Clinical and Hemodynamic Features of Aneurysm Rupture in Coil Embolization of Intracranial Aneurysms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Aneurysm Characteristics
2.2. Computational Fluid Dynamics Analysis
2.3. Pressure Elevation and Time-Averaged WSS (TAWSS) Were Computed
2.4. Statistical Analysis
3. Results
3.1. Representative Cases
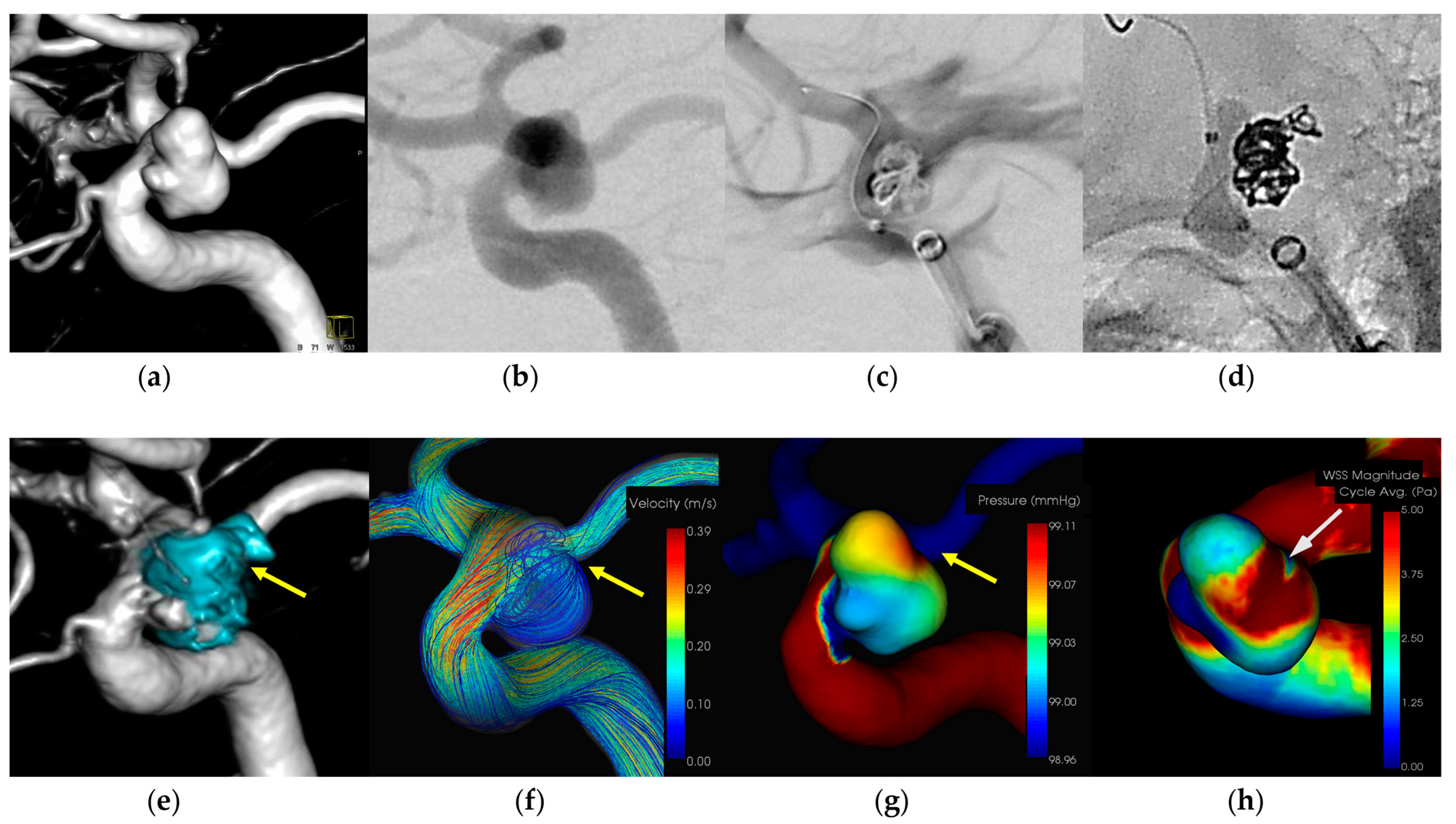
3.1.1. Case 1 (Figure 1)
- Patient: Female, 40s, unruptured right ICA aneurysm.
- Technique: Balloon-assisted coiling.
- Findings: Extravasation after initial coiling, hemostasis achieved with balloon inflation and additional coiling.
- Outcome: Discharge without neurological deficits.
- CFD Results: Pressure elevation and low WSS at the rupture site.
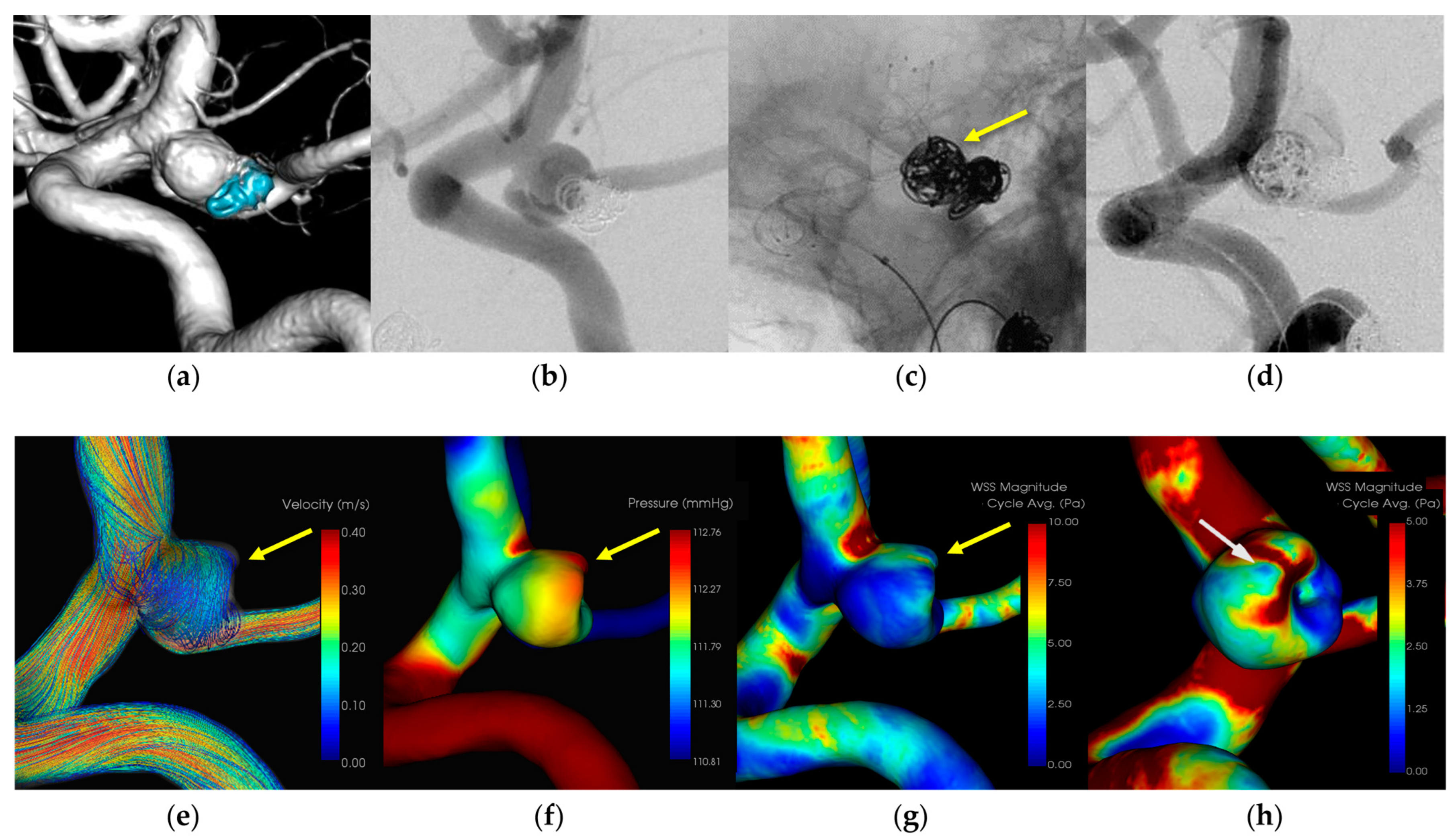
3.1.2. Case 2 (Figure 2)
- Patient: Female, 70s, unruptured left ICA aneurysm.
- Technique: Stent-assisted coiling.
- Findings: Extravasation during coil embolization, hemostasis achieved with additional coiling.
- Outcome: Discharge without neurological deficits.
- CFD Results: Pressure elevation and low WSS at the rupture site.
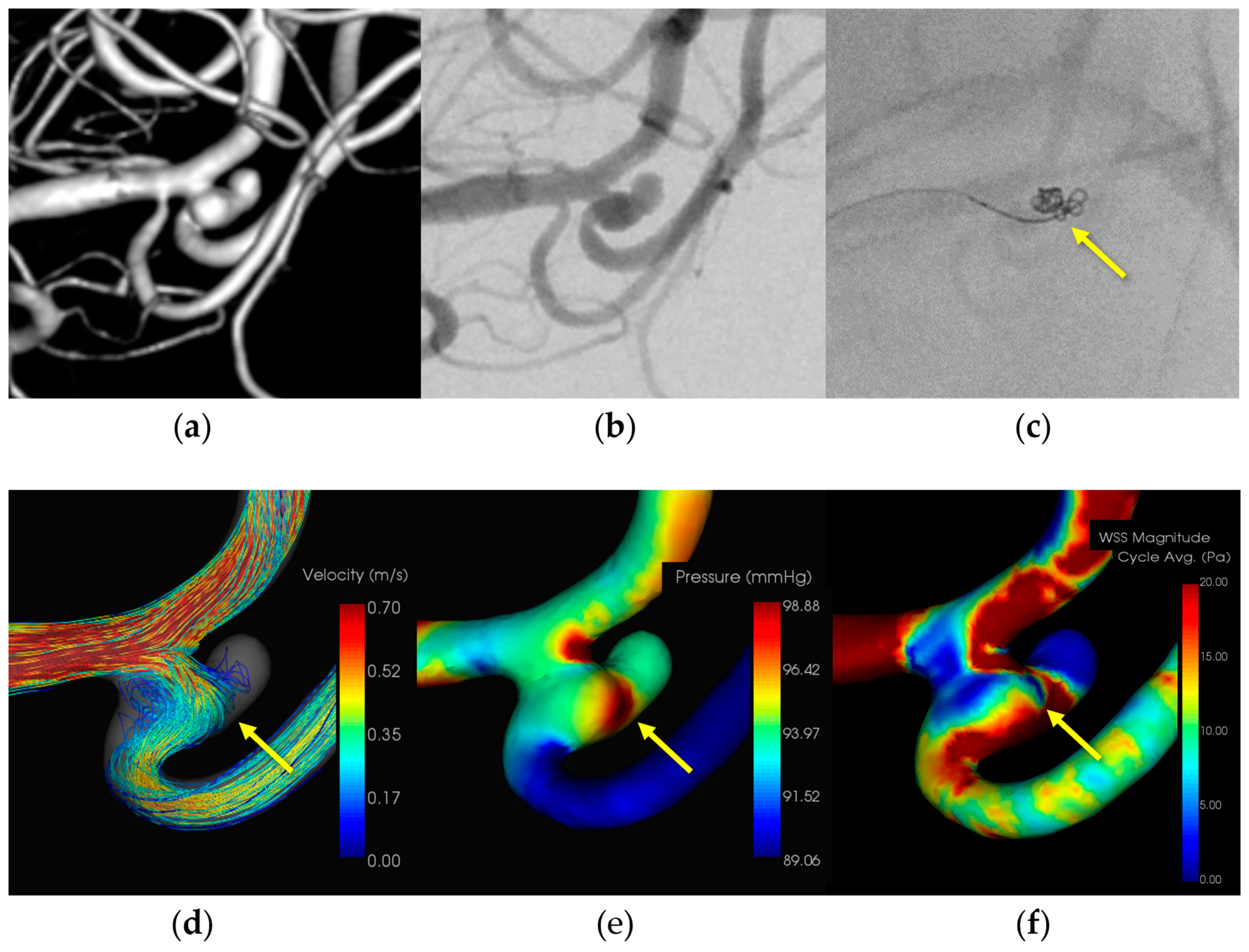
3.1.3. Case 3 (Figure 3)
- Patient: Female, 50s, ruptured left MCA M1–M2 bifurcation aneurysm.
- Technique: Simple coiling.
- Findings: Extravasation after initial coiling, hemostasis achieved with balloon inflation and additional coiling.
- Outcome: Discharge without neurological deficits due to IPR.
- CFD Results: Pressure elevation and low WSS at the rupture site.
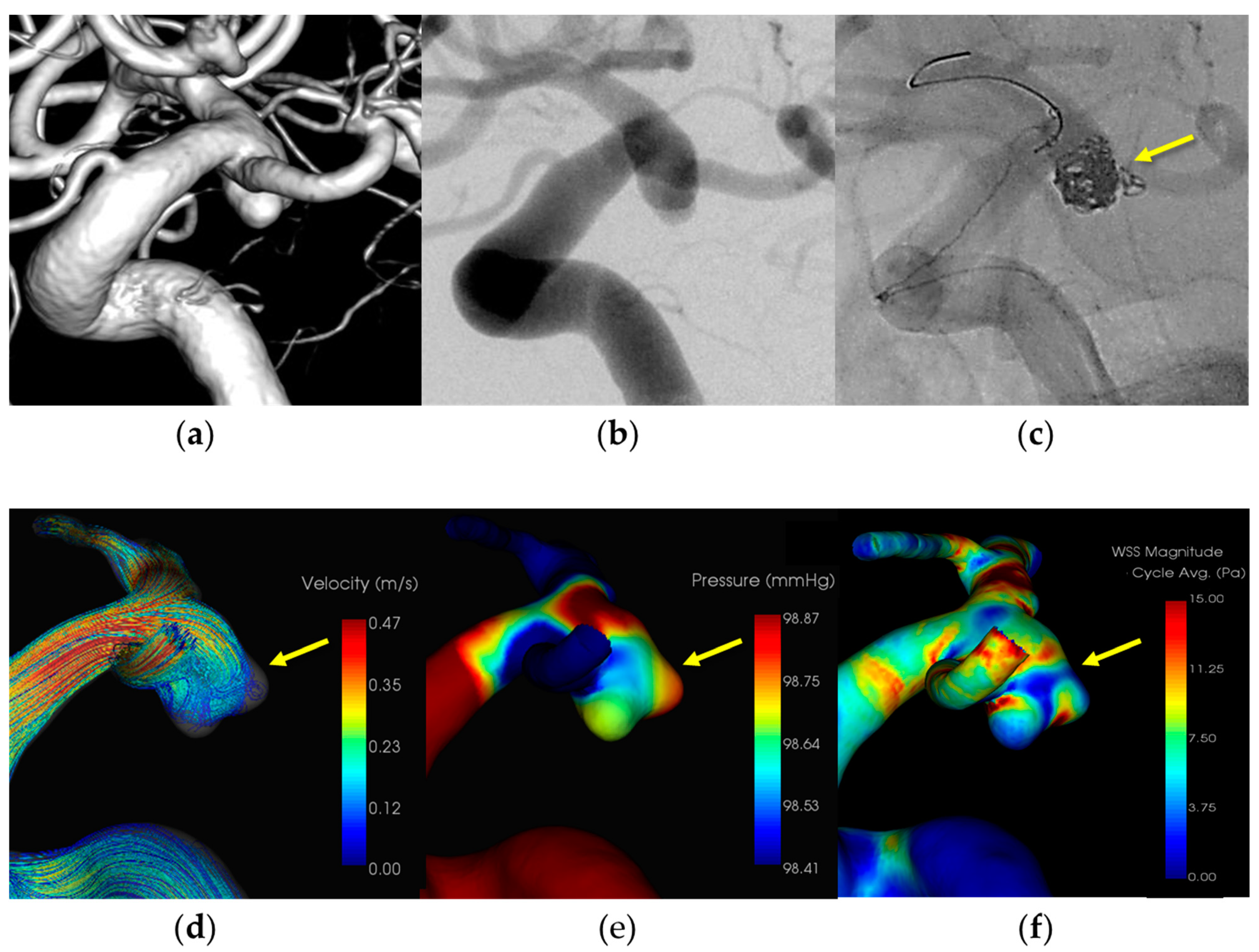
3.1.4. Case 4 (Figure 4)
- Patient: Female, 70s, ruptured left ICA aneurysm.
- Technique: Balloon-assisted coiling.
- Findings: Coil protruded from the aneurysm dome during coiling, hemostasis was achieved with balloon inflation, and additional coiling.
- Outcome: Discharge without neurological deficits.
- CFD Results: Pressure elevation and low WSS at the rupture site.
4. Discussion
4.1. Hemodynamic Findings and Aneurysmal Wall Condition with IPR
4.2. Mechanical and Hemodymanic Stress on Aneurysm Wall with IPR
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spetzler, R.F.; McDougall, C.G.; Zabramski, J.M.; Albuquerque, F.C.; Hills, N.K.; Nakaji, P.; Karis, J.P.; Wallace, R.C. Ten-year analysis of saccular aneurysms in the Barrow Ruptured Aneurysm Trial. J. Neurosurg. 2019, 132, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Luther, E.; McCarthy, D.J.; Brunet, M.C.; Sur, S.; Chen, S.H.; Sheinberg, D.; Hasan, D.; Jabbour, P.; Yavagal, D.R.; Peterson, E.C.; et al. Treatment and diagnosis of cerebral aneurysms in the post-International Subarachnoid Aneurysm Trial (ISAT) era: Trends and outcomes. J. Neurointerv. Surg. 2020, 12, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Calvanese, F.; Auricchio, A.M.; Pohjola, A.; Hafez, A.; Nurminen, V.; Korja, M.; Numminen, J.; Lehecka, M.; Raj, R.; Niemelä, M. Changes in treatment of intracranial aneurysms during the last decade in a large European neurovascular center. Acta Neurochir. 2024, 166, 173. [Google Scholar] [CrossRef]
- Salem, M.M.; Maragkos, G.A.; Gomez-Paz, S.; Ascanio, L.C.; Ngo, L.H.; Ogilvy, C.S.; Thomas, A.J.; Moore, J.M. Trends of ruptured and unruptured aneurysms treatment in the United States in post-ISAT era: A national inpatient sample analysis. J. Am. Heart Assoc. 2021, 10, e016998. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, C.J.; Walcott, B.P.; Butler, W.E.; Ogilvy, C.S. Neurological outcomes following intraprocedural rerupture during coil embolization of ruptured intracranial aneurysms. J. Neurosurg. 2015, 122, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.K.; Yi, H.J.; Choi, K.S.; Lee, Y.J.; Chun, H.J. Intraprocedural rupture during endovascular treatment of intracranial aneurysm: Clinical results and literature review. World Neurosurg. 2018, 114, e605–e615. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, S.; Imamura, H.; Adachi, H.; Tani, S.; Tokunaga, S.; Funatsu, T.; Suzuki, K.; Sakai, N. Risk factors for and outcomes of intraprocedural rupture during endovascular treatment of unruptured intracranial aneurysms. J. Neurointerv. Surg. 2018, 10, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Cloft, H.J.; Kallmes, D.F. Cerebral aneurysm perforations complicating therapy with Guglielmi detachable coils: A meta-analysis. AJNR Am. J. Neuroradiol. 2002, 23, 1706–1709. [Google Scholar] [PubMed]
- Elijovich, L.; Higashida, R.T.; Lawton, M.T.; Duckwiler, G.; Giannotta, S.; Johnston, S.C.; Cerebral Aneurysm Rerupture After Treatment (CARAT) Investigators. Predictors and outcomes of intraprocedural rupture in patients treated for ruptured intracranial aneurysms: The CARAT study. Stroke 2008, 39, 1501–1506. [Google Scholar] [CrossRef]
- Chen, X.L.; Chen, Y.; Ma, L.; Burkhardt, J.K.; Wardell, T.; Wang, C.; Guo, G.; Wang, S.; Zhao, Y.L. Translucent appearance of middle cerebral artery bifurcation aneurysms is risk factor for intraoperative aneurysm rupture during clipping. World Neurosurg. 2017, 101, 149–154. [Google Scholar] [CrossRef]
- Frösen, J.; Cebral, J.; Robertson, A.M.; Aoki, T. Flow-induced, inflammation-mediated arterial wall remodeling in the formation and progression of intracranial aneurysms. Neurosurg. Focus 2019, 47, E21. [Google Scholar] [CrossRef] [PubMed]
- Kadasi, L.M.; Dent, W.C.; Malek, A.M. Colocalization of thin-walled dome regions with low hemodynamic wall shear stress in unruptured cerebral aneurysms. J. Neurosurg. 2013, 119, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Takao, H.; Suzuki, T.; Kambayashi, Y.; Watanabe, M.; Sakamoto, H.; Kan, I.; Nishimura, K.; Kaku, S.; Ishibashi, T.; et al. Determining the presence of thin-walled regions at high-pressure areas in unruptured cerebral aneurysms by using computational fluid dynamics. Neurosurgery 2016, 79, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.X.; Geng, J.W.; He, C.; Hu, P.; Sun, L.Y.; Zhang, H.Q. Analysis of the wall thickness of intracranial aneurysms: Can computational fluid dynamics detect the translucent areas of saccular intracranial aneurysms and predict the rupture risk preoperatively? Front. Neurol. 2022, 13, 1075078. [Google Scholar] [CrossRef] [PubMed]
- Veeturi, S.S.; Patel, T.R.; Baig, A.A.; Chien, A.; Monteiro, A.; Waqas, M.; Snyder, K.V.; Siddiqui, A.H.; Tutino, V.M. Hemodynamic analysis shows high wall shear stress is associated with intraoperatively observed thin wall regions of intracranial aneurysms. J. Cardiovasc. Dev. Dis. 2022, 9, 424. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, S.I.; Niizuma, K.; Sato, K.; Rashad, S.; Kohama, M.; Endo, H.; Endo, T.; Matsumoto, Y.; Ohta, M.; Tominaga, T. Blood flow into basilar tip aneurysms: A predictor for recanalization after coil embolization. Stroke 2016, 47, 2541–2547. [Google Scholar] [CrossRef] [PubMed]
- Zarins, C.K.; Zatina, M.A.; Giddens, D.P.; Ku, D.N.; Glagov, S. Shear stress regulation of artery lumen diameter in experimental atherogenesis. J. Vasc. Surg. 1987, 5, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Yamagami, K.; Hatano, T.; Nakahara, I.; Ishii, A.; Ando, M.; Chihara, H.; Ogura, T.; Suzuki, K.; Kondo, D.; Kamata, T.; et al. Long-term outcomes after intraprocedural aneurysm rupture during coil embolization of unruptured intracranial aneurysms. World Neurosurg. 2020, 134, e289–e297. [Google Scholar] [CrossRef]
- Mo, X.; Meng, Q.; Yang, X.; Li, H. The impact of inflow angle on aneurysm hemodynamics: A simulation study based on patient-specific intracranial aneurysm models. Front. Neurol. 2020, 11, 534096. [Google Scholar] [CrossRef]
- Guo, H.; Yang, S.T.; Wang, J.W.; Li, H.; Gao, B.L.; Li, C.H. High hemodynamic stresses induce aneurysms at internal carotid artery bends. Medicine 2023, 102, e34587. [Google Scholar] [CrossRef]
- Murayama, Y.; Fujimura, S.; Suzuki, T.; Takao, H. Computational fluid dynamics as a risk assessment tool for aneurysm rupture. Neurosurg. Focus 2019, 47, E12. [Google Scholar] [CrossRef]
- Cebral, J.R.; Detmer, F.; Chung, B.J.; Choque-Velasquez, J.; Rezai, B.; Lehto, H.; Tulamo, R.; Hernesniemi, J.; Niemela, M.; Yu, A.; et al. Local hemodynamic conditions associated with focal changes in the intracranial aneurysm wall. AJNR Am. J. Neuroradiol. 2019, 40, 510–516. [Google Scholar] [CrossRef]
- Omodaka, S.; Sugiyama, S.I.; Inoue, T.; Funamoto, K.; Fujimura, M.; Shimizu, H.; Hayase, T.; Takahashi, A.; Tominaga, T. Local hemodynamics at the rupture point of cerebral aneurysms determined by computational fluid dynamics analysis. Cerebrovasc. Dis. 2012, 34, 121–129. [Google Scholar] [CrossRef]
- Jiang, P.; Liu, Q.; Wu, J.; Chen, X.; Li, M.; Li, Z.; Yang, S.; Guo, R.; Gao, B.; Cao, Y.; et al. Hemodynamic characteristics associated with thinner regions of intracranial aneurysm wall. J. Clin. Neurosci. 2019, 67, 185–190. [Google Scholar] [CrossRef]
- Suzuki, T.; Stapleton, C.J.; Koch, M.J.; Tanaka, K.; Fujimura, S.; Suzuki, T.; Yanagisawa, T.; Yamamoto, M.; Fujii, Y.; Murayama, Y.; et al. Decreased wall shear stress at high-pressure areas predicts the rupture point in ruptured intracranial aneurysms. J. Neurol. Surg. 2019, 132, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Tutino, V.M.; Xiang, J.; Siddiqui, A. High WSS or low WSS? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: Toward a unifying hypothesis. AJNR Am. J. Neuroradiol. 2014, 35, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Nordahl, E.R.; Uthamaraj, S.; Dennis, K.D.; Sejkorová, A.; Hejčl, A.; Hron, J.; Švihlová, H.; Carlson, K.D.; Suzen, Y.B.; Dragomir-Daescu, D. Morphological and hemodynamic changes during cerebral aneurysm growth. Brain Sci. 2021, 11, 520. [Google Scholar] [CrossRef]
- Sejkorová, A.; Dennis, K.D.; Švihlová, H.; Petr, O.; Lanzino, G.; Hejčl, A.; Dragomir-Daescu, D. Hemodynamic changes in a middle cerebral artery aneurysm at follow-up times before and after its rupture: A case report and a review of the literature. Neurosurg. Rev. 2017, 40, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jing, L.; Zhang, Y.; Liu, J.; Yang, X. Low wall shear stress is associated with the rupture of intracranial aneurysm with known rupture point: Case report and literature review. BMC Neurol. 2016, 16, 231. [Google Scholar] [CrossRef]
- Mitchell, P.J.; Muthusamy, S.; Dowling, R.; Yan, B. Does small aneurysm size predict intraoperative rupture during coiling in ruptured and unruptured aneurysms? J. Stroke Cerebrovasc. Dis. 2013, 22, 1298–1303. [Google Scholar] [CrossRef]
- Peng, F.; Feng, X.; He, X.; Niu, H.; Zhang, H.; Tong, X.; Zhang, B.; Xia, J.; Chen, X.; Xu, B.; et al. Independent predictors and risk score for intraprocedural rupture during endovascular treatment of small ruptured intracranial aneurysms (<5 mm). Front. Neurol. 2022, 13, 923645. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Luan, D.; Wang, C.; Liu, Q.; Han, J.; Li, G. Risk and prognostic factors for rupture of intracranial aneurysms during endovascular embolization. World Neurosurg. 2019, 129, e641–e649. [Google Scholar] [CrossRef] [PubMed]
| N = 6 | |
|---|---|
| Sex (female:male) | 5:1 |
| Mean age (years) | 62.8 ± 15.3 |
| Aneurysm Size | |
| Dome size (mm) | 4.7 ± 1.8 |
| Neck size (mm) | 3.2 ± 0.8 |
| D/N | 1.5 ± 0.5 |
| Aneurysm Location | |
| ICA | 4 |
| MCA | 1 |
| ACA | 1 |
| Treatment | |
| Simple coiling | 2 |
| Ballon-assisted coiling | 3 |
| Stent-assisted coiling | 1 |
| Flow impingement zone (Pmax) = IPR site | 5/6 (83.3%) |
| Pressure | |
| IPR site (mmHg) | 109.4 ± 6.4 |
| TAWSS | |
| IPR site (Pa) | * 1.3 ± 0.7 |
| Dome (Pa) | 5.1 ± 3.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, T.; Hasegawa, H.; Shibuya, K.; Fujiwara, H.; Oishi, M. Clinical and Hemodynamic Features of Aneurysm Rupture in Coil Embolization of Intracranial Aneurysms. Diagnostics 2024, 14, 1203. https://doi.org/10.3390/diagnostics14111203
Suzuki T, Hasegawa H, Shibuya K, Fujiwara H, Oishi M. Clinical and Hemodynamic Features of Aneurysm Rupture in Coil Embolization of Intracranial Aneurysms. Diagnostics. 2024; 14(11):1203. https://doi.org/10.3390/diagnostics14111203
Chicago/Turabian StyleSuzuki, Tomoaki, Hitoshi Hasegawa, Kohei Shibuya, Hidemoto Fujiwara, and Makoto Oishi. 2024. "Clinical and Hemodynamic Features of Aneurysm Rupture in Coil Embolization of Intracranial Aneurysms" Diagnostics 14, no. 11: 1203. https://doi.org/10.3390/diagnostics14111203
APA StyleSuzuki, T., Hasegawa, H., Shibuya, K., Fujiwara, H., & Oishi, M. (2024). Clinical and Hemodynamic Features of Aneurysm Rupture in Coil Embolization of Intracranial Aneurysms. Diagnostics, 14(11), 1203. https://doi.org/10.3390/diagnostics14111203






