Diffusion Tensor Imaging Identifies Cervical Spondylosis, Myelitis, and Spinal Cord Tumors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Clinical Assessment
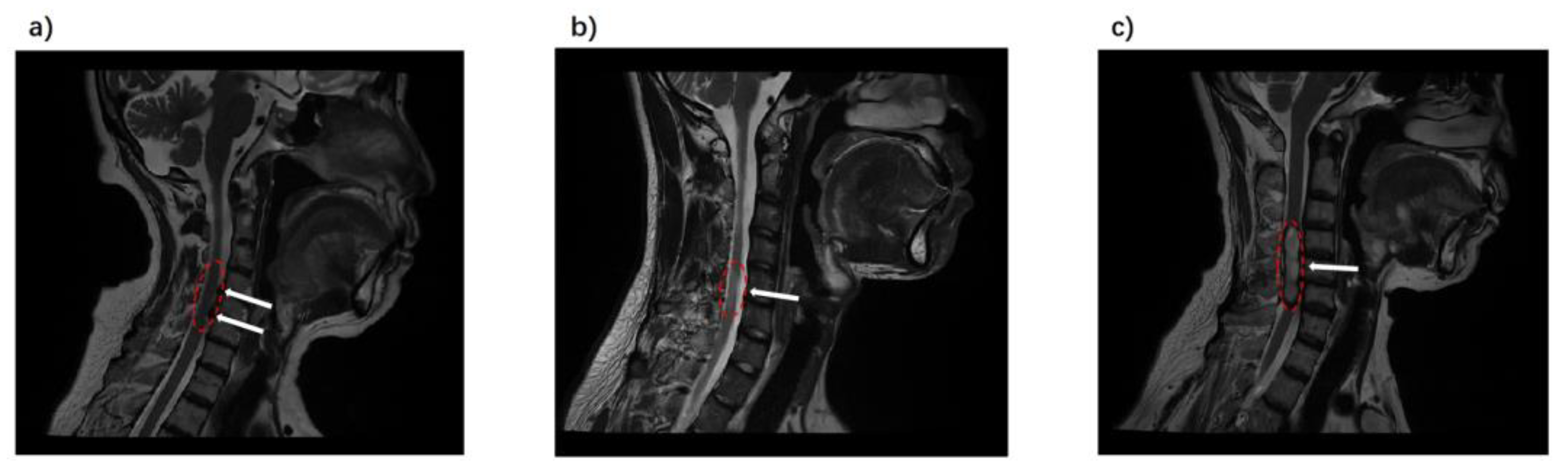
2.3. MRI Acquisition
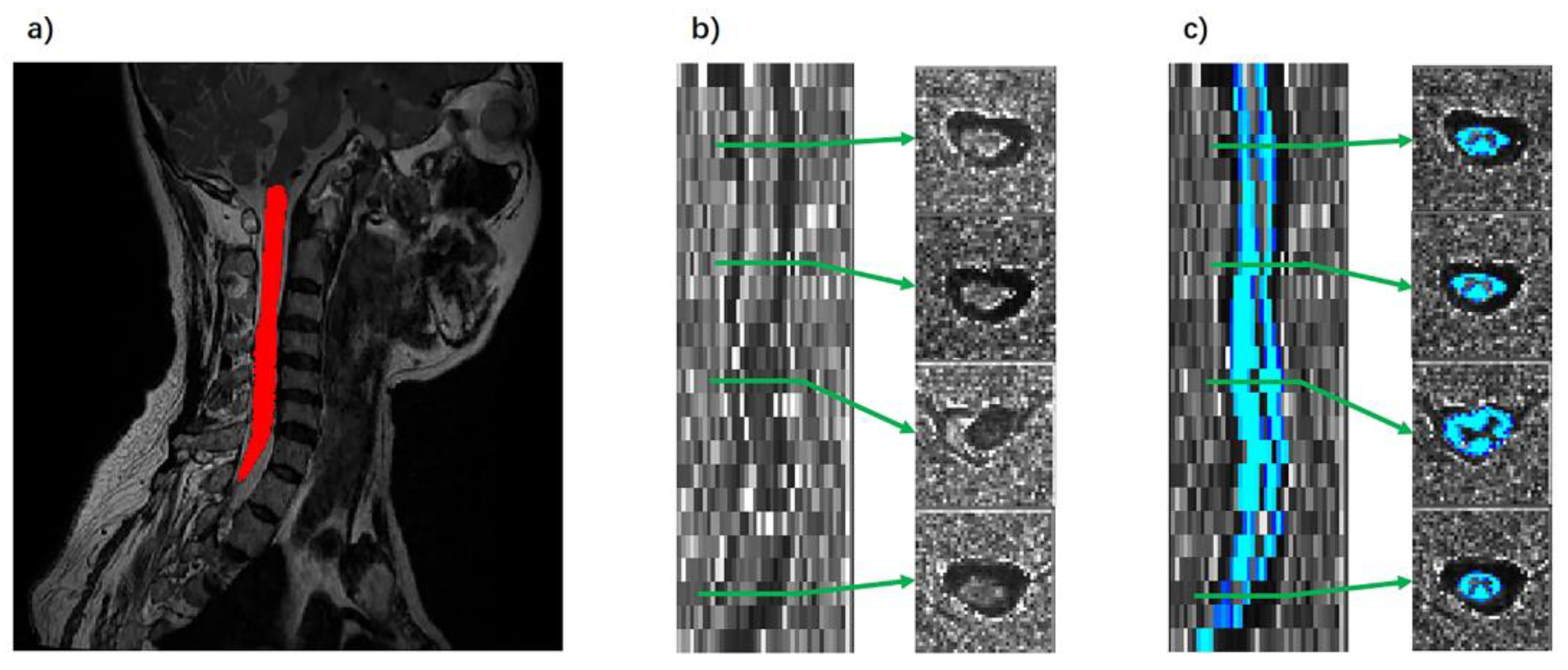
2.4. Data Processing
2.5. Statistics Analysis
3. Results
3.1. Patient Characteristics
3.2. DTI Indicators in Spondylosis, Myelitis, and Spinal Tumors
3.3. Discriminative Power of DTI Indicators in Spinal Pathologies
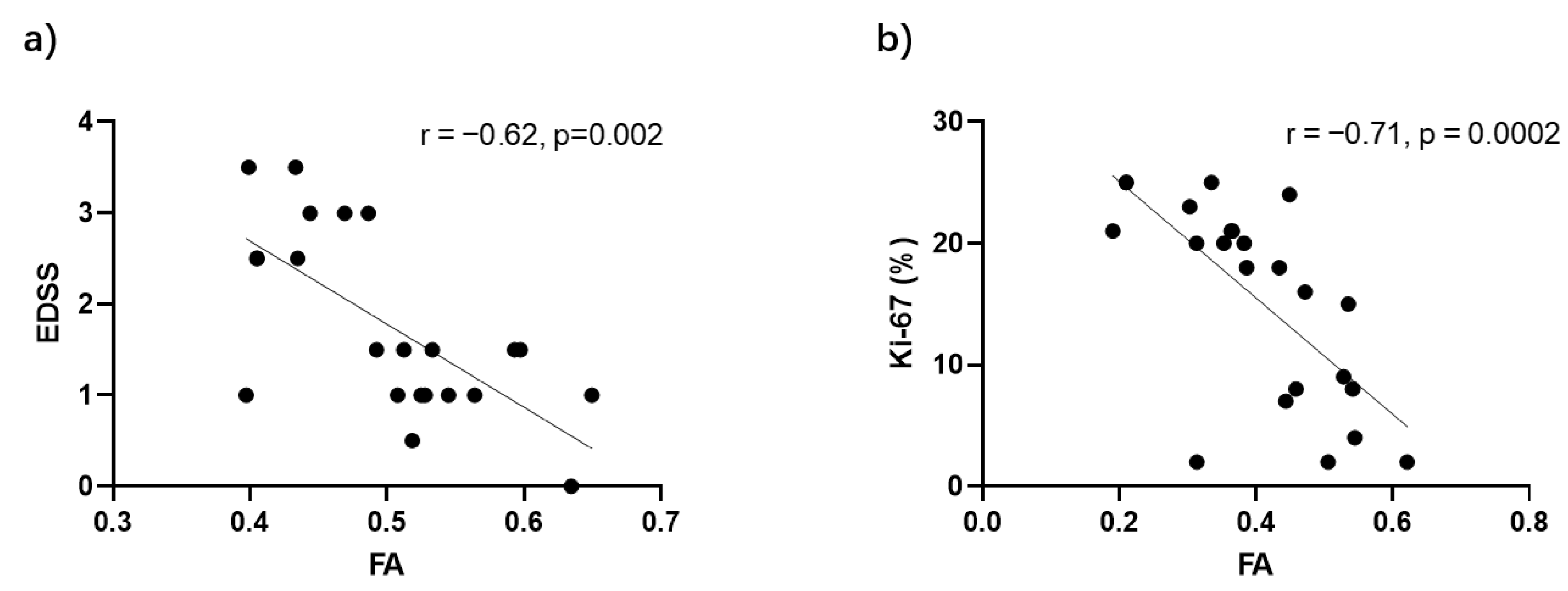
3.4. FA Correlations with Disease Severity in Myelitis and Spinal Tumors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.B.; Yang, X.S.; He, Z.G.; Li, J.; Li, Y.J.; Wu, Y.Q.; Manyande, A.; Feng, M.H.; Xiang, H.B. Spinal cord injury: Global burden from 1990 to 2019 and projections up to 2030 using Bayesian age-period-cohort analysis. Front. Neurol. 2023, 14, 1304153. [Google Scholar] [CrossRef] [PubMed]
- Parenteau, C.S.; Lau, E.C.; Campbell, I.C.; Courtney, A. Prevalence of spine degeneration diagnosis by type, age, gender, and obesity using Medicare data. Sci. Rep. 2021, 11, 5389. [Google Scholar] [CrossRef] [PubMed]
- Brinjikji, W.; Luetmer, P.H.; Comstock, B.; Bresnahan, B.W.; Chen, L.E.; Deyo, R.A.; Halabi, S.; Turner, J.A.; Avins, A.L.; James, K.; et al. Systematic Literature Review of Imaging Features of Spinal Degeneration in Asymptomatic Populations. AJNR Am. J. Neuroradiol. 2015, 36, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Teraguchi, M.; Yoshimura, N.; Hashizume, H.; Muraki, S.; Yamada, H.; Minamide, A.; Oka, H.; Ishimoto, Y.; Nagata, K.; Kagotani, R.; et al. Prevalence and distribution of intervertebral disc degeneration over the entire spine in a population-based cohort: The Wakayama Spine Study. Osteoarthr. Cartil. 2014, 22, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Fadda, G.; Flanagan, E.P.; Cacciaguerra, L.; Jitprapaikulsan, J.; Solla, P.; Zara, P.; Sechi, E. Myelitis features and outcomes in CNS demyelinating disorders: Comparison between multiple sclerosis, MOGAD, and AQP4-IgG-positive NMOSD. Front. Neurol. 2022, 13, 1011579. [Google Scholar] [CrossRef] [PubMed]
- Harada, G.K.; Siyaji, Z.; Younis, S.; Louie, P.; Samartzis, D.; An, H.S. Imaging in Spine Surgery: Current Concepts and Future Directions. Spine Surg. Relat. Res. 2020, 4, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.I.; Boto, J.; Meling, T.R. Imaging of the spine and spinal cord: An overview of magnetic resonance imaging (MRI) techniques. Rev. Neurol. 2021, 177, 451–458. [Google Scholar] [CrossRef]
- Meng, X.X.; Zhang, Y.Q.; Liao, H.Q.; Liu, H.C.; Jiang, H.L.; Ke, S.J.; Dong, W.H. Dynamic contrast-enhanced MRI for the assessment of spinal tumor vascularity: Correlation with angiography. Eur. Spine J. 2016, 25, 3952–3961. [Google Scholar] [CrossRef]
- Klein, J.P. A practical approach to spine imaging. Continuum 2015, 21, 36–51. [Google Scholar] [CrossRef]
- de Paiva, J.L.R.; Sabino, J.V.; Pereira, F.V.; Okuda, P.A.; Villarinho, L.D.; Queiroz, L.D.; França, M.C., Jr.; Reis, F. The Role of MRI in the Diagnosis of Spinal Cord Tumors. Semin. Ultrasound CT MRI 2023, 44, 436–451. [Google Scholar] [CrossRef]
- Jellison, B.J.; Field, A.S.; Medow, J.; Lazar, M.; Salamat, M.S.; Alexander, A.L. Diffusion tensor imaging of cerebral white matter: A pictorial review of physics, fiber tract anatomy, and tumor imaging patterns. AJNR Am. J. Neuroradiol. 2004, 25, 356–369. [Google Scholar]
- Cheng, S.J.; Tsai, P.H.; Lee, Y.T.; Li, Y.T.; Chung, H.W.; Chen, C.Y. Diffusion Tensor Imaging of the Spinal Cord. Magn. Reson. Imaging Clin. N. Am. 2021, 29, 195–204. [Google Scholar] [CrossRef]
- Figley, C.R.; Uddin, M.N.; Wong, K.H.; Kornelsen, J.; Puig, J.; Figley, T.D. Potential Pitfalls of Using Fractional Anisotropy, Axial Diffusivity, and Radial Diffusivity as Biomarkers of Cerebral White Matter Microstructure. Front. Neurosci. 2022, 15, 799576. [Google Scholar] [CrossRef]
- Valsasina, P.; Rocca, M.A.; Agosta, F.; Benedetti, B.; Horsfield, M.A.; Gallo, A.; Rovaris, M.; Comi, G.; Filippi, M. Mean diffusivity and fractional anisotropy histogram analysis of the cervical cord in MS patients. Neuroimage 2005, 26, 822–828. [Google Scholar] [CrossRef]
- Kara, B.; Celik, A.; Karadereler, S.; Ulusoy, L.; Ganiyusufoglu, K.; Onat, L.; Mutlu, A.; Ornek, I.; Sirvanci, M.; Hamzaoglu, A. The role of DTI in early detection of cervical spondylotic myelopathy: A preliminary study with 3-T MRI. Neuroradiology 2011, 53, 609–616. [Google Scholar] [CrossRef]
- Wheeler-Kingshott, C.A.; Stroman, P.W.; Schwab, J.M.; Bacon, M.; Bosma, R.; Brooks, J.; Cadotte, D.W.; Carlstedt, T.; Ciccarelli, O.; Cohen-Adad, J.; et al. The current state-of-the-art of spinal cord imaging: Applications. Neuroimage 2014, 84, 1082–1093. [Google Scholar] [CrossRef]
- Naismith, R.T.; Xu, J.; Klawiter, E.C.; Lancia, S.; Tutlam, N.T.; Wagner, J.M.; Qian, P.; Trinkaus, K.; Song, S.-K.; Cross, A.H. Spinal cord tract diffusion tensor imaging reveals disability substrate in demyelinating disease. Neurology 2013, 80, 2201–2209. [Google Scholar] [CrossRef]
- Renoux, J.; Facon, D.; Fillard, P.; Huynh, I.; Lasjaunias, P.; Ducreux, D. MR diffusion tensor imaging and fiber tracking in inflammatory diseases of the spinal cord. AJNR Am. J. Neuroradiol. 2006, 27, 1947–1951. [Google Scholar]
- Kauthankar, A.A.; Jaseemudheen, M. Diffusion Tensor Imaging in Spinal Cord Injury: A Review. J. Health Allied Sci. NU 2023, 13, 161–167. [Google Scholar] [CrossRef]
- Casseb, R.F.; de Paiva, J.L.R.; Branco, L.M.T.; Martinez, A.R.M.; Reis, F.; de Lima-Junior, J.C.; Castellano, G.; Junior, M.C.F. Spinal cord diffusion tensor imaging in patients with sensory neuronopathy. Neuroradiology 2016, 58, 1103–1108. [Google Scholar] [CrossRef]
- Liu, X.; Tian, W.; Chen, H.; LoStracco, T.A.; Zhang, J.; Li, M.Y.; Germin, B.; Wang, H.Z. Advanced neuroimaging in the evaluation of spinal cord tumors and tumor mimics: Diffusion tensor and perfusion-weighted imaging. Semin. Ultrasound CT MRI 2017, 38, 163–175. [Google Scholar] [CrossRef]
- Liu, X.; Tian, W.; Kolar, B.; Hu, R.; Huang, Y.; Huang, J.; Ekholm, S. Advanced MR diffusion tensor imaging and perfusion weighted imaging of intramedullary tumors and tumor like lesions in the cervicomedullary junction region and the cervical spinal cord. J. Neurooncol. 2014, 116, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Theodore, N. Degenerative Cervical Spondylosis. N. Engl. J. Med. 2020, 383, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Weidauer, S.; Hattingen, E.; Arendt, C.T. Cervical myelitis: A practical approach to its differential diagnosis on MR imaging. Rofo 2023, 195, 1081–1096. [Google Scholar] [CrossRef] [PubMed]
- Kurtzke, J.F. Rating neurologic impairment in multiple-sclerosis—An Expanded Disability Status Scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Scholzen, T.; Gerdes, J. The Ki-67 protein: From the known and the unknown. J. Cell. Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- De Leener, B.; Lévy, S.; Dupont, S.M.; Fonov, V.S.; Stikov, N.; Collins, D.L.; Callot, V.; Cohen-Adad, J. SCT: Spinal Cord Toolbox, an open-source software for processing spinal cord MRI data. Neuroimage 2017, 145, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Shenton, M.E.; Hamoda, H.M.; Schneiderman, J.S.; Bouix, S.; Pasternak, O.; Rathi, Y.; Vu, M.A.; Purohit, M.P.; Helmer, K.; Koerte, I.; et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 2012, 6, 137–192. [Google Scholar] [CrossRef] [PubMed]
- Mousa, M.I.; Youssef, A.; Hamed, M.R.; Mousa, W.B.; Al Ajerami, Y.; Akhdar, H.; Eisa, M.H.; Ibnaouf, K.H.; Sulieman, A. Mapping high-grade glioma response to chemoradiotherapy: Insights from fractional anisotropy and mean diffusivity. J. Radiat. Res. Appl. Sci. 2023, 16, 100706. [Google Scholar] [CrossRef]
- MacKay, A.L.; Laule, C. Magnetic resonance of myelin water: An in vivo marker for myelin. Brain Plast. 2016, 2, 71–91. [Google Scholar] [CrossRef]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef] [PubMed]
- Hohenhaus, M.; Merz, Y.; Klingler, J.-H.; Scholz, C.; Hubbe, U.; Beck, J.; Wolf, K.; Egger, K.; Reisert, M.; Kremers, N. Diffusion tensor imaging in unclear intramedullary tumor-suspected lesions allows separating tumors from inflammation. Spinal Cord. 2022, 60, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Brooks, L.J.; Clements, M.P.; Burden, J.J.; Kocher, D.; Richards, L.; Devesa, S.C.; Zakka, L.; Woodberry, M.; Ellis, M.; Jaunmuktane, Z.; et al. The white matter is a pro-differentiative niche for glioblastoma. Nat. Commun. 2021, 12, 2184. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q. Diffusion Magnetic Resonance Imaging of Central Nervous System Diseases: Structure, Function and Pathology. Ph.D. Thesis, Washington University in St. Louis, St. Louis, MO, USA, 2011. [Google Scholar]
- Facon, D.; Ozanne, A.; Fillard, P.; Lepeintre, J.F.; Tournoux-Facon, C.; Ducreux, D. MR diffusion tensor imaging and fiber tracking in spinal cord compression. AJNR Am. J. Neuroradiol. 2005, 26, 1587–1594. [Google Scholar] [PubMed]
- Agosta, F.; Absinta, M.; Sormani, M.P.; Ghezzi, A.; Bertolotto, A.; Montanari, E.; Comi, G.; Filippi, M. In vivo assessment of cervical cord damage in MS patients: A longitudinal diffusion tensor MRI study. Brain 2007, 130, 2211–2219. [Google Scholar] [CrossRef]
- Liu, X.; Tian, W.; Kolar, B.; Johnson, M.D.; Milano, M.T.; Jiang, H.H.; Lin, S.; Li, D.M.; Mohile, N.A.; Li, Y.M.; et al. The correlation of fractional anisotropy parameters with Ki-67 index, and the clinical implication in grading of non-enhancing gliomas and neuronalglial tumors. Magn. Reson. Imaging 2020, 65, 129–135. [Google Scholar] [CrossRef]


| Characteristics | Spondylosis (n = 22) | Myelitis (n = 23) | Tumor (n = 23) |
|---|---|---|---|
| Gender (M/F) a | 9/13 | 10/13 | 9/14 |
| Age (years) a | 53.68 ± 13.11 | 48.64 ± 17.48 | 46.74 ± 14.68 |
| Height (cm) a | 165.18 ± 6.03 | 164.85 ± 9.70 | 158.83 ± 22.34 |
| Weight (kg) a | 67.68 ± 11.65 | 66.77 ± 13.14 | 62.22 ± 18.67 |
| EDSS | - | 1.63 ± 1.03 | - |
| Ki-67 (%) | - | - | 15.30 ± 9.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Huang, J.; Cui, B.; Yang, H.; Tian, D.; Ma, J.; Duan, W.; Dong, H.; Chen, Z.; Lu, J. Diffusion Tensor Imaging Identifies Cervical Spondylosis, Myelitis, and Spinal Cord Tumors. Diagnostics 2024, 14, 1225. https://doi.org/10.3390/diagnostics14121225
Wang J, Huang J, Cui B, Yang H, Tian D, Ma J, Duan W, Dong H, Chen Z, Lu J. Diffusion Tensor Imaging Identifies Cervical Spondylosis, Myelitis, and Spinal Cord Tumors. Diagnostics. 2024; 14(12):1225. https://doi.org/10.3390/diagnostics14121225
Chicago/Turabian StyleWang, Jiyuan, Jing Huang, Bixiao Cui, Hongwei Yang, Defeng Tian, Jie Ma, Wanru Duan, Huiqing Dong, Zan Chen, and Jie Lu. 2024. "Diffusion Tensor Imaging Identifies Cervical Spondylosis, Myelitis, and Spinal Cord Tumors" Diagnostics 14, no. 12: 1225. https://doi.org/10.3390/diagnostics14121225






