High-Frequency Vestibular Function Is Vulnerable to Presbyvestibulopathy
Abstract
:1. Introduction
2. Methods
2.1. Subject Enrollment
2.2. Vestibular Function Tests
2.3. Statistical Analysis
3. Results
3.1. Information of Enrolled Subjects
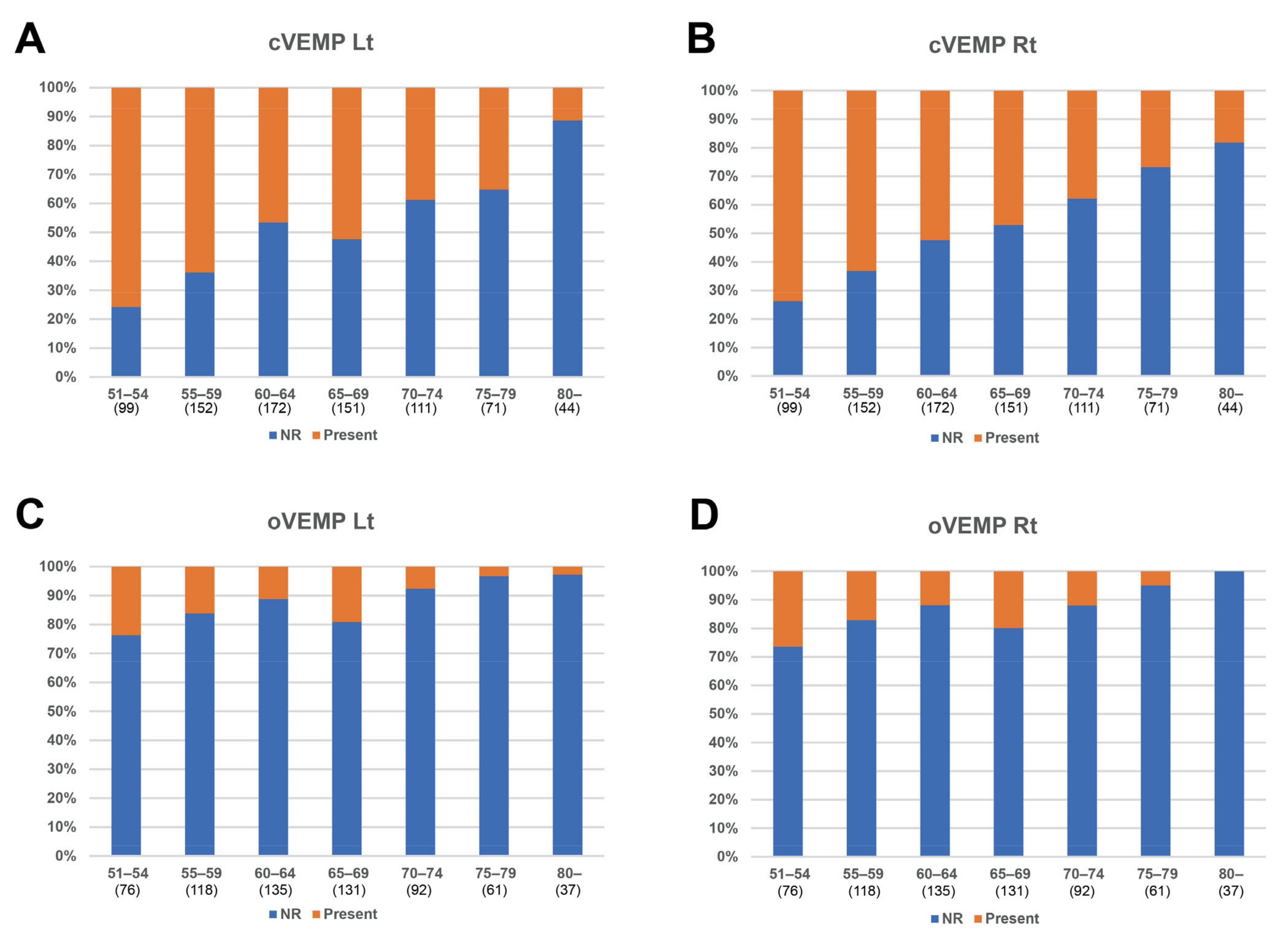
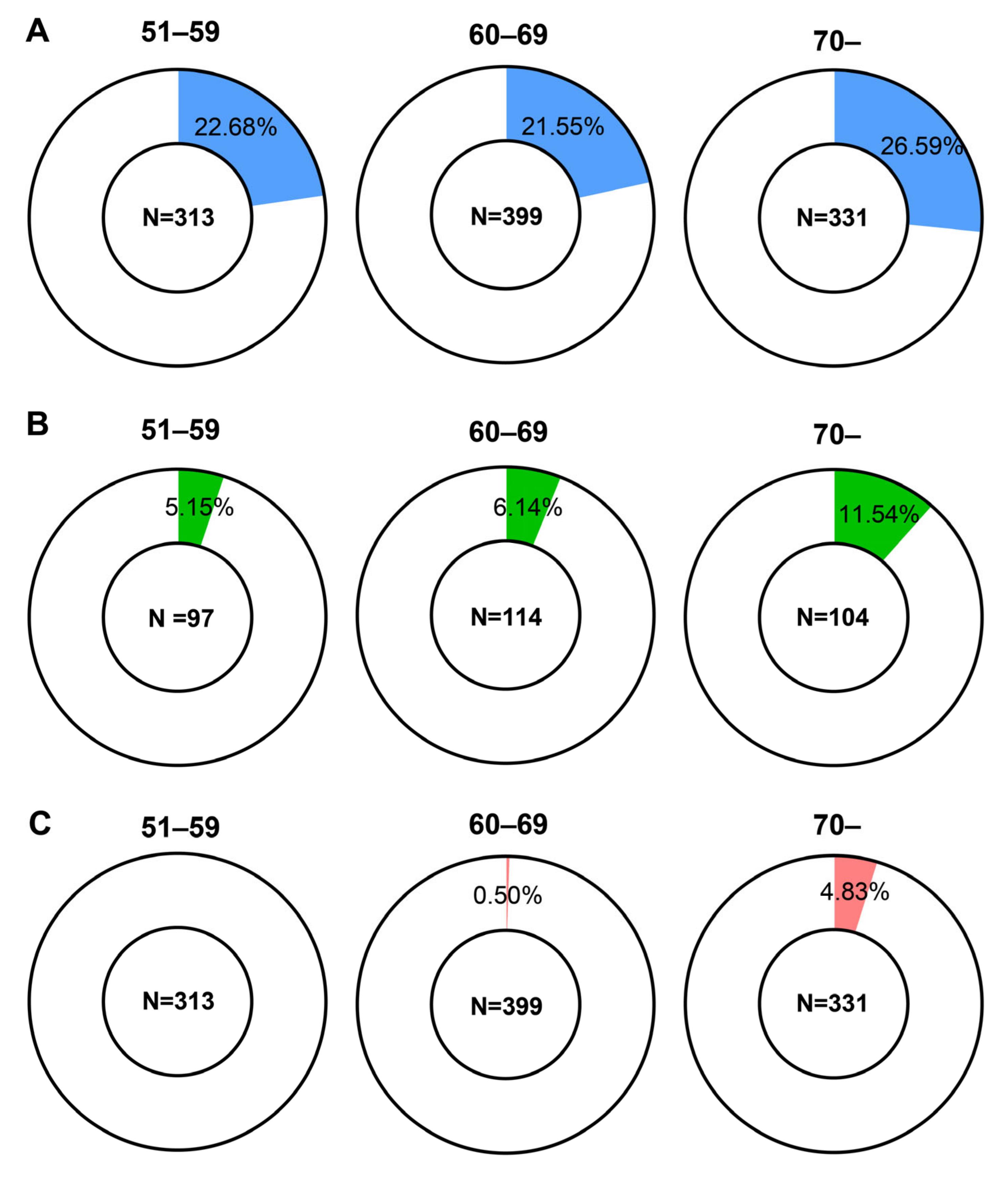
3.2. Relation between VFT Results and Aging
3.3. Analysis of Groups Categorized by Age
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Graafmans, W.C.; Ooms, M.E.; Hofstee, H.M.W.; Bezemer, P.D.; Bouter, L.M.; Lips, P. Falls in the elderly: A prospective study of risk factors and risk profiles. Am. J. Epidemiol. 1996, 143, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Tinetti, M.E.; Williams, C.S.; Gill, T.M. Dizziness among older adults: A possible geriatric syndrome. Ann. Intern. Med. 2000, 132, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, S.; Yamasoba, T. Dizziness and Imbalance in the Elderly: Age-related Decline in the Vestibular System. Aging Dis. 2015, 6, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, Y.; Van de Berg, R.; Wuyts, F.; Walther, L.; Magnusson, M.; Oh, E.; Sharpe, M.; Strupp, M. Presbyvestibulopathy: Diagnostic criteria Consensus document of the classification committee of the Barany Society. J. Vestibul. Res. Equil. 2019, 29, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.U.; Park, S.H.; Kim, H.J.; Koo, J.W.; Kim, J.S. Normal Caloric Responses during Acute Phase of Vestibular Neuritis. J. Clin. Neurol. 2016, 12, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Halmagyi, G.M.; Curthoys, I.S.; Cremer, P.D.; Henderson, C.J.; Todd, M.J.; Staples, M.J.; Dcruz, D.M. The Human Horizontal Vestibuloocular Reflex in Response to High-Acceleration Stimulation before and after Unilateral Vestibular Neurectomy. Exp. Brain Res. 1990, 81, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.M.; Galatioto, J.; Amato, M.; Kim, A.H. Normative data for rotational chair stratified by age. Laryngoscope 2016, 126, 460–463. [Google Scholar] [CrossRef]
- Zuniga, S.A.; Adams, M.E. Efficient Use of Vestibular Testing. Otolaryngol. Clin. N. Am. 2021, 54, 875–891. [Google Scholar] [CrossRef]
- Su, H.C.; Huang, T.W.; Young, Y.H.; Cheng, P.W. Aging effect on vestibular evoked myogenic potential. Otol. Neurotol. 2004, 25, 977–980. [Google Scholar] [CrossRef]
- Janky, K.L.; Shepard, N. Vestibular evoked myogenic potential (VEMP) testing: Normative threshold response curves and effects of age. J. Am. Acad. Audiol. 2009, 20, 514–522. [Google Scholar] [CrossRef]
- Ochi, K.; Ohashi, T. Age-related changes in the vestibular-evoked myogenic potentials. Otolaryngol. Head Neck Surg. 2003, 129, 655–659. [Google Scholar] [CrossRef] [PubMed]
- You, S.H. Joint position sense in elderly fallers: A preliminary investigation of the validity and reliability of the SENSERite measure. Arch. Phys. Med. Rehabil. 2005, 86, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Jongkees, L.B.; Maas, J.P.; Philipszoon, A.J. Clinical nystagmography. A detailed study of electro-nystagmography in 341 patients with vertigo. Pract. Otorhinolaryngol. 1962, 24, 65–93. [Google Scholar]
- Jeong, J.; Jung, J.; Lee, J.M.; Suh, M.J.; Kwak, S.H.; Kim, S.H. Effects of Saccular Function on Recovery of Subjective Dizziness After Vestibular Rehabilitation. Otol. Neurotol. 2017, 38, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Grossman, G.E.; Leigh, R.J.; Abel, L.A.; Lanska, D.J.; Thurston, S.E. Frequency and velocity of rotational head perturbations during locomotion. Exp. Brain Res. 1988, 70, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Hirasaki, E.; Moore, S.T.; Raphan, T.; Cohen, B. Effects of walking velocity on vertical head and body movements during locomotion. Exp. Brain Res. 1999, 127, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Halmagyi, G.M.; Chen, L.; MacDougall, H.G.; Weber, K.P.; McGarvie, L.A.; Curthoys, I.S. The Video Head Impulse Test. Front. Neurol. 2017, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, N.D.; Hougaard, D.D. Age- and gender-specific normative data on computerized dynamic posturography in a cohort of Danish adults. Eur. Arch. Otorhinolaryngol. 2023, 280, 2191–2200. [Google Scholar] [CrossRef] [PubMed]
- Matino-Soler, E.; Esteller-More, E.; Martin-Sanchez, J.C.; Martinez-Sanchez, J.M.; Perez-Fernandez, N. Normative data on angular vestibulo-ocular responses in the yaw axis measured using the video head impulse test. Otol. Neurotol. 2015, 36, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, M.B. Effect of aging and direction of impulse in video head impulse test. Laryngoscope 2018, 128, E228–E233. [Google Scholar] [CrossRef]
- Li, C.; Layman, A.J.; Geary, R.; Anson, E.; Carey, J.P.; Ferrucci, L.; Agrawal, Y. Epidemiology of vestibulo-ocular reflex function: Data from the Baltimore Longitudinal Study of Aging. Otol. Neurotol. 2015, 36, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Janky, K.L.; Patterson, J.N.; Shepard, N.T.; Thomas, M.L.A.; Honaker, J.A. Effects of Device on Video Head Impulse Test (vHIT) Gain. J. Am. Acad. Audiol. 2017, 28, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Maes, L.; Dhooge, I.; D’Haenens, W.; Bockstael, A.; Keppler, H.; Philips, B.; Swinnen, F.; Vinck, B.M. The effect of age on the sinusoidal harmonic acceleration test, pseudorandom rotation test, velocity step test, caloric test, and vestibular-evoked myogenic potential test. Ear Hear. 2010, 31, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Bruner, A.; Norris, T.W. Age-related changes in caloric nystagmus. Acta Otolaryngol. Suppl. 1971, 282, 1–24. [Google Scholar] [PubMed]
- Mulch, G.; Petermann, W. Influence of age on results of vestibular function tests. Review of literature and presentation of caloric test results. Ann. Otol. Rhinol. Laryngol. Suppl. 1979, 88, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, E.A.; Hassanein, R.M.; Goetzinger, C.P. The effects of age, sex, hearing loss and water temperature on caloric nystagmus. Laryngoscope 1981, 91, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Zangemeister, W.H.; Bock, O. The influence of pneumatization of mastoid bone on caloric nystagmus response. A clinical study and a mathematical model. Acta Otolaryngol. 1979, 88, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Patki, A.U.; Ronen, O.; Kaylie, D.M.; Frank-Ito, D.O.; Piker, E.G. Anatomic Variations in Temporal Bones Affect the Intensity of Nystagmus During Warm Caloric Irrigation. Otol. Neurotol. 2016, 37, 1111–1116. [Google Scholar] [CrossRef]
- Lee, D.H.; Jun, B.C.; Kim, D.G.; Jung, M.K.; Yeo, S.W. Volume variation of mastoid pneumatization in different age groups: A study by three-dimensional reconstruction based on computed tomography images. Surg. Radiol. Anat. 2005, 27, 37–42. [Google Scholar] [CrossRef]
- Chatterjee, D.; Ghosh, T.B.; Ghosh, B.B. Size Variation of Mastoid Air Cell System in Indian People at Different Age-Groups—A Radiographic Planimetric Study. J. Laryngol. Otol. 1990, 104, 603–605. [Google Scholar] [CrossRef]
- Kristinsdottir, E.K.; Nordell, E.; Jarnlo, G.B.; Tjader, A.; Thorngren, K.G.; Magnusson, M. Observation of vestibular asymmetry in a majority of patients over 50 years with fall-related wrist fractures. Acta Otolaryngol. 2001, 121, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.W.; Bhattacharyya, N. Balance disorders in the elderly: Epidemiology and functional impact. Laryngoscope 2012, 122, 1858–1861. [Google Scholar] [CrossRef] [PubMed]
- Dabiri, S.; Yazdani, N.; Esfahani, M.; Tari, N.; Adil, S.; Mahvi, Z.; Rezazadeh, N. Analysis of Saccular Function with Vestibular Evoked Myogenic Potential Test in Meniere’s Disease. Acta Med. Iran. 2017, 55, 123–127. [Google Scholar] [PubMed]

| Factors | Value |
|---|---|
| Mean Age, years (SD) | 65.19 (8.89) |
| Male:Female, n (%) | 306 (29.3):737 (70.7) |
| Caloric test | |
| Mean Lt SPV, deg/sec (SD) | 33.75 (15.50) |
| Mean Rt SPV, deg/sec (SD) | 33.35 (15.25) |
| Mean CP, % (SD) | 8.33 (5.41) |
| Number, n (%) | 1043 (100) |
| vHIT, LSCC | |
| Mean Lt gain (SD) | 0.964 (0.099) |
| Mean Rt gain (SD) | 1.023 (0.104) |
| Number, n (%) | 1043 (100) |
| RCT, SHA | |
| Mean 0.1 Hz gain (SD) | 0.521 (0.157) |
| Mean 1.0 Hz gain (SD) | 0.774 (0.156) |
| Number, n (%) | 315 (30.2) |
| Posturography | |
| Mean Composite score (SD) | 68.30 (11.94) |
| Number, n (%) | 841 (80.6) |
| cVEMP | |
| Lt Present, n (%) | 404 (50.5) |
| Lt Absent, n (%) | 396 (49.5) |
| Rt Present, n (%) | 399 (49.9) |
| Rt Absent, n (%) | 401 (50.1) |
| Number, n (%) | 800 (76.3) |
| oVEMP | |
| Lt Present, n (%) | 87 (13.4) |
| Lt Absent, n (%) | 563 (86.6) |
| Rt Present, n (%) | 96 (14.8) |
| Rt Absent, n (%) | 554 (85.2) |
| Number, n (%) | 650 (62.0) |
| Total, n (%) | 1043 (100) |
| R p-Value n | Caloric Lt | Caloric Rt | vHIT Lt | vHIT Rt | RCT 0.12 | RCT 1.0 | Posturo |
|---|---|---|---|---|---|---|---|
| Caloric Lt | 1 N/A 1043 | ||||||
| Caloric Rt | 0.892 *** <0.001 1043 | 1 N/A 1043 | |||||
| vHIT Lt | 0.163 *** <0.001 1043 | 0.165 *** <0.001 1043 | 1 N/A 1043 | ||||
| vHIT Rt | 0.115 *** <0.001 1043 | 0.115 *** <0.001 1043 | 0.762 *** <0.001 1043 | 1 N/A 1043 | |||
| RCT 0.12 | 0.225 *** <0.001 315 | 0.164 ** 0.004 315 | 0.149 ** 0.008 315 | 0.165 ** 0.003 315 | 1 N/A 315 | ||
| RCT 1.0 | 0.058 0.301 315 | 0.057 0.314 315 | 0.171 ** 0.002 315 | 0.188 ** 0.001 315 | 0.438 *** <0.001 315 | 1 N/A 315 | |
| Posturo | 0.028 0.413 841 | 0.024 0.490 841 | 0.136 *** <0.001 841 | 0.123 *** <0.001 841 | −0.049 0.422 276 | 0.058 0.335 276 | 1 N/A 841 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, S.; Yun, J.; Kwak, S.; Jung, H.; Lee, H.; Kim, J.; Kim, C.; Lee, Y.; Kim, S. High-Frequency Vestibular Function Is Vulnerable to Presbyvestibulopathy. Diagnostics 2024, 14, 1224. https://doi.org/10.3390/diagnostics14121224
Bae S, Yun J, Kwak S, Jung H, Lee H, Kim J, Kim C, Lee Y, Kim S. High-Frequency Vestibular Function Is Vulnerable to Presbyvestibulopathy. Diagnostics. 2024; 14(12):1224. https://doi.org/10.3390/diagnostics14121224
Chicago/Turabian StyleBae, Seonghoon, Jimin Yun, Seungmin Kwak, Hyuntaek Jung, Hancheol Lee, Juyoung Kim, Chanhee Kim, Yujin Lee, and Sunghuhn Kim. 2024. "High-Frequency Vestibular Function Is Vulnerable to Presbyvestibulopathy" Diagnostics 14, no. 12: 1224. https://doi.org/10.3390/diagnostics14121224





