Myocardial Work Indices Predict Hospitalization in Patients with Advanced Heart Failure
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Myocardial Work Analysis
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Patient Population
3.2. Patients with Composite Endpoints Versus No Composite Endpoints
3.3. Prognostic Analysis
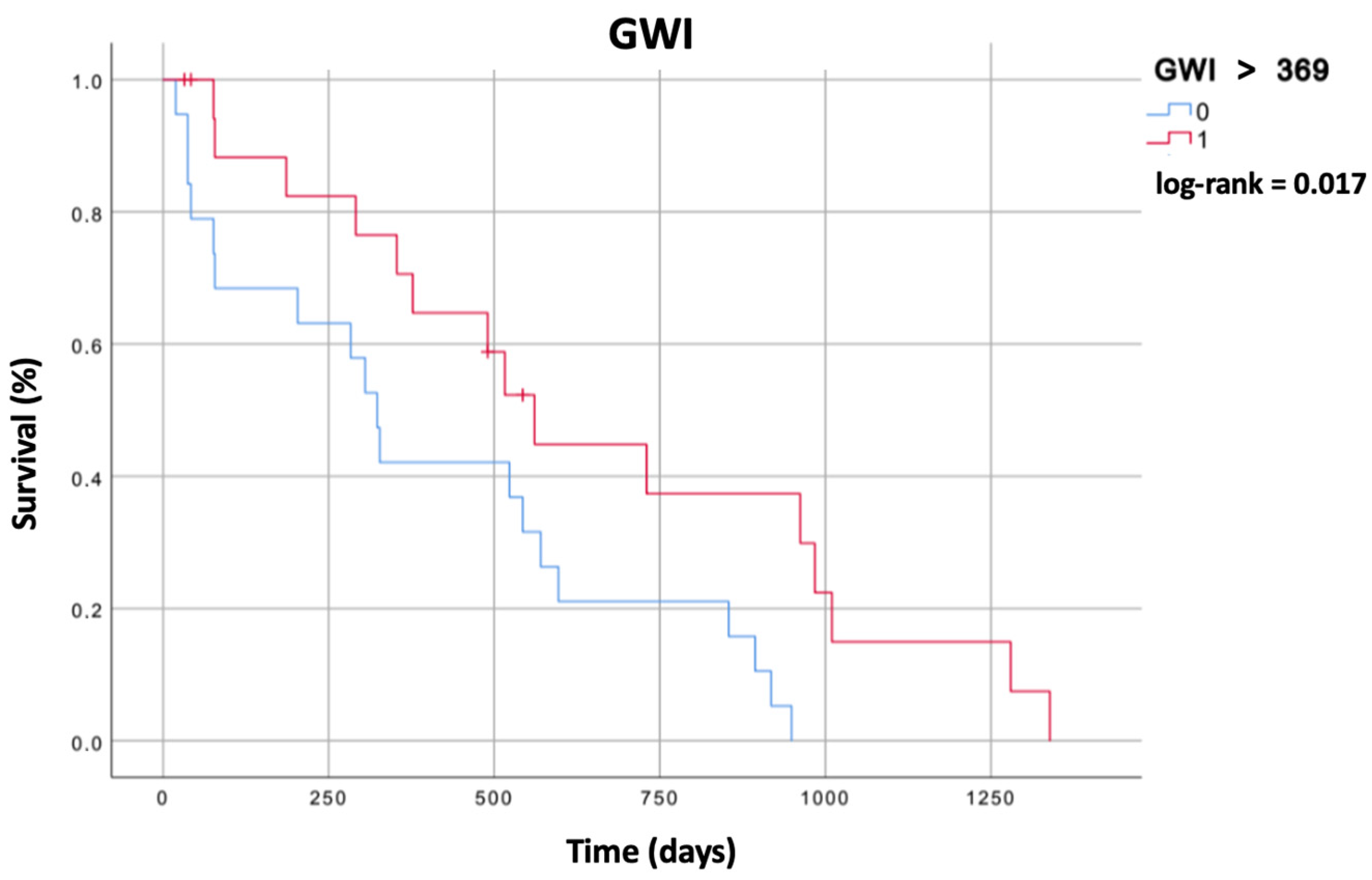
3.4. Survival Analysis
4. Discussion
Clinical Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–37262. [Google Scholar] [CrossRef] [PubMed]
- van Riet, E.E.; Hoes, A.W.; Wagenaar, K.P.; Limburg, A.; Landman, M.A.; Rutten, F.H. Epidemiology of heart failure: The prev-alence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur. J. Heart Fail. 2016, 18, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure. Card. Fail. Rev. 2017, 3, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A compre-hensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Leiro, M.G.; Metra, M.; Lund, L.H.; Milicic, D.; Costanzo, M.R.; Filippatos, G.; Gustafsson, F.; Tsui, S.; Barge-Caballero, E.; De Jonge, N.; et al. Advanced heart failure: A position statement of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 1505–1535. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.P.; Stewart, G.C. Advanced Heart Failure: Prevalence, Natural History, and Prognosis. Heart Fail Clin. 2016, 12, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Pastore, M.C.; Mandoli, G.E.; Aboumarie, H.S.; Santoro, C.; Bandera, F.; D’andrea, A.; Benfari, G.; Esposito, R.; Evola, V.; Sorrentino, R.; et al. Basic and advanced echocardiography in advanced heart failure: An overview. Heart Fail. Rev. 2020, 25, 937–948. [Google Scholar] [CrossRef]
- Grayburn, P.A.; Appleton, C.P.; DeMaria, A.N.; Greenberg, B.; Lowes, B.; Oh, J.; Plehn, J.F.; Rahko, P.; Sutton, M.S.J.; Eichhorn, E.J. Echocardiographic predictors of morbidity and mortality in patients with advanced heart failure: The Beta-blocker Evaluation of Survival Trial (BEST). J. Am. Coll. Cardiol. 2005, 45, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Marwick, T.H. Ejection Fraction Pros and Cons: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2360–2379. [Google Scholar] [CrossRef]
- Ilardi, F.; D’Andrea, A.; D’Ascenzi, F.; Bandera, F.; Benfari, G.; Esposito, R.; Malagoli, A.; Mandoli, G.E.; Santoro, C.; Russo, V.; et al. Myocardial Work by Echocardiography: Principles and Applications in Clinical Practice. J. Clin. Med. 2021, 10, 4521. [Google Scholar] [CrossRef]
- D’andrea, A.; the Echocardiography Study Group of the Italian Society of Cardiology; Radmilovic, J.; Carbone, A.; Mandoli, G.E.; Santoro, C.; Evola, V.; Bandera, F.; D’ascenzi, F.; Bossone, E.; et al. Speckle tracking evaluation in endurance athletes: The “optimal” myocardial work. Int. J. Cardiovasc. Imaging 2020, 36, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Manganaro, R.; Marchetta, S.; Dulgheru, R.; Ilardi, F.; Sugimoto, T.; Robinet, S.; Cimino, S.; Go, Y.Y.; Bernard, A.; Kacharava, G.; et al. Echocardiographic reference ranges for normal non-invasive myocardial work indices: Results from the EACVI NORRE study. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Mandoli, G.E.; Sciaccaluga, C.; Mondillo, S. More than 10 years of speckle tracking echocardiography: Still a novel technique or a definite tool for clinical practice? Echocardiography 2019, 36, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Remme, E.W.; Haugaa, K.H.; Opdahl, A.; Fjeld, J.G.; Gjesdal, O.; et al. A novel clinical method for quantification of regional left ventricular pressure-strain loop area: A non-invasive index of myocardial work. Eur. Heart J. 2012, 33, 724–733. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Ilardi, F.; D’Ascenzi, F.; Bandera, F.; Benfari, G.; Esposito, R.; Malagoli, A.; Mandoli, G.E.; Santoro, C.; Russo, V.; et al. Impaired myocardial work efficiency in heart failure with preserved ejection fraction. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Landra, F.; Mandoli, G.E.; Chiantini, B.; Barilli, M.; Merello, G.; De Carli, G.; Sciaccaluga, C.; Lisi, M.; Flamigni, F.; D’ascenzi, F.; et al. Correlation of left ventricular myocardial work indices with invasive measurement of stroke work in patients with advanced heart failure. Front. Cardiovasc. Med. 2022, 9, 946540. [Google Scholar] [CrossRef] [PubMed]
- Landra, F.; Mandoli, G.E.; Sciaccaluga, C.; Gallone, G.; Bruno, F.; Fusi, C.; Barilli, M.; Focardi, M.; Cavigli, L.; D’Ascenzi, F.; et al. Pressure–strain loops unveil haemodynamics behind mechanical circulatory support systems. ESC Heart Fail. 2023, 10, 2607–2620. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270, Erratum in: Eur. Heart J. Cardiovasc. Imaging 2016, 17, 412. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Abawi, D.; Rinaldi, T.; Faragli, A.; Pieske, B.; Morris, D.A.; Kelle, S.; Tschöpe, C.; Zito, C.; Alogna, A. The non-invasive assessment of myocardial work by pressure-strain analysis: Clinical applications. Heart Fail. Rev. 2022, 27, 1261–1279. [Google Scholar] [CrossRef]
- Smiseth, O.A.; Donal, E.; Penicka, M.; Sletten, O.J. How to measure left ventricular myocardial work by pressure–strain loops. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 259–261. [Google Scholar] [CrossRef] [PubMed]
- van der Bijl, P.; Kostyukevich, M.; El Mahdiui, M.; Hansen, G.; Samset, E.; Ajmone Marsan, N.; Bax, J.J.; Delgado, V. A Roadmap to Assess Myocardial Work: From Theory to Clinical Practice. J. Am. Coll. Cardiol. Imaging 2019, 12, 2549–2554. [Google Scholar] [CrossRef] [PubMed]
- Hedwig, F.; Nemchyna, O.; Stein, J.; Knosalla, C.; Merke, N.; Knebel, F.; Hagendorff, A.; Schoenrath, F.; Falk, V.; Knierim, J. Myocardial Work Assessment for the Prediction of Prognosis in Advanced Heart Failure. Front. Cardiovasc. Med. 2021, 8, 691611. [Google Scholar] [CrossRef] [PubMed]

| All Patients (n = 138) | Composite Endpoint (n = 35) | No Composite Endpoint (n = 103) | p-Value | |
|---|---|---|---|---|
| Age (years) | 58 [50–62] | 60 [52–64] | 57 [50–62] | 0.169 |
| Gender | 0.307 | |||
| Male, n (%) | 110 (80%) | 30 (85%) | 80 (78%) | |
| Female, n (%) | 28 (20%) | 5 (15%) | 23 (22%) | |
| Smoking Status | 0.674 | |||
| Non-smoker, n (%) | 83 (61%) | 19 (56%) | 64 (62%) | |
| Active, n (%) | 19 (13%) | 5 (12%) | 14 (14%) | |
| Former, n (%) | 36 (26%) | 11 (32%) | 25 (24%) | |
| Hyperlipidemia, n (%) | 101 (73%) | 27 (76%) | 74 (72%) | 0.541 |
| Diabetes mellitus, n (%) | 35 (26%) | 8 (23%) | 27 (26%) | 0.693 |
| Arterial hypertension, n (%) | 31 (22%) | 4 (11%) | 27 (26%) | 0.081 |
| Obesity (BMI > 30 kg/m2), n (%) | 35 (25%) | 8 (23%) | 27 (26%) | 0.693 |
| CKD (eGFR < 60 mL/min/1.73 m2), n (%) | 25 (18%) | 11 (31%) | 14 (14%) | 0.018 |
| Etiology | 0.428 | |||
| Ischemic, n (%) | 47 (34%) | 10 (29%) | 37 (36%) | |
| Non-ischemic, n (%) | 91 (66%) | 25 (71%) | 66 (64%) | |
| NYHA class | <0.001 | |||
| I, n (%) | 16 (12%) | 1 (3%) | 15 (15%) | |
| II, n (%) | 83 (60%) | 15 (43%) | 68 (66%) | |
| III, n (%) | 37 (27%) | 17 (49%) | 20 (19%) | |
| IV, n (%) | 2 (2%) | 2 (6%) | 0 (0%) | |
| ICD at baseline, n (%) | 122 (88%) | 32 (91%) | 90 (87%) | 0.275 |
| CRT at baseline, n (%) | 76 (55%) | 20 (57%) | 56 (54%) | 0.650 |
| ARB/ACEi, n (%) | 63 (46%) | 16 (46%) | 47 (46%) | 0.993 |
| MRA, n (%) | 126 (91%) | 33 (95%) | 93 (90%) | 0.469 |
| β-blockers, n (%) | 135 (98%) | 35 (100%) | 100 (97%) | 0.307 |
| ARNI, n (%) | 61 (44%) | 14 (40%) | 47 (45%) | 0.562 |
| Ivabradine, n (%) | 18 (13%) | 5 (14%) | 13 (13%) | 0.801 |
| Loop diuretic, n (%) | 119 (86%) | 34 (97%) | 85 (82%) | 0.030 |
| Digoxin, n (%) | 22 (16%) | 7 (20%) | 15 (15%) | 0.448 |
| SGLT2i, n (%) | 8 (6%) | 1 (3%) | 7 (7%) | 0.389 |
| Hemoglobin (g/dL) | 14 [13–15] | 14 [14–15] | 14 [13–15] | 0.259 |
| Serum creatinine (mg/dL) | 1.0 [1.0–1.3] | 1.2 [1.0–1.4] | 1 [0.8–1.2] | 0.001 |
| Total bilirubin (mg/dL) | 0.5 [0.4–0.8] | 0.8 [0.5–1.4] | 0.5 [0.4–0.7] | <0.001 |
| GOT (UI/L) | 20 [17–24] | 23 [18–27] | 19 [16–23] | 0.009 |
| GPT (UI/L) | 20 [15–26] | 20 [15–30] | 19 [15–26] | 0.493 |
| NT-proBNP (pg/mL) | 919 [410–2007] | 2419 [1405–6609] | 645 [288–1253] | <0.001 |
| Serum iron (μg/dL) | 81 ± 29 | 73 ± 25 | 83 ± 30 | 0.082 |
| Systolic blood pressure (mmHg) | 105 [95–115] | 95 [90–110] | 105 [100–120] | 0.004 |
| Diastolic blood pressure (mmHg) | 65 [60–75] | 60 [60–70] | 70 [60–75] | 0.041 |
| All Patients (n = 138) | Composite Endpoint (n = 35) | No Composite Endpoint (n = 103) | p-Value | |
|---|---|---|---|---|
| LV EDD (mm) | 67 ± 10 | 72 ± 11 | 66 ± 10 | 0.004 |
| LV EF (%) | 30 [23–35] | 23 [20–28] | 30 [25–37] | <0.001 |
| LA volume (mL) | 99 [77–127] | 121 [94–138] | 93 [72–116] | 0.001 |
| PALS (%) | 12.7 [7.9–19.5] | 8.8 [6.7–13.1] | 15.0 [9.7–21.5] | 0.004 |
| E/A | 0.89 [0.65–1.45] | 1.50 [0.74–2.98] | 0.82 [0.65–1.37] | 0.018 |
| E/e’ | 11 [8–15] | 15 [9–22] | 11 [7–14] | 0.064 |
| sPAP (mmHg) | 32 [25–45] | 46 [32–55] | 30 [25–37] | 0.001 |
| TAPSE (mm) | 18 ± 4 | 17 ± 4 | 19 ± 4 | 0.043 |
| LV GLS (%) | −7 [−11–−5] | −5 [−8–−3] | −8 [−11–−5] | <0.001 |
| LV GWE (%) | 76 ± 11 | 74 ± 11 | 77 ± 11 | 0.202 |
| LV GWI (mmHg%) | 598 [353–867] | 346 [239–612] | 660 [424–908] | <0.001 |
| LV GCW (mmHg%) | 800 [587–1107] | 573 [433–803] | 939 [653–1184] | <0.001 |
| LV GWW (mmHg%) | 196 [138–282] | 168 [97–229] | 209 [142–287] | 0.016 |
| Secondary Endpoint (n = 19) | No Secondary Endpoint (n = 119) | p-Value | |
|---|---|---|---|
| LVEDD (mm) | 70 ± 9 | 67 ± 10 | 0.167 |
| LVEF (%) | 25 [20–30] | 30 [23–35] | 0.039 |
| LA volume (mL) | 126 [90–156] | 95 [75–121 | 0.084 |
| PALS (%) | 7.2 [5.5–17.0] | 13.4 [9.0–19.6] | 0.050 |
| E/A | 1.11 [0.72–1.88] | 0.89 [0.63–1.42] | 0.839 |
| E/e’ | 15 [10–19] | 11 [7–15] | 0.114 |
| sPAP (mmHg) | 40 [30–55] | 30 [25–43] | 0.084 |
| TAPSE (mm) | 17 ± 3 | 18 ± 4 | 0.194 |
| LV GLS (%) | −6 [−8–−4] | −8 [−11–−5] | 0.033 |
| LV GWE (%) | 76 ± 9 | 76 ± 11 | 0.849 |
| LV GWI (mmHg%) | 481 [287–651] | 609 [362–880] | 0.065 |
| LV GCW (mmHg%) | 614 [573–970] | 814 [592–1182] | 0.050 |
| LV GWW (mmHg%) | 167 [89–293] | 198 [139–280] | 0.575 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (CI 95%) | p-Value | HR (CI 95%) | p-Value | |
| Creatinine | 1.93 (0.75–4.96) | 0.172 | ||
| CKD | 4.13 (1.71–9.97) | 0.002 | ||
| Bilirubin | 2.06 (1.16–3.66) | 0.013 | ||
| GOT | 0.99 (0.98–1.01) | 0.868 | ||
| NYHA | 0.031 | |||
| I | 1.00 | / | ||
| II | 2.91 (0.36–23.58) | 0.316 | 2.79 (0.92–8.44) | 0.069 |
| III | 8.50 (0.96–74.96) | 0.054 | 0.89 (0.36–2.17) | 0.797 |
| IV | 11.13 (0.85–146.59) | 0.067 | ||
| NTproBNP | 1.00 (1.00–1.00) | <0.001 | 0.103 | |
| EDD | 0.98 (0.95–1.02) | 0.375 | ||
| LV EF | (0.97–1.06) | 0.624 | 2.95 (0.36–24.51) | 0.316 |
| E/A | 1.23 (0.87–1.73) | 0.244 | 6.35 (0.64–63.42) | 0.115 |
| LA volume | 1.00 (0.99–1.02) | 0.442 | 17.44 (1.15–264.16) | 0.039 |
| PALS | 0.95 (0.86–1.05) | 0.293 | 1.00 (1.00–1.00) | 0.166 |
| E/e’ | 1.00 (0.96–1.03) | 0.833 | ||
| sPAP | 1.02 (0.99–1.05) | 0.149 | ||
| Systolic BP | 1.00 (0.98–1.02) | 0.893 | ||
| Diastolic BP | 0.99 (0.95–1.04) | 0.691 | ||
| Loop diuretic | 0.84 (0.11–6.27) | 0.861 | ||
| LV GLS | 1.03 (0.89–1.19) | 0.676 | ||
| LV GWE | 0.95 (0.90–1.05) | 0.422 | ||
| LV GWI 50 mmHg% | 0.95 (0.90–1.05) | 0.326 | ||
| LV GCW 50 mmHg% | 1.00 (0.90–1.05) | 0.599 | ||
| LV GWW 50 mmHg% | 1.11 (0.90–1.35) | 0.316 | ||
| Univariate | ||
|---|---|---|
| HR (CI 95%) | p-Value | |
| LV EF | 0.92 (0.85–0.98) | 0.037 |
| LV GLS | 1.19 (1.02–1.40) | 0.028 |
| LV GWE | 0.99 (0.94–1.04) | 0.718 |
| LV GWI 50 mmHg% | 0.90 (0.78–0.95) | 0.025 |
| LV GCW 50 mmHg% | 0.90 (0.82–0.95) | 0.022 |
| LV GWW 50 mmHg% | 0.95 (0.78–1.16) | 0.583 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandoli, G.E.; Landra, F.; Chiantini, B.; Bonadiman, L.; Pastore, M.C.; Focardi, M.; D’Ascenzi, F.; Lisi, M.; Diviggiano, E.E.; Martini, L.; et al. Myocardial Work Indices Predict Hospitalization in Patients with Advanced Heart Failure. Diagnostics 2024, 14, 1196. https://doi.org/10.3390/diagnostics14111196
Mandoli GE, Landra F, Chiantini B, Bonadiman L, Pastore MC, Focardi M, D’Ascenzi F, Lisi M, Diviggiano EE, Martini L, et al. Myocardial Work Indices Predict Hospitalization in Patients with Advanced Heart Failure. Diagnostics. 2024; 14(11):1196. https://doi.org/10.3390/diagnostics14111196
Chicago/Turabian StyleMandoli, Giulia Elena, Federico Landra, Benedetta Chiantini, Lorenzo Bonadiman, Maria Concetta Pastore, Marta Focardi, Flavio D’Ascenzi, Matteo Lisi, Enrico Emilio Diviggiano, Luca Martini, and et al. 2024. "Myocardial Work Indices Predict Hospitalization in Patients with Advanced Heart Failure" Diagnostics 14, no. 11: 1196. https://doi.org/10.3390/diagnostics14111196
APA StyleMandoli, G. E., Landra, F., Chiantini, B., Bonadiman, L., Pastore, M. C., Focardi, M., D’Ascenzi, F., Lisi, M., Diviggiano, E. E., Martini, L., Bernazzali, S., Valente, S., Maccherini, M., Cameli, M., & Henein, M. Y. (2024). Myocardial Work Indices Predict Hospitalization in Patients with Advanced Heart Failure. Diagnostics, 14(11), 1196. https://doi.org/10.3390/diagnostics14111196












