2006, 12, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Akarapornkrailert, P.; Muangpaisan, W.; Boonpeng, A.; Daengdee, D. Validation of the Thai version of SARC-F, MSRA-7, and MSRA-5 questionnaires compared to AWGS 2019 and sarcopenia risks in older patients at a medical outpatient clinic. Osteoporos. Sarcopenia 2020, 6, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Krzymińska-Siemaszko, R.; Deskur-Śmielecka, E.; Kaluźniak-Szymanowska, A.; Styszyński, A.; Wieczorowska-Tobis, K. Polish version of SARC-F to assess sarcopenia in older adults: An examination of reliability and validity. PLoS ONE 2020, 15, e0244001. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hou, L.; Zhao, W.; Xia, X.; Hu, F.; Zhang, G.; Hao, Q.; Zhou, L.; Liu, Y.; Ge, M.; et al. The Comparison of Sarcopenia Diagnostic Criteria using AWGS 2019 with the Other Five Criteria in West China. Gerontology 2021, 67, 386–396. [Google Scholar] [CrossRef]
- He, X.; Song, Y.; Ma, L.; Ainsworth, B.E.; Liu, Y.; Chen, N. Prevalence and Factors Influencing Sarcopenia Among Community-Dwelling Older Adults Using the Asian Working Group for Sarcopenia Definition. Clin. Interv. Aging 2022, 17, 1707–1727. [Google Scholar] [CrossRef]
- Voulgaridou, G.; Tyrovolas, S.; Detopoulou, P.; Tsoumana, D.; Drakaki, M.; Apostolou, T.; Chatziprodromidou, I.P.; Papandreou, D.; Giaginis, C.; Papadopoulou, S.K. Diagnostic Criteria and Measurement Techniques of Sarcopenia: A Critical Evaluation of the Up-to-Date Evidence. Nutrients 2024, 16, 436. [Google Scholar] [CrossRef] [PubMed]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle. 2022, 13, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Gasparik, A.; Demián, M.B.; Pascanu, I. Romanian Translation and Validation of the SARC-F Questionnaire. Acta Endocrinol. 2020, 16, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Locquet, M.; Bornheima, S.; Reginster, J.Y.; Bruyère, O. French translation and validation of the sarcopenia screening tool SARC-F. Eur. Geriatr. Med. 2018, 9, 29–37. [Google Scholar] [CrossRef] [PubMed]
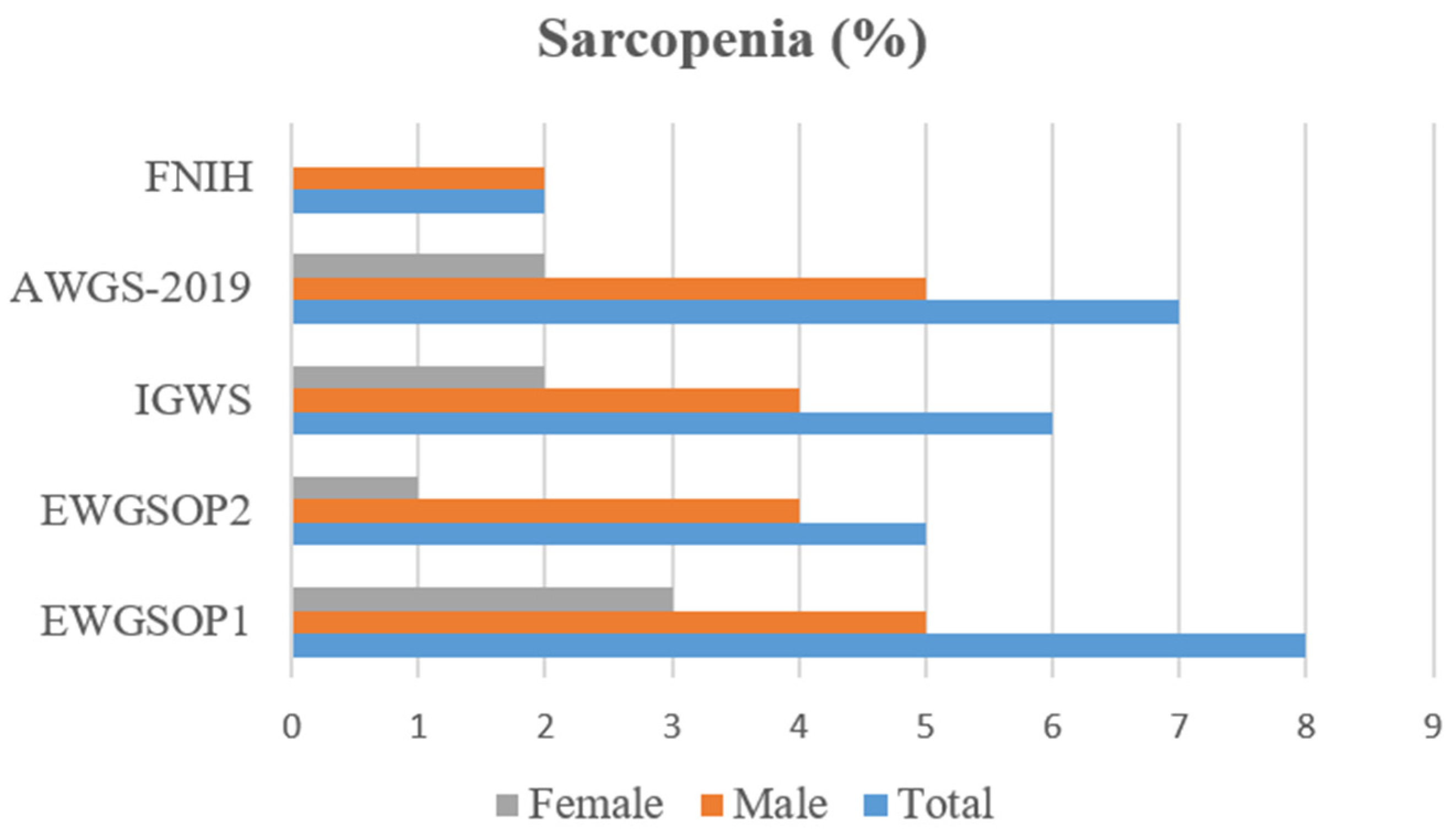
| I. Low Muscle Strength (HGS) (1) | II. Low Muscle Quantity (ASMI (2)/h2, Except for FNIH (3), ASMI/BMI (4)) | III. Low Physical Performance (Gait Speed) | Sarcopenia Diagnosis | ||||
|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | ||
| EWGSOP (5) | <30 kg | <20 kg | <7.4 kg/m2 | <5.6 kg/m2 | ≤0.8 m/s | II + I or II + III | |
| EWGSOP2 (6) | <27 kg | <16 kg | <7 kg/m2 | <5.5 kg/m2 | ≤0.8 m/s | I + II | |
| IWGS (7) | - | - | <7.23 kg/m2 | <5.67 kg/m2 | ≤1 m/s | II + III | |
| AWGS-2019 | <27 kg | <18 kg | <7 kg/m2 | <5.7 kg/m2 | ≤1 m/s | II + I or II + III | |
| FNIH | <26 kg | <16 kg | <0.79 | <0.51 | ≤0.8 m/s | I + II + III | |
| All the Participants (n = 100) | Men (n = 27) | Women (n = 73) | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| Age (years) a | 77.1 | 7.36 | 78.3 | 6.38 | 76.6 | 7.69 | 0.314 | |
| Marital status b | Married | 66 | 66 | 22 | 81.48 | 44 | 60.3 | 0.204 |
| Divorced/separated | 6 | 6 | 1 | 3.7 | 5 | 6.85 | ||
| widowed | 23 | 23 | 4 | 14.8 | 19 | 26.0 | ||
| Single | 5 | 5 | 0 | 0 | 5 | 6.85 | ||
| Education b | No level | 8 | 8 | 2 | 7.41 | 6 | 8.22 | 0.497 |
| Basic | 77 | 77 | 19 | 70.4 | 58 | 79.5 | ||
| Secondary | 8 | 8 | 4 | 14.8 | 4 | 5.48 | ||
| Higher | 7 | 7 | 2 | 7.41 | 5 | 6.85 | ||
| BMI (1) (kg/m2) a | 27.0 | 4.26 | 26.3 | 4.45 | 27.3 | 4.2 | 0.311 | |
| Fat mass percentage a | 32.8 | 9.7 | 26.7 | 9.17 | 35.1 | 8.94 | <0.001 | |
| SARC-F (2) total score a | 2.25 | 2.61 | 1.07 | 1.17 | 2.68 | 2.86 | <0.001 | |
| Calf circumference (cm) a | 35.3 | 3.34 | 35.4 | 3.53 | 35.3 | 3.29 | 0.944 | |
| ASMI (3)-height2 (kg/m2) a | 7.32 | 1.06 | 7.92 | 1.13 | 7.1 | 0.94 | <0.001 | |
| ASMI-BMI a | 0.67 | 0.13 | 0.83 | 0.1 | 0.61 | 0.08 | <0.001 | |
| Handgrip strength (kg) a | 22.1 | 9.24 | 32.2 | 10.1 | 18.4 | 5.15 | <0.001 | |
| TUG (4) test (s) a | 11.0 | 8.86 | 11.0 | 9.11 | 11.0 | 8.83 | 0.974 | |
| Gait speed (m/s) a | 1.19 | 0.51 | 1.27 | 0.59 | 1.16 | 0.48 | 0.326 | |
| SARC-F Items | SARC-F (1) Total Score | |
|---|---|---|
| Spearman’s Coefficient | p-Value | |
| 1. Strength | 0.77 | <0.001 |
| 2. Assistance with walking | 0.67 | <0.001 |
| 3. Rising from a chair | 0.73 | <0.001 |
| 4. Climbing stairs | 0.87 | <0.001 |
| 5. Falls | 0.63 | <0.001 |
| SARC-F (1) | ||||||
|---|---|---|---|---|---|---|
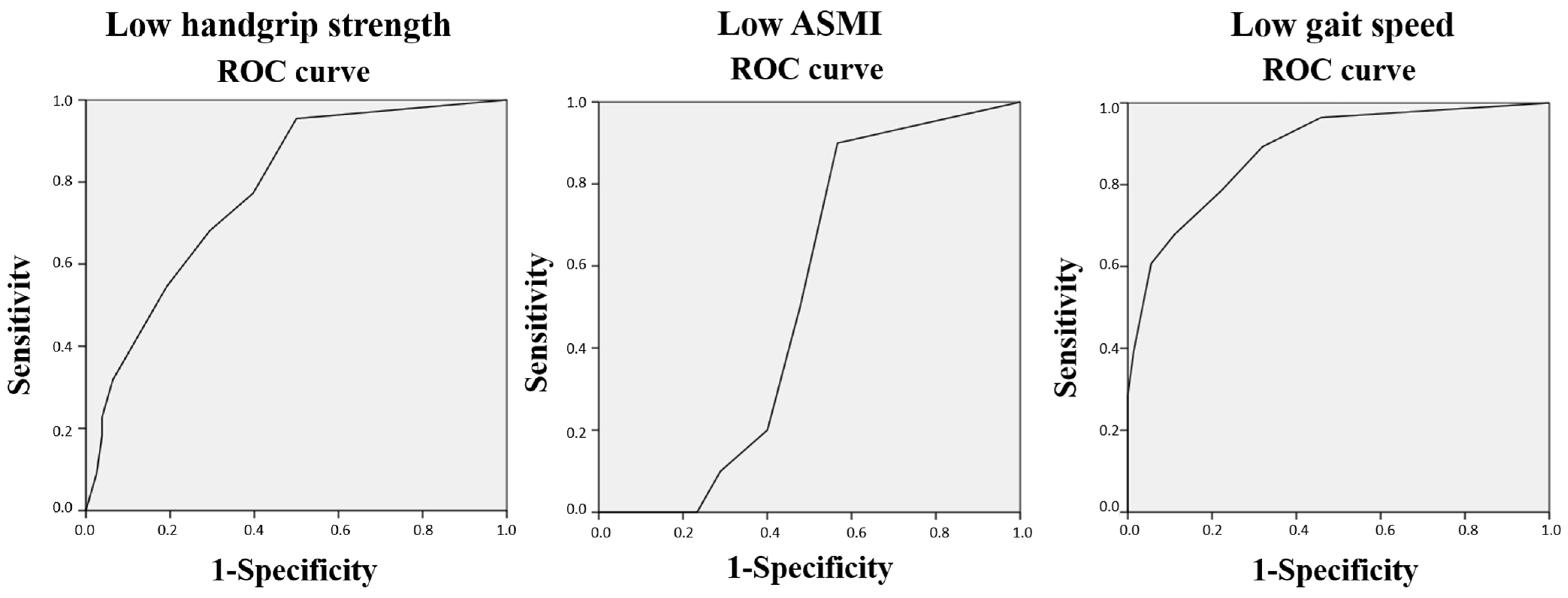
| Se (2) | Sp (3) | PPV (4) | NPV (5) | Acc (6) | AUC (7) | |
| Sarcopenia EWGSOP1 (8) | 25 | 72.8 | 7.41 | 91.9 | 69 | 0.56 |
| Sarcopenia EWGSOP2 (9) | 20 | 72.6 | 3.7 | 94.5 | 70 | 0.58 |
| Sarcopenia IGWS (10) | 33.3 | 73.4 | 7.41 | 94.5 | 71 | 0.64 |
| Sarcopenia AWGS (11)-2019 | 28.6 | 73.1 | 7.41 | 93.1 | 70 | 0.61 |
| Sarcopenia FNIH (12) | 0 | 72.5 | 0 | 97.3 | 71 | 0.52 |
| SARC-F (1) Total Score | ||
|---|---|---|
| Spearman’s Coefficient | p-Value | |
| BMI (2) | −0.23 | 0.02 |
| Fat mass percentage | −0.23 | 0.025 |
| Calf circumference | −0.26 | 0.01 |
| ASMI (3)-height2 | −0.12 | 0.248 |
| ASMI-BMI | −0.11 | 0.264 |
| Handgrip strength | −0.4 | <0.001 |
| TUG (4) test | 0.62 | <0.001 |
| Gait speed | −0.68 | <0.001 |
| βa (1) | Βb (2) (95% CI (3)) | p-Value | ||
|---|---|---|---|---|
| SARC-F (4) | Handgrip strength | −3.13 | −0.61 (−3.92, −2.34) | >0.001 |
| Gait speed | −0.05 | −0.19 (−0.10, −0.01) | 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boteta-Gomes, M.I.; Aibar-Almazán, A.; Hita-Contreras, F.; de Loureiro, N.E.M.; Brandão-Loureiro, V.A.F. Cross-Cultural Adaptation and Validation of the Portuguese Version of the SARC-F in Community-Dwelling Older Adults. Diagnostics 2024, 14, 1096. https://doi.org/10.3390/diagnostics14111096
Boteta-Gomes MI, Aibar-Almazán A, Hita-Contreras F, de Loureiro NEM, Brandão-Loureiro VAF. Cross-Cultural Adaptation and Validation of the Portuguese Version of the SARC-F in Community-Dwelling Older Adults. Diagnostics. 2024; 14(11):1096. https://doi.org/10.3390/diagnostics14111096
Chicago/Turabian StyleBoteta-Gomes, Margarida Isabel, Agustín Aibar-Almazán, Fidel Hita-Contreras, Nuno Eduardo Marques de Loureiro, and Vânia Azevedo Ferreira Brandão-Loureiro. 2024. "Cross-Cultural Adaptation and Validation of the Portuguese Version of the SARC-F in Community-Dwelling Older Adults" Diagnostics 14, no. 11: 1096. https://doi.org/10.3390/diagnostics14111096
APA StyleBoteta-Gomes, M. I., Aibar-Almazán, A., Hita-Contreras, F., de Loureiro, N. E. M., & Brandão-Loureiro, V. A. F. (2024). Cross-Cultural Adaptation and Validation of the Portuguese Version of the SARC-F in Community-Dwelling Older Adults. Diagnostics, 14(11), 1096. https://doi.org/10.3390/diagnostics14111096










