The Stress Index as a Predictor of Mortality in Patients with Isolated Moderate to Severe Traumatic Brain Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Enrollment and Study Design
2.2. Statistical Analysis
3. Results
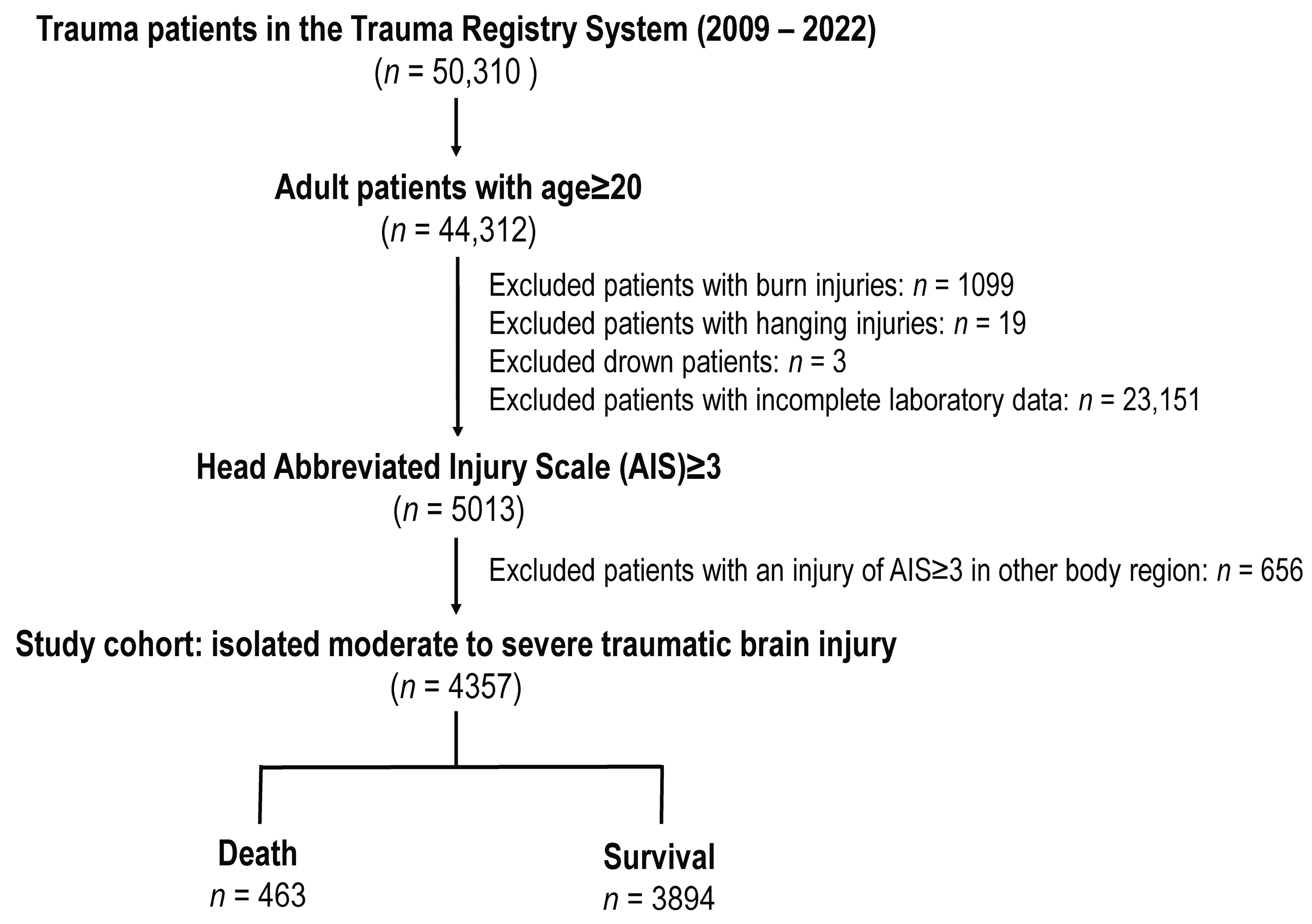
3.1. Patient Enrollment
3.2. Patient and Injury Characteristics
3.3. Univariate and Multivariate Analysis of Mortality Risk Factors
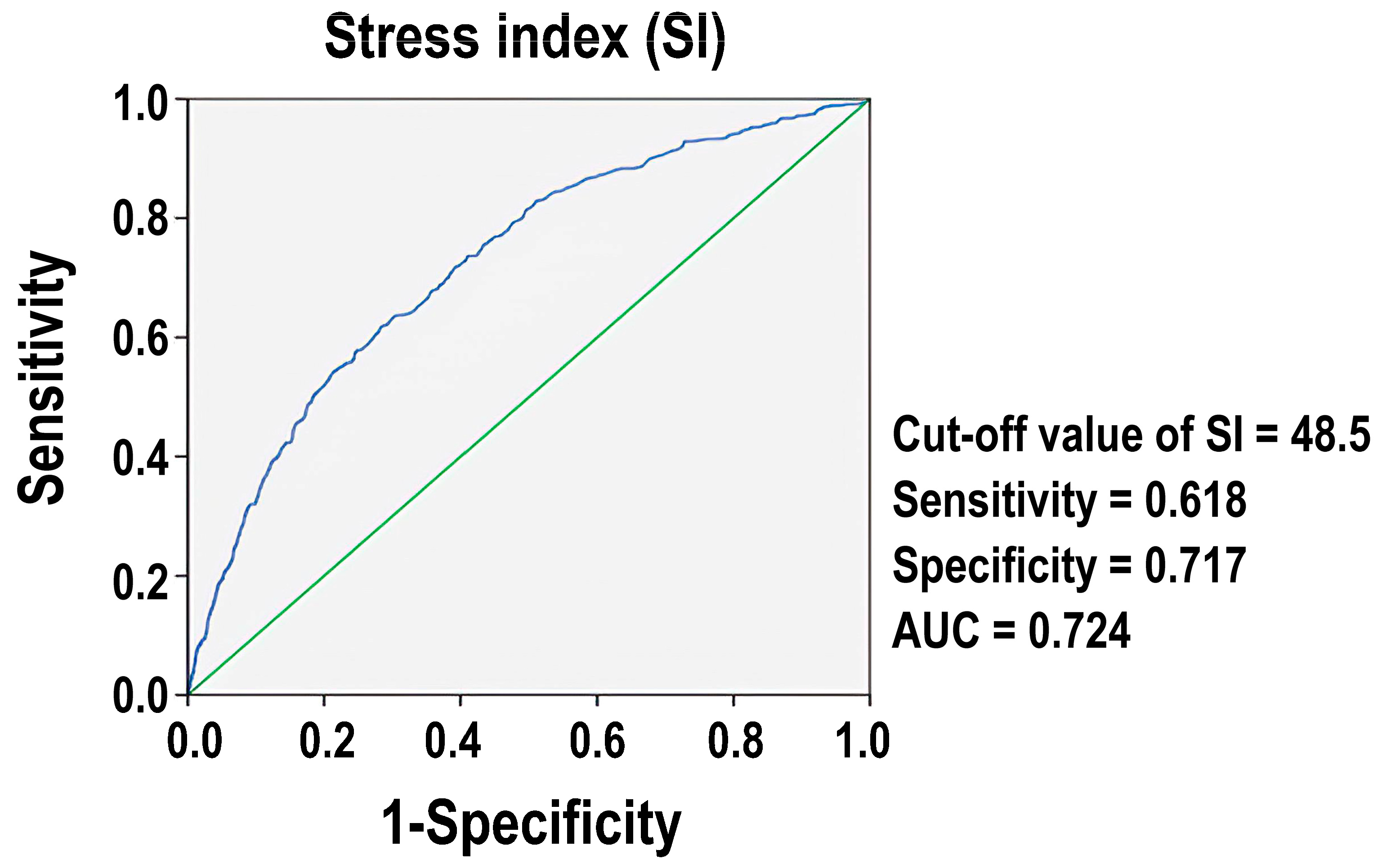
3.4. The Mortality Predictive Performance of SI
3.5. Comparative Analysis of the Group of Patients Divided by the Optimal Cut-Off SI Value
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palmer, B.F.; Clegg, D.J. Physiology and pathophysiology of potassium homeostasis. Adv. Physiol. Educ. 2016, 40, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Vanek, V.W.; Seballos, R.M.; Chong, D.; Bourguet, C.C. Serum potassium concentrations in trauma patients. South Med. J. 1994, 87, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Morell, V.; Lundgren, E.; Gillott, A. Predicting severity of trauma by admission white blood cell count, serum potassium level, and arterial pH. South Med. J. 1993, 86, 658–659. [Google Scholar] [CrossRef] [PubMed]
- Ookuma, T.; Miyasho, K.; Kashitani, N.; Beika, N.; Ishibashi, N.; Yamashita, T.; Ujike, Y. The clinical relevance of plasma potassium abnormalities on admission in trauma patients: A retrospective observational study. J. Intensive Care 2015, 3, 37. [Google Scholar] [CrossRef] [PubMed]
- Perkins, R.M.; Aboudara, M.C.; Abbott, K.C.; Holcomb, J.B. Resuscitative hyperkalemia in noncrush trauma: A prospective, observational study. Clin. J. Am. Soc. Nephrol. 2007, 2, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, K.A.; Brown, R.O.; Maish, G.O., 3rd; Croce, M.A.; Minard, G.; Dickerson, R.N. Influence of traumatic brain injury on potassium and phosphorus homeostasis in critically ill multiple trauma patients. Nutrition 2010, 26, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Godinjak, A.; Iglica, A.; Burekovic, A.; Jusufovic, S.; Ajanovic, A.; Tancica, I.; Kukuljac, A. Hyperglycemia in Critically Ill Patients: Management and Prognosis. Med. Arch. 2015, 69, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Vedantam, D.; Poman, D.S.; Motwani, L.; Asif, N.; Patel, A.; Anne, K.K. Stress-Induced Hyperglycemia: Consequences and Management. Cureus 2022, 14, e26714. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.W.; Huang, C.Y.; Liu, H.T.; Chen, Y.C.; Hsieh, C.H. Stress-Induced and Diabetic Hyperglycemia Associated with Higher Mortality among Intensive Care Unit Trauma Patients: Cross-Sectional Analysis of the Propensity Score-Matched Population. Int. J. Environ. Res. Public Health 2018, 15, 992. [Google Scholar] [CrossRef] [PubMed]
- Rau, C.S.; Wu, S.C.; Chen, Y.C.; Chien, P.C.; Hsieh, H.Y.; Kuo, P.J.; Hsieh, C.H. Higher Mortality in Trauma Patients Is Associated with Stress-Induced Hyperglycemia, but Not Diabetic Hyperglycemia: A Cross-Sectional Analysis Based on a Propensity-Score Matching Approach. Int. J. Environ. Res. Public Health 2017, 14, 1161. [Google Scholar] [CrossRef]
- Boyuk, F. The Predictor Potential Role of the Glucose to Potassium Ratio in the Diagnostic Differentiation of Massive and Non-Massive Pulmonary Embolism. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221076146. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.M.; Paik, J.H.; Kim, S.Y.; Hong, D.Y. Association of Plasma Glucose to Potassium Ratio and Mortality After Aneurysmal Subarachnoid Hemorrhage. Front. Neurol. 2021, 12, 661689. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ma, X.; Zhou, X.; Wang, Y. The association between serum glucose to potassium ratio on admission and short-term mortality in ischemic stroke patients. Sci. Rep. 2022, 12, 8233. [Google Scholar] [CrossRef] [PubMed]
- Marini, J.I.; Sein, M.E. The Role of the Glucose Potassium Ratio in the Management of Traumatic Brain Injury. Korean J. Neurotrauma 2023, 19, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Satoh, A.; Nakamura, H.; Kobayashi, S.; Miyata, A.; Matsutani, M. Management of severe subarachnoid hemorrhage; significance of assessment of both neurological and systemic insults at acute stage. Acta Neurochir. Suppl. 2005, 94, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Shibata, A.; Matano, F.; Saito, N.; Fujiki, Y.; Matsumoto, H.; Mizunari, T.; Morita, A. Serum Glucose-To-Potassium Ratio as a Prognostic Predictor for Severe Traumatic Brain Injury. J. Nippon. Med. Sch. 2021, 88, 342–346. [Google Scholar] [CrossRef]
- Taniguchi, H.; Doi, T.; Abe, T.; Takeuchi, I. Trauma severity associated with stress index in emergency settings: An observational prediction-and-validation study. Acute Med. Surg. 2020, 7, e493. [Google Scholar] [CrossRef]
- Alamri, F.F.; Almarghalani, D.A.; Alraddadi, E.A.; Alharbi, A.; Algarni, H.S.; Mulla, O.M.; Alhazmi, A.M.; Alotaibi, T.A.; Beheiry, D.H.; Alsubaie, A.S.; et al. The utility of serum glucose potassium ratio as a predictive factor for haemorrhagic transformation, stroke recurrence, and mortality among ischemic stroke patients. Saudi Pharm. J. 2024, 32, 102082. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Le Gall, J.R.; Lemeshow, S.; Saulnier, F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. Jama 1993, 270, 2957–2963. [Google Scholar] [CrossRef]
- Lemeshow, S.; Teres, D.; Klar, J.; Avrunin, J.S.; Gehlbach, S.H.; Rapoport, J. Mortality Probability Models (MPM II) based on an international cohort of intensive care unit patients. Jama 1993, 270, 2478–2486. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Smejkal, R.; Civil, I.; Unkle, D.; Ross, S.E. Injury severity scoring: A comparison of early clinical versus discharge diagnosis. Accid. Anal. Prev. 1989, 21, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Aharonson-Daniel, L.; Giveon, A.; Stein, M.; Israel Trauma, G.; Peleg, K. Different AIS triplets: Different mortality predictions in identical ISS and NISS. J. Trauma. 2006, 61, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Østrem, M.; Thorsen, K.; Wisborg, T.; Røise, O.; Helseth, E.; Jeppesen, E. Care pathways and factors associated with interhospital transfer to neurotrauma centers for patients with isolated moderate-to-severe traumatic brain injury: A population-based study from the Norwegian trauma registry. Scand. J. Trauma. Resusc. Emerg. Med. 2023, 31, 34. [Google Scholar] [CrossRef]
- Rau, C.S.; Wu, S.C.; Chen, Y.C.; Chien, P.C.; Hsieh, H.Y.; Kuo, P.J.; Hsieh, C.H. Stress-Induced Hyperglycemia, but Not Diabetic Hyperglycemia, Is Associated with Higher Mortality in Patients with Isolated Moderate and Severe Traumatic Brain Injury: Analysis of a Propensity Score-Matched Population. Int. J. Environ. Res. Public Health 2017, 14, 1340. [Google Scholar] [CrossRef] [PubMed]
- Reith, F.C.M.; Lingsma, H.F.; Gabbe, B.J.; Lecky, F.E.; Roberts, I.; Maas, A.I.R. Differential effects of the Glasgow Coma Scale Score and its Components: An analysis of 54,069 patients with traumatic brain injury. Injury 2017, 48, 1932–1943. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.P.; O’Neill, B.; Haddon, W., Jr.; Long, W.B. The injury severity score: A method for describing patients with multiple injuries and evaluating emergency care. J. Trauma 1974, 14, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.I.R.; Menon, D.K.; Manley, G.T.; Abrams, M.; Åkerlund, C.; Andelic, N.; Aries, M.; Bashford, T.; Bell, M.J.; Bodien, Y.G.; et al. Traumatic brain injury: Progress and challenges in prevention, clinical care, and research. Lancet Neurol. 2022, 21, 1004–1060. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Mushkudiani, N.; Perel, P.; Butcher, I.; Lu, J.; McHugh, G.S.; Murray, G.D.; Marmarou, A.; Roberts, I.; Habbema, J.D.; et al. Predicting outcome after traumatic brain injury: Development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008, 5, e165, discussion e165. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, C.-S.; Shen, L.-J.; Lv, Q.-W.; Xu, Q.-C. Usefulness of serum glucose and potassium ratio as a predictor for 30-day death among patients with severe traumatic brain injury. Clinica Chimica Acta 2020, 506, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Turan, E.; Şahin, A. Role of glucose/potassium ratio and shock index in predicting mortality in patients with isolated thoracoabdominal blunt trauma. Ulus. Travma Acil Cerrahi Derg. 2022, 28, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Katipoğlu, B.; Demirtaş, E. Assessment of serum glucose potassium ratio as a predictor for morbidity and mortality of blunt abdominal trauma. Ulus. Travma Acil Cerrahi Derg. 2022, 28, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Van Beek, J.G.; Mushkudiani, N.A.; Steyerberg, E.W.; Butcher, I.; McHugh, G.S.; Lu, J.; Marmarou, A.; Murray, G.D.; Maas, A.I. Prognostic value of admission laboratory parameters in traumatic brain injury: Results from the IMPACT study. J. Neurotrauma 2007, 24, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, T.; Morganti-Kossmann, M.C. The role of markers of inflammation in traumatic brain injury. Front. Neurol. 2013, 4, 18. [Google Scholar] [CrossRef]
- Hinson, H.E.; Rowell, S.; Schreiber, M. Clinical evidence of inflammation driving secondary brain injury: A systematic review. J. Trauma. Acute Care Surg. 2015, 78, 184–191. [Google Scholar] [CrossRef]
| Variables | Death n = 463 | Survival n = 3894 | OR (95%CI) | p |
|---|---|---|---|---|
| Sex | 0.001 * | |||
| Male, n (%) | 311 (67.2) | 2307 (59.2) | 1.41 (1.15–1.73) | |
| Female, n (%) | 152 (32.8) | 1587 (40.8) | 0.71 (0.58–0.87) | |
| Age, years | 62.8 ± 18.8 | 59.4 ± 18.9 | - | <0.001 * |
| Stress index | 61.7 ± 30.6 | 44.1 ± 19.0 | - | <0.001 * |
| Glucose (mg/dL) | 213.2 ± 95.7 | 158.4 ± 63.7 | - | <0.001 * |
| Potassium (mEq/L) | 3.6 ± 0.7 | 3.7 ± 2.3 | - | 0.225 |
| Comorbidities | ||||
| CVA, n (%) | 23 (5.0) | 236 (6.1) | 0.81 (0.52–1.26) | 0.347 |
| HTN, n (%) | 181 (39.1) | 1508 (38.7) | 1.02 (0.83–1.24) | 0.878 |
| CAD, n (%) | 54 (11.7) | 282 (7.2) | 1.69 (1.24–2.30) | 0.001 * |
| CHF, n (%) | 2 (0.4) | 29 (0.7) | 0.58 (0.14–2.43) | 0.449 |
| DM, n (%) | 95 (20.5) | 823 (21.1) | 0.96 (0.76–1.22) | 0.758 |
| ESRD, n (%) | 36 (7.8) | 105 (2.7) | 3.04 (2.06–4.50) | <0.001 * |
| GCS, median (IQR) | 4 (3–9) | 15 (11–15) | - | <0.001 * |
| 3–8 | 333 (71.9) | 641 (16.5) | 13.00 (10.44–16.19) | <0.001 * |
| 9–12 | 43 (9.3) | 458 (11.8) | 0.77 (0.55–1.07) | 0.115 |
| 13–15 | 87 (18.8) | 2795 (71.8) | 0.09 (0.07–0.12) | <0.001 * |
| ISS, median (IQR) | 25 (25–29) | 16 (16–21) | - | <0.001 * |
| 1–15 | 16 (3.5) | 863 (22.2) | 0.13 (0.08–0.21) | <0.001 * |
| 16–24 | 88 (19.0) | 2298 (59.0) | 0.16 (0.13–0.21) | <0.001 * |
| ≥25 | 359 (77.5) | 733 (18.8) | 14.89 (11.80–18.78) | <0.001 * |
| Hospital stay (days) | 8.5 ± 11.7 | 13.3 ± 13.4 | - | <0.001 * |
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| OR | CI | p | OR | CI | p | |
| Male, yes | 1.41 | (1.15–1.73) | 0.001 | 1.70 | (1.34–2.15) | <0.001 * |
| Age, year | 1.01 | (1.00–1.02) | <0.001 | 1.02 | (1.02–1.03) | <0.001 * |
| Stress index | 1.03 | (1.02–1.03) | <0.001 | 6.70 | (1.66–26.99) | 0.007 * |
| Glucose(mg/dL) | 2.20 | (1.96–2.46) | <0.001 | 1.07 | (0.72–1.60) | 0.728 |
| Potassium (mEq/L) | 0.75 | (0.62–0.89) | 0.001 | 0.86 | (0.66–1.13) | 0.281 |
| CAD, yes | 1.69 | (1.24–2.30) | 0.001 | 1.26 | (0.88–1.80) | 0.217 |
| ESRD, yes | 3.04 | (2.06–4.50) | 0.001 | 4.33 | (2.71–6.91) | <0.001 * |
| ISS | 1.14 | (1.12–1.15) | <0.001 | 1.14 | (1.12–1.15) | <0.001 * |
| Predictor Variables | VIF |
|---|---|
| Age | 1.12 |
| Sex | 1.07 |
| SI | 1.15 |
| Glucose | 1.09 |
| Potassium | 1.08 |
| CAD | 1.10 |
| ESRD | 1.14 |
| ISS | 1.20 |
| Stress Index (SI) | ||||
|---|---|---|---|---|
| Variables | ≥48.5 n = 1385 | <48.5 n = 2972 | OR (95%CI) | p |
| Sex | 0.002 * | |||
| Male, n (%) | 785 (56.7) | 1833 (61.7) | 0.81 (0.71–0.93) | |
| Female, n (%) | 600 (43.3) | 1139 (38.3) | 1.23 (1.08–1.40) | |
| Age, years | 60.1 ± 17.8 | 59.6 ± 19.4 | - | 0.407 |
| Comorbidities | ||||
| CVA, n (%) | 70 (5.1) | 189 (6.4) | 0.78 (0.59–1.04) | 0.090 |
| HTN, n (%) | 582 (42.0) | 1107 (37.2) | 1.22 (1.07–1.39) | 0.003 * |
| CAD, n (%) | 117 (8.4) | 219 (7.4) | 1.16 (0.92–1.47) | 0.214 |
| CHF, n (%) | 14 (1.0) | 17 (0.6) | 1.78 (0.87–3.61) | 0.109 |
| DM, n (%) | 485 (35.0) | 433 (14.6) | 3.16 (2.72–3.67) | <0.001 * |
| ESRD, n (%) | 40 (2.9) | 101 (3.4) | 0.85 (0.58–1.23) | 0.375 |
| GCS, median (IQR) | 12 (6–15) | 15 (12–15) | - | <0.001 |
| 3–8 | 536 (38.7) | 438 (14.7) | 3.65 (3.15–4.24) | <0.001 |
| 9–12 | 171 (12.3) | 330 (11.1) | 1.13 (0.93–1.37) | 0.231 |
| 13–15 | 678 (49.0) | 2204 (74.2) | 0.33 (0.29–0.38) | <0.001 * |
| ISS, median (IQR) | 20 (16–25) | 16 (16–20) | - | <0.001 * |
| 1–15 | 183 (13.2) | 696 (23.4) | 0.50 (0.42–0.59) | <0.001 * |
| 16–24 | 641 (46.3) | 1745 (58.7) | 0.61 (0.53–0.69) | <0.001 * |
| ≥25 | 561 (40.5) | 531 (17.9) | 3.13 (2.71–3.61) | <0.001 * |
| Hospital stay (days) | 15.6 ± 15.8 | 11.5 ± 11.7 | - | <0.001 * |
| Mortality, n (%) | 285 (20.6) | 178 (6.0) | 4.07 (3.33–4.97) | <0.001 * |
| AOR of mortality * | 1.94 (1.51–2.50) | <0.001 * | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-Y.; Rau, C.-S.; Huang, C.-Y.; Su, W.-T.; Hsu, S.-Y.; Hsieh, C.-H. The Stress Index as a Predictor of Mortality in Patients with Isolated Moderate to Severe Traumatic Brain Injury. Diagnostics 2024, 14, 1244. https://doi.org/10.3390/diagnostics14121244
Huang C-Y, Rau C-S, Huang C-Y, Su W-T, Hsu S-Y, Hsieh C-H. The Stress Index as a Predictor of Mortality in Patients with Isolated Moderate to Severe Traumatic Brain Injury. Diagnostics. 2024; 14(12):1244. https://doi.org/10.3390/diagnostics14121244
Chicago/Turabian StyleHuang, Ching-Ya, Cheng-Shyuan Rau, Chun-Ying Huang, Wei-Ti Su, Shiun-Yuan Hsu, and Ching-Hua Hsieh. 2024. "The Stress Index as a Predictor of Mortality in Patients with Isolated Moderate to Severe Traumatic Brain Injury" Diagnostics 14, no. 12: 1244. https://doi.org/10.3390/diagnostics14121244





