Magnetic Resonance Imaging Features of Anterolateral Ligament in Young Adults without Anterior Cruciate Ligament Injury: Preliminary Evaluation
Abstract
:1. Introduction
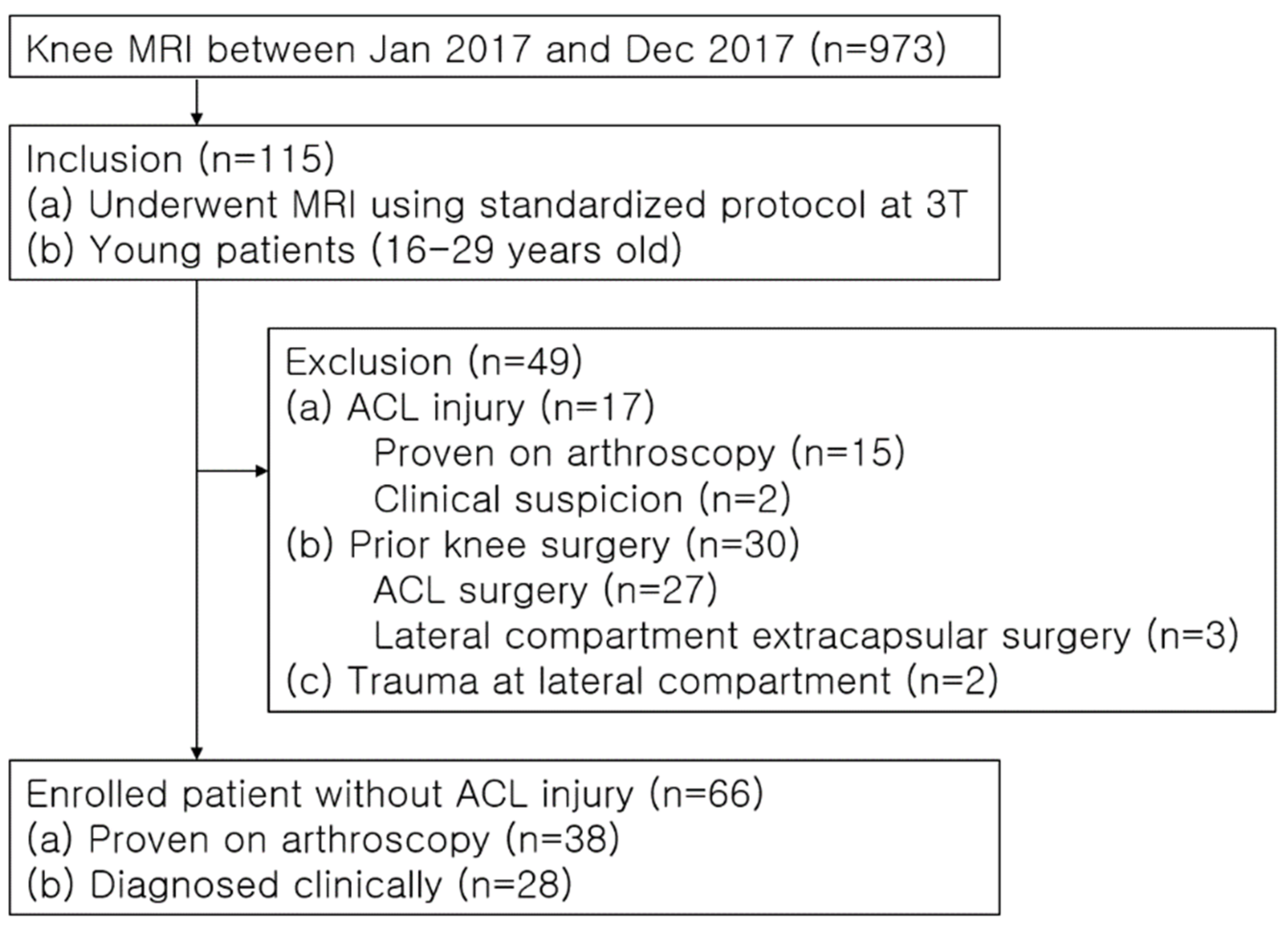
2. Materials and Methods
2.1. Study Population
2.2. MRI Protocol
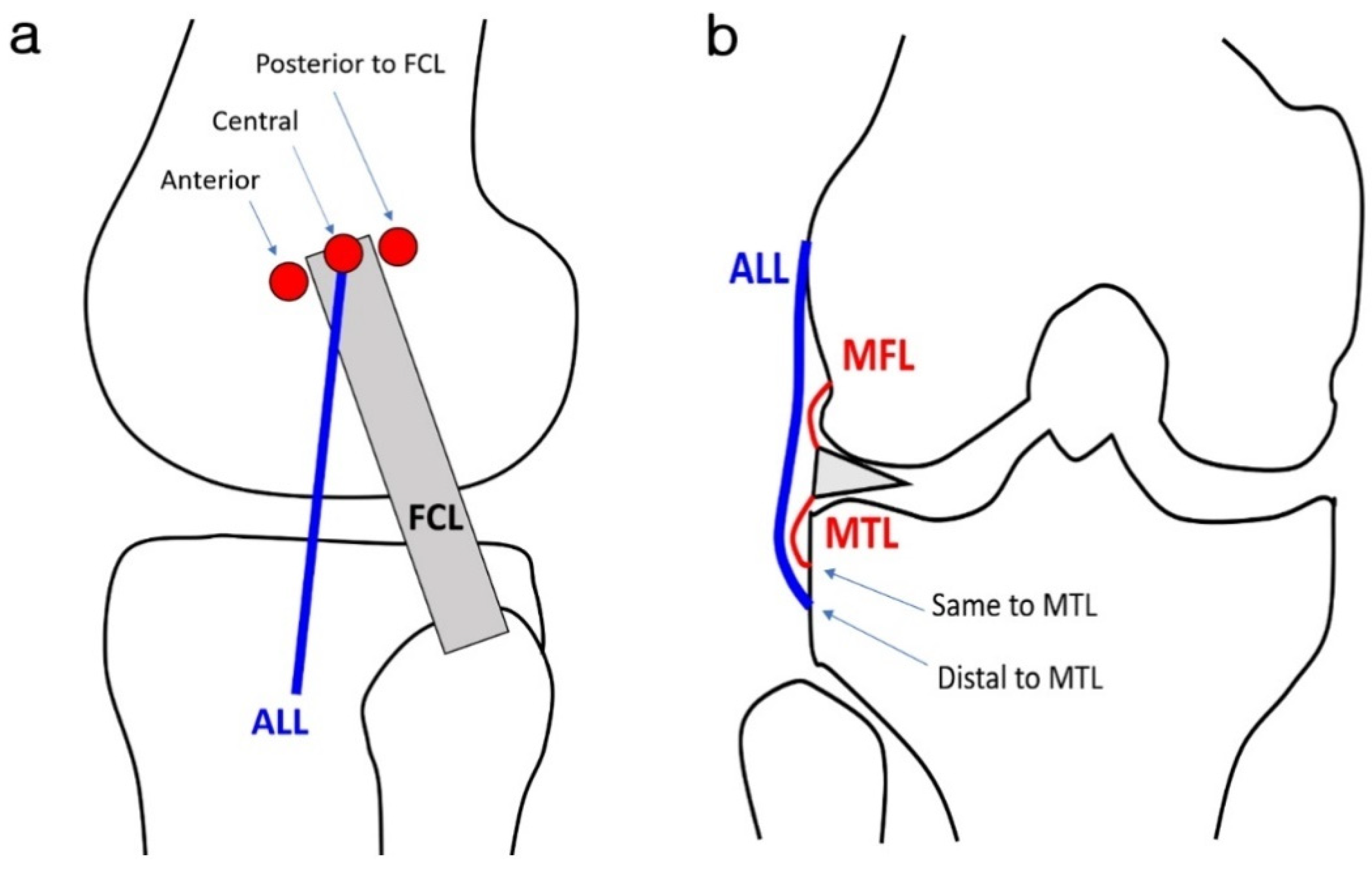
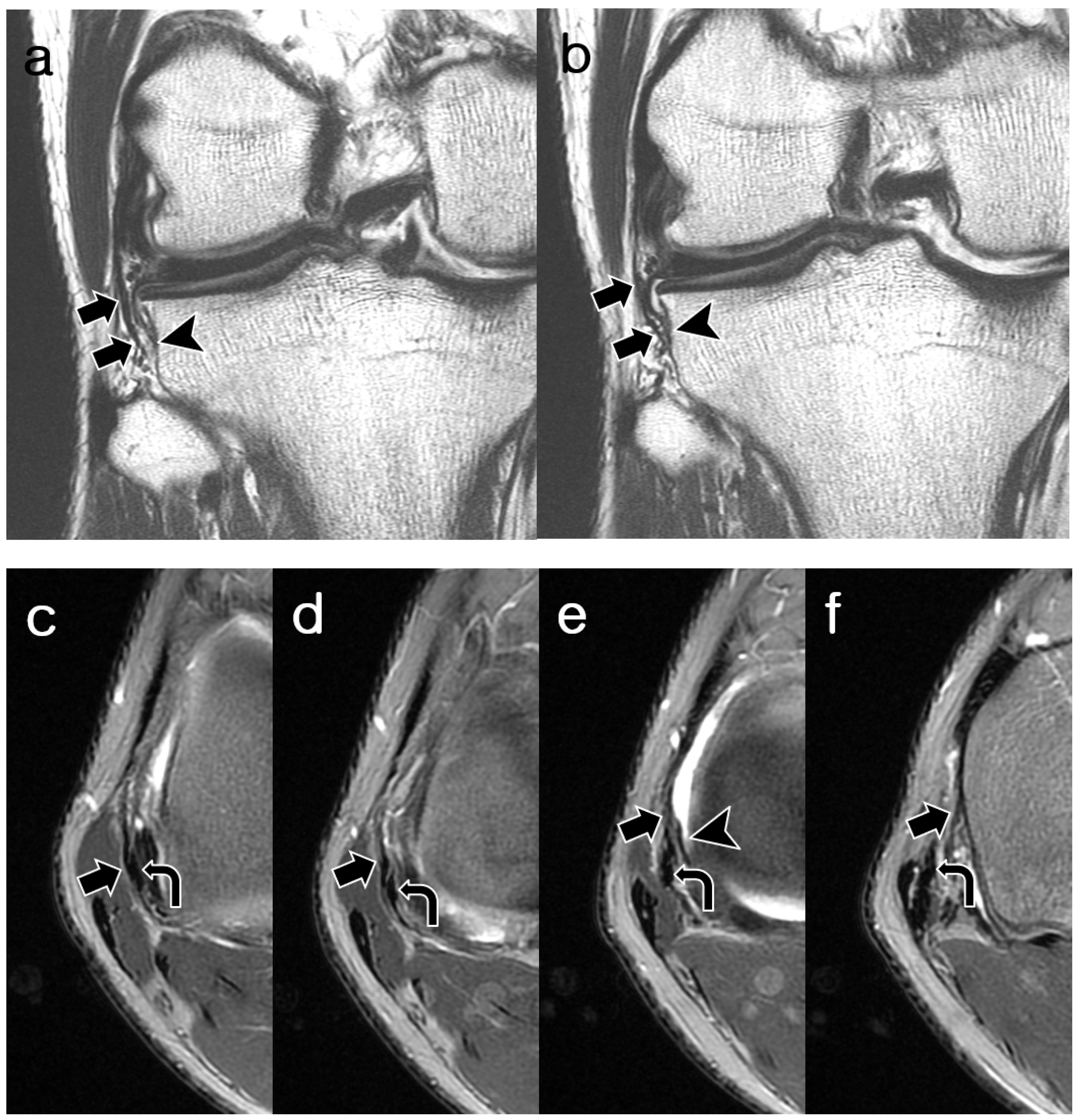
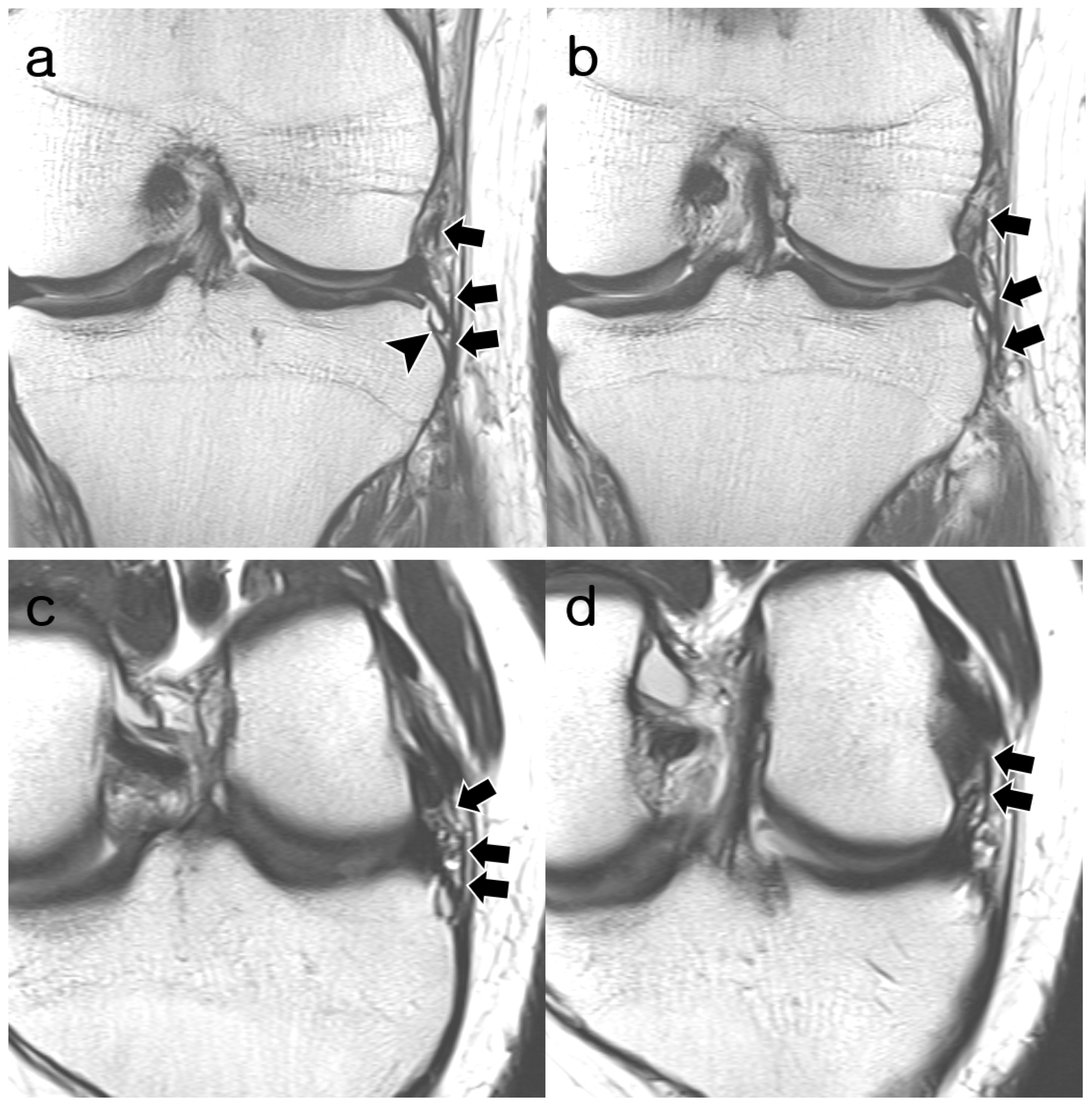
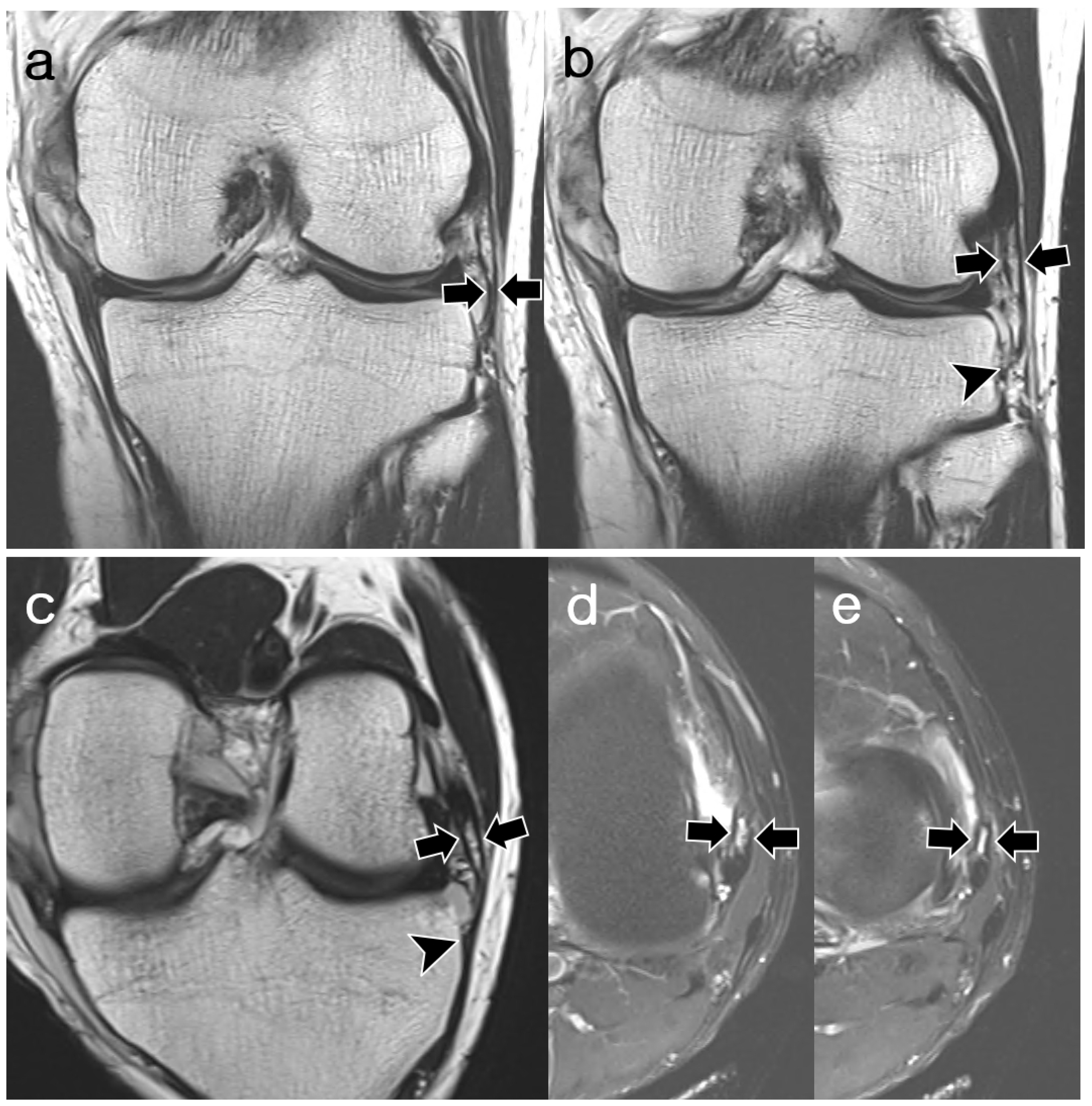
2.3. Image Analysis
2.4. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. ALL Visibility
3.3. ALL Morphology
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vincent, J.P.; Magnussen, R.A.; Gezmez, F.; Uguen, A.; Jacobi, M.; Weppe, F.; Al-Saati, M.F.; Lustig, S.; Demey, G.; Servien, E.; et al. The anterolateral ligament of the human knee: An anatomic and histologic study. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 147–152. [Google Scholar] [CrossRef]
- Helito, C.P.; Demange, M.K.; Bonadio, M.B.; Tirico, L.E.; Gobbi, R.G.; Pecora, J.R.; Camanho, G.L. Anatomy and Histology of the Knee Anterolateral Ligament. Orthop. J. Sports Med. 2013, 1, 2325967113513546. [Google Scholar] [CrossRef]
- Claes, S.; Luyckx, T.; Vereecke, E.; Bellemans, J. The Segond fracture: A bony injury of the anterolateral ligament of the knee. Arthroscopy 2014, 30, 1475–1482. [Google Scholar] [CrossRef]
- Sonnery-Cottet, B.; Daggett, M.; Fayard, J.M.; Ferretti, A.; Helito, C.P.; Lind, M.; Monaco, E.; de Padua, V.B.C.; Thaunat, M.; Wilson, A.; et al. Anterolateral Ligament Expert Group consensus paper on the management of internal rotation and instability of the anterior cruciate ligament—Deficient knee. J. Orthop. Traumatol. 2017, 18, 91–106. [Google Scholar] [CrossRef]
- Sabzevari, S.; Rahnemai-Azar, A.A.; Albers, M.; Linde, M.; Smolinski, P.; Fu, F.H. Anatomic and Histological Investigation of the Anterolateral Capsular Complex in the Fetal Knee. Am. J. Sports Med. 2017, 45, 1383–1387. [Google Scholar] [CrossRef]
- Shea, K.G.; Milewski, M.D.; Cannamela, P.C.; Ganley, T.J.; Fabricant, P.D.; Terhune, E.B.; Styhl, A.C.; Anderson, A.F.; Polousky, J.D. Anterolateral Ligament of the Knee Shows Variable Anatomy in Pediatric Specimens. Clin. Orthop. Relat. Res. 2017, 475, 1583–1591. [Google Scholar] [CrossRef]
- Herbst, E.; Albers, M.; Burnham, J.M.; Fu, F.H.; Musahl, V. The Anterolateral Complex of the Knee. Orthop. J. Sports Med. 2017, 5, 2325967117730805. [Google Scholar] [CrossRef]
- Fardin, P.B.A.; Lizardo, J.H.F.; Baptista, J.D.S. Study of the Anterolateral Ligament of the Knee in Formalin-Embedded Cadavers. Acta Ortop. Bras. 2017, 25, 89–92. [Google Scholar] [CrossRef]
- Potu, B.K.; Salem, A.H.; Abu-Hijleh, M.F. Morphology of Anterolateral Ligament of the Knee: A Cadaveric Observation with Clinical Insight. Adv. Med. 2016, 2016, 9182863. [Google Scholar] [CrossRef]
- Getgood, A.; Brown, C.; Lording, T.; Amis, A.; Claes, S.; Geeslin, A.; Musahl, V.; Group, A.L.C.C. The anterolateral complex of the knee: Results from the International ALC Consensus Group Meeting. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 166–176. [Google Scholar] [CrossRef]
- Urban, S.; Pretterklieber, B.; Pretterklieber, M.L. The anterolateral ligament of the knee and the lateral meniscotibial ligament—Anatomical phantom versus constant structure within the anterolateral complex. Ann. Anat. 2019, 226, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Claes, S.; Vereecke, E.; Maes, M.; Victor, J.; Verdonk, P.; Bellemans, J. Anatomy of the anterolateral ligament of the knee. J. Anat. 2013, 223, 321–328. [Google Scholar] [CrossRef]
- Toro-Ibarguen, A.N.; Pretell-Mazzini, J.; Perez, E.; Pedrajas, I.; Cano-Egea, J.M.; Ramon Sanudo, J. The anterolateral ligament: A cadaveric study in fetuses. Clin. Anat. 2017, 30, 625–634. [Google Scholar] [CrossRef]
- Dodds, A.L.; Halewood, C.; Gupte, C.M.; Williams, A.; Amis, A.A. The anterolateral ligament: Anatomy, length changes and association with the Segond fracture. Bone Jt. J. 2014, 96, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.A.; Chhabra, A.; Goodwin, J.A.; Hartigan, D.E. Identification of the Anterolateral Ligament on Magnetic Resonance Imaging. Arthrosc. Tech. 2017, 6, e137–e141. [Google Scholar] [CrossRef]
- Van Dyck, P.; De Smet, E.; Lambrecht, V.; Heusdens, C.H.; Van Glabbeek, F.; Vanhoenacker, F.M.; Gielen, J.L.; Parizel, P.M. The Anterolateral Ligament of the Knee: What the Radiologist Needs to Know. Semin. Musculoskelet. Radiol. 2016, 20, 26–32. [Google Scholar] [CrossRef]
- Taneja, A.K.; Miranda, F.C.; Braga, C.A.; Gill, C.M.; Hartmann, L.G.; Santos, D.C.; Rosemberg, L.A. MRI features of the anterolateral ligament of the knee. Skelet. Radiol. 2015, 44, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Klontzas, M.E.; Maris, T.G.; Zibis, A.H.; Karantanas, A.H. Normal Magnetic Resonance Imaging Anatomy of the Anterolateral Knee Ligament With a T2/T1-Weighted 3-Dimensional Sequence: A Feasibility Study. Can. Assoc. Radiol. J. 2016, 67, 52–59. [Google Scholar] [CrossRef]
- Ariel de Lima, D.; Helito, C.P.; Lacerda de Lima, L.; de Castro Silva, D.; Costa Cavalcante, M.L.; Dias Leite, J.A. Anatomy of the Anterolateral Ligament of the Knee: A Systematic Review. Arthroscopy 2019, 35, 670–681. [Google Scholar] [CrossRef]
- Khanna, M.; Gupte, C.; Dodds, A.; Williams, A.; Walker, M. Magnetic resonance imaging appearances of the capsulo-osseous layer of the iliotibial band and femoral attachments of the iliotibial band in the normal and pivot-shift ACL injured knee. Skelet. Radiol. 2019, 48, 729–740. [Google Scholar] [CrossRef]
- Caterine, S.; Litchfield, R.; Johnson, M.; Chronik, B.; Getgood, A. A cadaveric study of the anterolateral ligament: Re-introducing the lateral capsular ligament. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 3186–3195. [Google Scholar] [CrossRef]
- Claes, S.; Bartholomeeusen, S.; Bellemans, J. High prevalence of anterolateral ligament abnormalities in magnetic resonance images of anterior cruciate ligament-injured knees. Acta Orthop. Belg. 2014, 80, 45–49. [Google Scholar] [PubMed]
- D’Ambrosi, R.; Sconfienza, L.M.; Albano, D.; Messina, C.; Mangiavini, L.; Ursino, N.; Rinaldi, S.; Zanirato, A.; Tagliafico, A.; Formica, M. High incidence of RAMP lesions and a nonnegligible incidence of anterolateral ligament and posterior oblique ligament rupture in acute ACL injury. Knee Surg. Sports Traumatol. Arthrosc. 2024, in press. [CrossRef]
- Stranger, N.; Kaulfersch, C.; Mattiassich, G.; Mandl, J.; Hausbrandt, P.A.; Szolar, D.; Schollnast, H.; Tillich, M. Frequency of anterolateral ligament tears and ramp lesions in patients with anterior cruciate ligament tears and associated injuries indicative for these lesions-a retrospective MRI analysis. Eur. Radiol. 2023, 33, 4833–4841. [Google Scholar] [CrossRef]
- Mogos, S.; Antonescu, D.; Stoica, I.C.; D’Ambrosi, R. Superior rotational stability and lower re-ruptures rate after combined anterolateral and anterior cruciate ligament reconstruction compared to isolated anterior cruciate ligament reconstruction: A 2-year prospective randomized clinical trial. Phys. Sportsmed. 2023, 51, 371–378. [Google Scholar] [CrossRef]
- Rezansoff, A.J.; Caterine, S.; Spencer, L.; Tran, M.N.; Litchfield, R.B.; Getgood, A.M. Radiographic landmarks for surgical reconstruction of the anterolateral ligament of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 3196–3201. [Google Scholar] [CrossRef]
- Daggett, M.; Helito, C.; Cullen, M.; Ockuly, A.; Busch, K.; Granite, J.; Wright, B.; Sonnery-Cottet, B. The Anterolateral Ligament: An Anatomic Study on Sex-Based Differences. Orthop. J. Sports Med. 2017, 5, 2325967116689387. [Google Scholar] [CrossRef]
- Helito, C.P.; Helito, P.V.; Costa, H.P.; Bordalo-Rodrigues, M.; Pecora, J.R.; Camanho, G.L.; Demange, M.K. MRI evaluation of the anterolateral ligament of the knee: Assessment in routine 1.5-T scans. Skelet. Radiol. 2014, 43, 1421–1427. [Google Scholar] [CrossRef]
- Watanabe, J.; Suzuki, D.; Mizoguchi, S.; Yoshida, S.; Fujimiya, M. The anterolateral ligament in a Japanese population: Study on prevalence and morphology. J. Orthop. Sci. 2016, 21, 647–651. [Google Scholar] [CrossRef]
- Campos, J.C.; Chung, C.B.; Lektrakul, N.; Pedowitz, R.; Trudell, D.; Yu, J.; Resnick, D. Pathogenesis of the Segond fracture: Anatomic and MR imaging evidence of an iliotibial tract or anterior oblique band avulsion. Radiology 2001, 219, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Helito, C.P.; Helito, P.V.; Bonadio, M.B.; Pecora, J.R.; Bordalo-Rodrigues, M.; Camanho, G.L.; Demange, M.K. Correlation of Magnetic Resonance Imaging With Knee Anterolateral Ligament Anatomy: A Cadaveric Study. Orthop. J. Sports Med. 2015, 3, 2325967115621024. [Google Scholar] [CrossRef] [PubMed]
- Helito, C.P.; Demange, M.K.; Bonadio, M.B.; Tirico, L.E.; Gobbi, R.G.; Pecora, J.R.; Camanho, G.L. Radiographic landmarks for locating the femoral origin and tibial insertion of the knee anterolateral ligament. Am. J. Sports Med. 2014, 42, 2356–2362. [Google Scholar] [CrossRef]
- Helito, C.P.; Bonadio, M.B.; Soares, T.Q.; da Mota e Albuquerque, R.F.; Natalino, R.J.; Pecora, J.R.; Camanho, G.L.; Demange, M.K. The meniscal insertion of the knee anterolateral ligament. Surg. Radiol. Anat. 2016, 38, 223–228. [Google Scholar] [CrossRef]
- Runer, A.; Birkmaier, S.; Pamminger, M.; Reider, S.; Herbst, E.; Kunzel, K.H.; Brenner, E.; Fink, C. The anterolateral ligament of the knee: A dissection study. Knee 2016, 23, 8–12. [Google Scholar] [CrossRef]
- Helito, C.P.; do Amaral, C., Jr.; Nakamichi, Y.D.; Gobbi, R.G.; Bonadio, M.B.; Natalino, R.J.; Pecora, J.R.; Cardoso, T.P.; Camanho, G.L.; Demange, M.K. Why Do Authors Differ With Regard to the Femoral and Meniscal Anatomic Parameters of the Knee Anterolateral Ligament?: Dissection by Layers and a Description of Its Superficial and Deep Layers. Orthop. J. Sports Med. 2016, 4, 2325967116675604. [Google Scholar] [CrossRef]


| Parameters | Magnetom Skyra | Discovery MR750 | ||||
|---|---|---|---|---|---|---|
| Cor-T2 | Ax-T2-FS | Obl-cor-T2 | Cor-T2 | Ax-T2-FS | Obl-cor-T2 | |
| TR/TE (ms) | 3900/76 | 4130/58 | 3400/68 | 3750/73 | 3500/64 | 2500/71 |
| Flip angle (°) | 150 | 150 | 150 | 142 | 142 | 142 |
| Matrix size | 512 × 281 | 384 × 269 | 384 × 211 | 640 × 320 | 352 × 288 | 416 × 256 |
| Field of view (cm) | 16 | 15 | 14 | 16 | 15 | 14 |
| Section thickness (mm) | 3 | 4 | 3 | 3 | 4 | 3 |
| Intersection gap (mm) | 0 | 0 | 0 | 0 | 0 | 0 |
| Bandwidth (kHz/pixel) | 200 | 205 | 205 | 98 | 98 | 195 |
| Echo train length | 11 | 13 | 15 | 10 | 14 | 12 |
| Number of excitation | 2 | 3 | 4 | 1 | 2 | 2 |
| Session 1 | Session 2 | |||
|---|---|---|---|---|
| Reader 1 | Reader 2 | Reader 1 | Reader 2 | |
| Normal | 24 (36.4) | 20 (30.3) | 25 (37.9) | 24 (36.4) |
| Probably normal | 20 (30.3) | 27 (40.9) | 18 (27.3) | 18 (27.3) |
| abnormal | 19 (28.8) | 13 (19.7) | 20 (30.3) | 16 (24.2) |
| Non-visualized | 3 (4.5) | 6 (9.1) | 3 (4.5) | 8 (12.1) |
| Proximal attachment of ALL | |
| Anterior to FCL | 37 (63.8) |
| Central to FCL | 15 (25.9) |
| Posterior to FCL | 1 (1.7) |
| Mid of FCL | 4 (6.9) |
| Blended into meniscofemoral ligament | 1(1.7) |
| Distal attachment of ALL | |
| Same insertion as MTL | 29 (50) |
| Distal insertion to MTL | 29 (50) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.-H.; Moon, S.-G.; Lee, D.-W. Magnetic Resonance Imaging Features of Anterolateral Ligament in Young Adults without Anterior Cruciate Ligament Injury: Preliminary Evaluation. Diagnostics 2024, 14, 1226. https://doi.org/10.3390/diagnostics14121226
Kang J-H, Moon S-G, Lee D-W. Magnetic Resonance Imaging Features of Anterolateral Ligament in Young Adults without Anterior Cruciate Ligament Injury: Preliminary Evaluation. Diagnostics. 2024; 14(12):1226. https://doi.org/10.3390/diagnostics14121226
Chicago/Turabian StyleKang, Ji-Hee, Sung-Gyu Moon, and Dhong-Won Lee. 2024. "Magnetic Resonance Imaging Features of Anterolateral Ligament in Young Adults without Anterior Cruciate Ligament Injury: Preliminary Evaluation" Diagnostics 14, no. 12: 1226. https://doi.org/10.3390/diagnostics14121226






