Classical Orbital Floor Post-Traumatic Reconstruction vs. Customized Reconstruction with the Support of “In-House” 3D-Printed Models: A Retrospective Study with an Analysis of Volumetric Measurement
Abstract
:1. Introduction
2. Materials and Methods
- -
- A diagnosis of unilateral isolated OFF in male patients aged >18 years;
- -
- Pre- and postoperative ophthalmological examination with preoperative diplopia confirmed by an Orthoptic examination with Hess Lancaster screens;
- -
- Pre- and postoperative CT in our Radiology Center with slice of 0.5 mm;
- -
- An absence of muscle deficits and nerve or vascular damage;
- -
- Surgery performed within 2 weeks from trauma;
- -
- Three-dimensional stereolithographic printed model, volumetric measurements, and reconstruction with titanium mesh (both modeled intraoperatively and preoperatively on a 3D model) performed by the same surgeon.
- -
- Patients with multiple maxilla-facial fractures;
- -
- Prior surgery for orbital trauma or ophthalmic surgery;
- -
- Patients who were not cooperating;
- -
- Patients with incomplete clinical-radiological documentation;
- -
- A reconstruction of the orbital floor with other materials.
2.1. Procedure
- Group 1 (G1): patients surgically treated for isolated OFF, divided into 2 subgroups: G1a (patients undergoing orbital floor reconstruction with an intraoperatively shaped mesh) and G1b (patients undergoing orbital floor reconstruction with a preoperative mesh shaped on a 3D-printed stereolithographic model, obtained from the patient’s CT scan and using the Ultimaker S5 3D printer—Manufat Engineering Srl, 160 23900 Lecco (LC), Italy).
- Group 2 (G2): patients treated for other traumatic pathologies such as mandibular or middle third fracture not involving orbit (zygmatic arch, Le Fort I, etc.).
- -
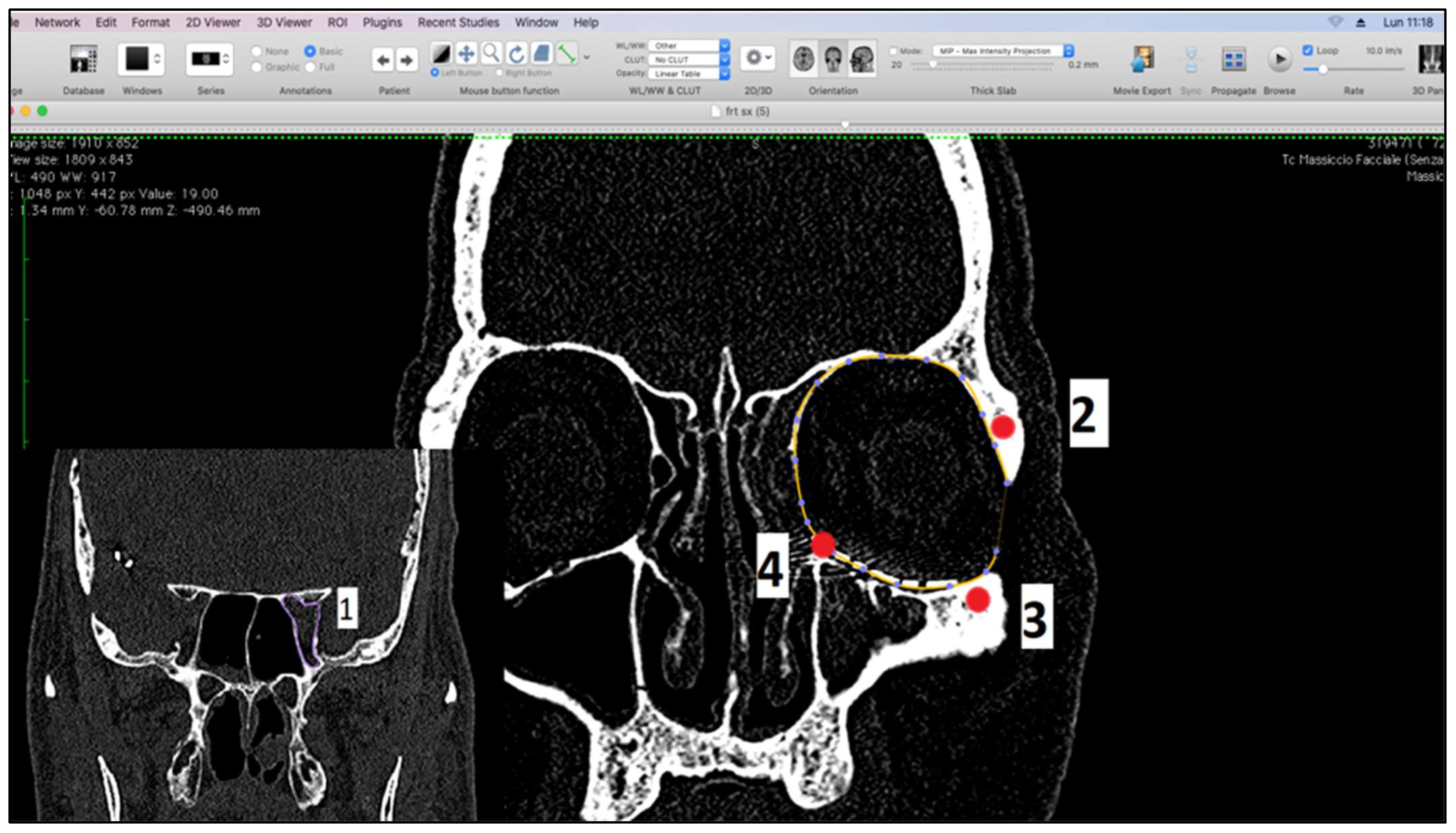
- Measurement of orbital volume using Osirix software (Version 12.0) (Pixmeo SARL, CH-1233 Bernex, Switzerland) on the new CT examination. This was carried out both semi-automatically and manually. For each patient, the orbital volume on the preoperative CT was calculated for both healthy and fractured orbits. On the postoperative CT, however, the orbital volume reconstructed with titanium mesh was calculated. We then calculated the difference in volumes between healthy and traumatized orbit, both before and after surgery. Finally, we evaluated the recovery difference, which is made up of the difference between the two volumes, pre-op and post-op, of the traumatized orbit.
- -
- Post-surgery thin layer CT (0.5 mm) with axial, coronal, and sagittal acquisitions and 3D reconstructions.
2.2. Measurement of Orbital Volumes
2.3. Production of the 3D Model
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Megafu, M.N.; Megafu, E.C.; Nguyen, J.T.; Mian, H.S.; Singhal, S.S.; Parisien, R.L. The Statistical Fragility of Orbital Fractures: A Systematic Review of Randomized Controlled Trials. J. Oral Maxillofac. Surg. 2023, 81, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Colangeli, W.; Ferragina, F.; Kallaverja, E.; Celano, C.; Cristofaro, M.G. Orbital fractures treated in a university hospital of southern Italy: Epidemiology, outcomes and prognostic factors resulting from 538 retrospectively analyzed cases. Oral Maxillofac. Surg. 2024, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Lim, J.S.; Yoo, G.; Byeon, J.H. An Analysis of Pure Blowout Fractures and Associated Ocular Symptoms. J. Craniofacial Surg. 2013, 24, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Manson, P.N.; Iiff, N. Management of blow-out fractures of the orbital floor II. Early repair for selected injuries. Surv. Ophthalmol. 1991, 35, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Kunz, C.; Sigron, G.R.; Jaquiéry, C. Functional outcome after non-surgical management of orbital fractures—The bias of decisionmaking according to size of defect: Critical review of 48 patients. Br. J. Oral Maxillofac. Surg. 2013, 51, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Burnstine, M.A. Clinical recommendations for repair of isolated orbital floor fractures: An evidence-based analysis. Ophthalmology 2002, 109, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Hammer, B.; Prein, J. Correction of post-traumatic orbital deformities: Operative techniques and review of 26 patients. J. Cranio-Maxillofac. Surg. 1995, 23, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Potter, J.K.; Ellis, E. Biomaterials for reconstruction of the internal orbit. J. Oral Maxillofac. Surg. 2004, 62, 1280–1297. [Google Scholar] [CrossRef] [PubMed]
- Sugar, A.W.; Kurlakose, M.; Walshaw, N.D. Titanium mesh in orbital wall reconstruction. Int. J. Oral Maxillofac. Surg. 1992, 21, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Metzger, M.C.; Schön, R.; Weyer, N.; Rafii, A.; Gellrich, N.-C.; Schmelzeisen, R.; Strong, B.E. Anatomical 3-dimensional Pre-bent Titanium Implant for Orbital Floor Fractures. Ophthalmology 2006, 113, 1863–1868. [Google Scholar] [CrossRef]
- Kärkkäinen, M.; Wilkman, T.; Mesimäki, K.; Snäll, J. Primary reconstruction of orbital fractures using patient-specific titanium milled implants: The Helsinki protocol. Br. J. Oral Maxillofac. Surg. 2018, 56, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Strong, E.B.; Fuller, S.C.; Wiley, D.F.; Zumbansen, J.; Wilson, M.D.; Metzger, M.C. Preformed vs Intraoperative Bending of Titanium Mesh for Orbital Reconstruction. Otolaryngol. Head Neck Surg. 2013, 149, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Kozakiewicz, M.; Elgalal, M.; Loba, P.; Komuński, P.; Arkuszewski, P.; Broniarczyk-Loba, A.; Stefańczyk, L. Clinical application of 3D pre-bent titanium implants for orbital floor fractures. J. Cranio-Maxillofac. Surg. 2009, 37, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Msallem, B.; Sharma, N.; Cao, S.; Halbeisen, F.S.; Zeilhofer, H.-F.; Thieringer, F.M. Evaluation of the Dimensional Accuracy of 3D-Printed Anatomical Mandibular Models Using FFF, SLA, SLS, MJ, and BJ Printing Technology. J. Clin. Med. 2020, 9, 817. [Google Scholar] [CrossRef] [PubMed]
- Hatz, C.R.; Msallem, B.; Aghlmandi, S.; Brantner, P.; Thieringer, F.M. Can an entry-level 3D printer create high-quality anatomical models? Accuracy assessment of mandibular models printed by a desktop 3D printer and a professional device. Int. J. Oral Maxillofac. Surg. 2020, 49, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Maglitto, F.; Orabona, G.D.; Committeri, U.; Salzano, G.; De Fazio, G.R.; Vaira, L.A.; Abbate, V.; Bonavolontà, P.; Piombino, P.; Califano, L. Virtual Surgical Planning and the “In-House” Rapid Prototyping Technique in Maxillofacial Surgery: The Current Situation and Future Perspectives. Appl. Sci. 2021, 11, 1009. [Google Scholar] [CrossRef]
- Kwon, J.; Barrera, J.E.; Jung, T.Y.; Most, S.P. Measurements of orbital volume change using computed tomography in isolated orbital blowout fractures. Arch. Facial Plast. Surg. 2009, 11, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Koppel, D.A.; Foy, R.H.; McCaul, J.A.; Logan, J.; Hadley, D.M.; Ayoub, A. The reliability of “Analyze” software in measuring orbital volume utilizing CT-derived data. J. Craniomaxillofac. Surg. 2003, 31, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Andrades, P.; Cuevas, P.; Hernández, R.; Danilla, S.; Villalobos, R. Characterization of the orbital volume in normal population. J. Craniomaxillofac. Surg. 2018, 46, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Ellis, E.; Tan, Y. Assessment of internal orbital reconstructions for pure blowout fractures: Cranial bone grafts versus titanium mesh. J. Oral Maxillofac. Surg. 2003, 61, 442–453. [Google Scholar] [CrossRef]
- Ellis, E.; Messo, E. Use of nonresorbable alloplastic implants for internal orbital reconstruction. J. Oral Maxillofac. Surg. 2004, 62, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Dubois, L.; Steenen, S.A.; Gooris, P.J.J.; Bos, R.R.M.; Becking, A.G. Controversies in orbital reconstruction—III. Biomaterials for orbital reconstruction: A review with clinical recommendations. Int. J. Oral Maxillofac. Surg. 2016, 45, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Chen, H.; Sun, Y.-J.; Wang, B.-F.; Che, L.; Liu, S.-Y.; Li, G.-Y. Clinical effects of 3-D printing-assisted personalized reconstructive surgery for blowout orbital fractures. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 2051–2057. [Google Scholar] [CrossRef] [PubMed]
- Schön, R.; Metzger, M.C.; Zizelmann, C.; Weyer, N.; Schmelzeisen, R. Individually preformed titanium mesh implants for a true-to-original repair of orbital fractures. Int. J. Oral Maxillofac. Surg. 2006, 35, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.G.T.; Campbell, D.I.; Clucas, D.M. Rapid Prototyping Technology in Orbital Floor Reconstruction: Application in Three Patients. Craniomaxillofac. Trauma Reconstr. 2014, 7, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Callahan, A.B.; Campbell, A.A.; Petris, C.; Kazim, M. Low-Cost 3D Printing Orbital Implant Templates in Secondary Orbital Reconstructions. Ophthalmic Plast. Reconstr. Surg. 2017, 33, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.-S.; Shin, H.J. Comparison of Orbital Reconstructive Effect between Customized Orbital Implants Using Three-Dimensional Printed Templates and Conventional Manual-Bending Implants in Blowout Fracture Surgery. Appl. Sci. 2023, 13, 9012. [Google Scholar] [CrossRef]
- Holmes, S.; Schlittler, F.L. Going beyond the limitations of the non-patient-specific implant in titanium reconstruction of the orbit. Br. J. Oral Maxillofac. Surg. 2021, 59, 1074–1078. [Google Scholar] [CrossRef]
- Zimmerer, R.M.; Ellis, E.; Aniceto, G.S.; Schramm, A.; Wagner, M.E.H.; Grant, M.P.; Cornelius, C.-P.; Strong, E.B.; Rana, M.; Chye, L.T. A prospective multicenter study to compare the precision of posttraumatic internal orbital reconstruction with standard preformed and individualized orbital implants. J. Cranio-Maxillofac. Surg. 2016, 44, 1485–1497. [Google Scholar] [CrossRef]
- Kozakiewicz, M.; Elgalal, M.; Piotr, L.; Broniarczyk-Loba, A.; Stefanczyk, L. Treatment with individual orbital wall implants in humans—1-Year ophthalmologic evaluation. J. Cranio-Maxillofac. Surg. 2011, 39, 30–36. [Google Scholar] [CrossRef]
- Kim, Y.C.; Jeong, W.S.; Park, T.-K.; Choi, J.W.; Koh, K.S.; Oh, T.S. The accuracy of patient specific implant prebented with 3D-printed rapid prototype model for orbital wall reconstruction. J. Cranio-Maxillofac. Surg. 2017, 45, 928–936. [Google Scholar] [CrossRef]
- Sigron, G.R.; Rüedi, N.; Chammartin, F.; Meyer, S.; Msallem, B.; Kunz, C.; Thieringer, F.M. Three-Dimensional Analysis of Isolated Orbital Floor Fractures Pre- and Post-Reconstruction with Standard Titanium Meshes and “Hybrid” Patient-Specific Implants. J. Clin. Med. 2020, 9, 1579. [Google Scholar] [CrossRef]
- Murray-Douglass, A.; Snoswell, C.; Winter, C.; Harris, R. Three-dimensional (3D) printing for post-traumatic orbital reconstruction, a systematic review and meta-analysis. Br. J. Oral Maxillofac. Surg. 2022, 60, 1176–1183. [Google Scholar] [CrossRef]
- Zieli’nski, R.; Mali’nska, M.; Kozakiewicz, M. Classical versus custom orbital wall reconstruction: Selected factors regarding surgery and hospitalization. J. Cranio-Maxillofac. Surg. 2017, 45, 710–715. [Google Scholar] [CrossRef]
- Msallem, D.M.B.; Beiglboeck, F.; Honigmann, P.; Jaquiéry, D.M.C.; Thieringer, F. Craniofacial Reconstruction by a Cost-Efficient Template-Based Process Using 3D Printing. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1582. [Google Scholar] [CrossRef] [PubMed]
- Legocki, A.T.; Duffy-Peter, A.; Scott, A.R. Benefits and Limitations of Entry-Level 3-Dimensional Printing of Maxillofacial Skeletal Models. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 389–394. [Google Scholar] [CrossRef] [PubMed]





| 10 patients isolated blow out fracture | |
| CT scans and Hess Lancaster screen | |
| G1a (intraoperative shaping mesh) | G1b (preoperative shaping mesh on 3D printed stereolithographic model) |
| Import Dicom blow out fracture Into Osirix MD software | |
| Blow Out fracture identification, first volume measurements, export the Dicom file in the stl file | |
| 3D Printing of the stereolithographic model | |
| Preoperative shaping of the mesh | |
| Sterilization of the pre shaped mesh | |
| Titanium mesh insertion without further adjustment | |
| Postoperative CT | |
| Import the new Dicom into Osirix MD software | |
| Second volume measurement | |
| Creating Table 2 and Table 3 | |
| Intraoperative shaping, mesh insertion, and evaluation if shape and insertion are correct | Analysis |
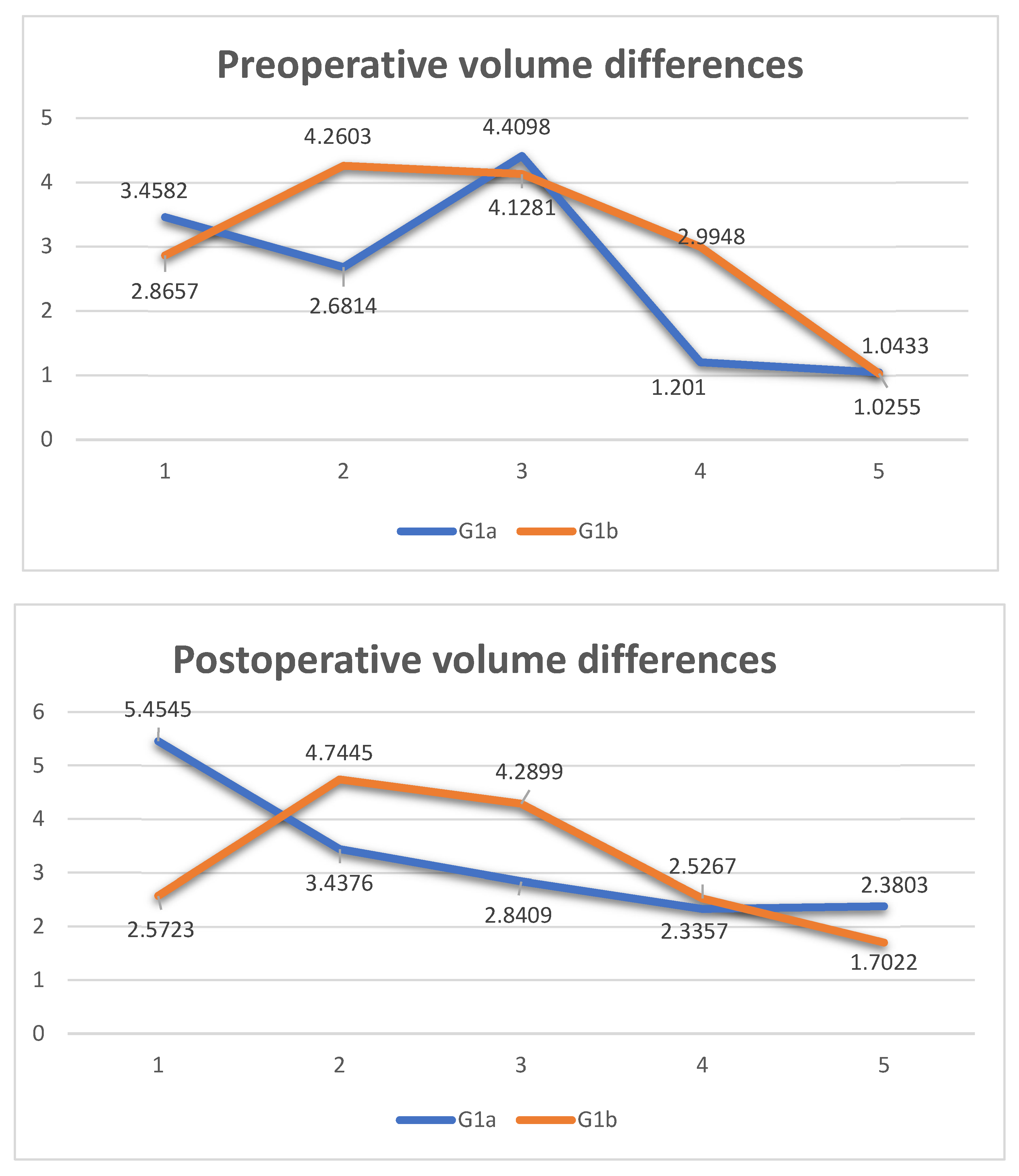
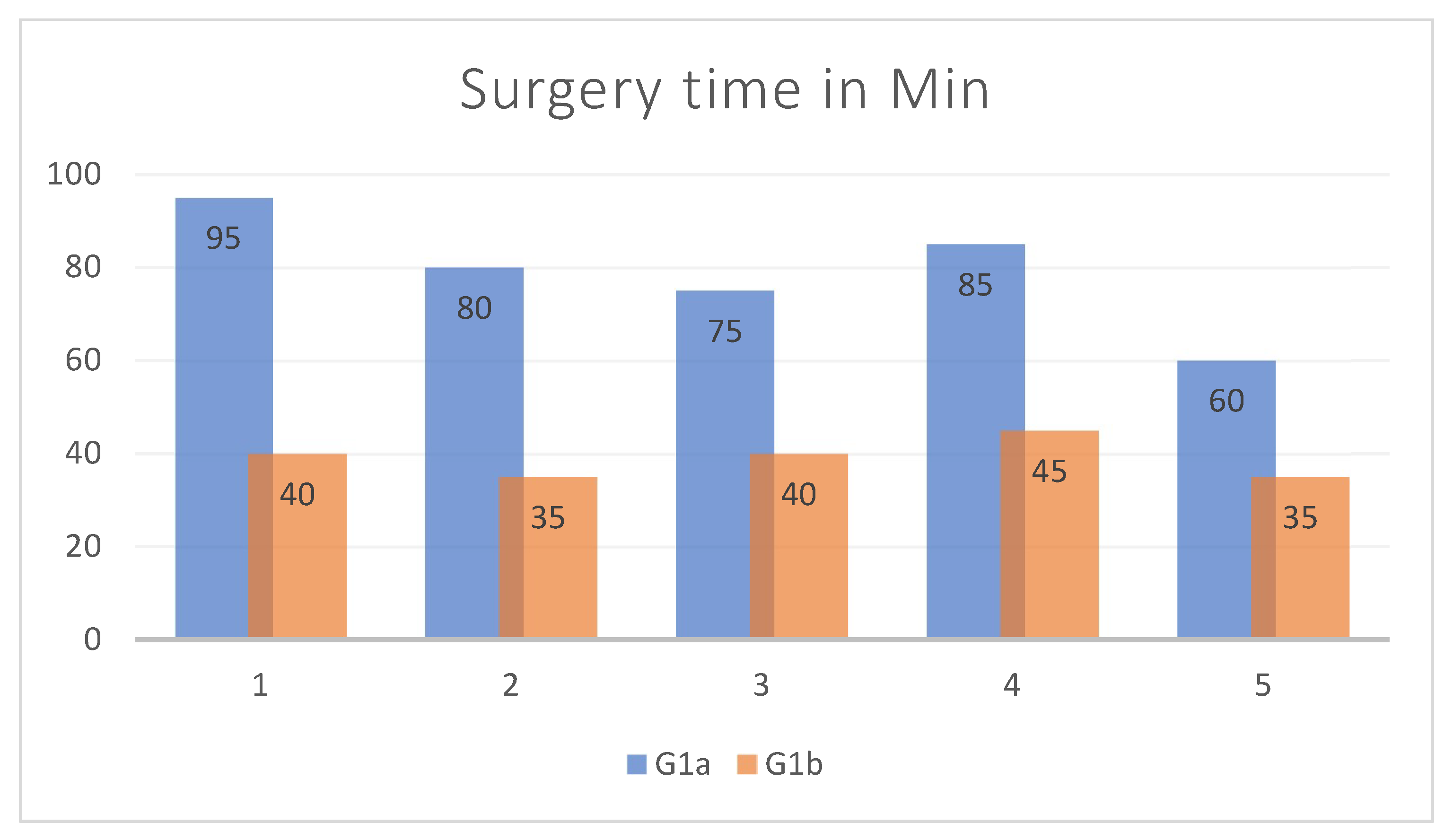
| Age | Sex | Type of Incidente | Fracture Side | Volume cm3 Right Orbit | Volume cm3 Left Orbit | Volume cm3 Mesh Orbit | Vol- cm3 Difference Pre-op | Vol- cm3 Difference Post-op | Vol- cm3 Recovery Difference | Surgery Time in Min | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 33 | Male | aggression | Right | 29.0359 | 25.5777 | 23.5814 | 3.4582 | 5.4545 | −1.9963 | 95 |
| 2 | 59 | Male | aggression | Right | 23.9741 | 21.2927 | 20.5365 | 2.6814 | 3.4376 | −0.7562 | 80 |
| 3 | 37 | Male | car accident | Left | 23.204 | 27.6138 | 24.7729 | 4.4098 | 2.8409 | 1.5689 | 75 |
| 4 | 74 | Male | accidental fall | Left | 25.2797 | 26.4807 | 24.145 | 1.201 | 2.3357 | −1.1347 | 85 |
| 5 | 41 | Male | aggression | Left | 25.9815 | 27.0248 | 24.6445 | 1.0433 | 2.3803 | −1.337 | 60 |
| Age | Sex | Type of Incidente | Fracture Side | Volume cm3 Right Orbit | Volume cm3 Left Orbit | Volume cm3 Mesh Orbit | Vol- cm3 Difference Pre-op | Vol- cm3 Difference Post-op | Vol- cm3 Recovery Difference | Surgery Time in Min | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 19 | Male | sports trauma | Right | 27.6954 | 24.8297 | 25.1231 | 2.8657 | 2.5723 | 0.2934 | 40 |
| 2 | 72 | Male | car accident | Left | 24.0521 | 28.3124 | 23.5679 | 4.2603 | 4.7445 | −0.4842 | 35 |
| 3 | 37 | Male | aggression | Right | 21.9546 | 26.0827 | 21.7928 | 4.1281 | 4.2899 | −0.1618 | 40 |
| 4 | 30 | Male | sports trauma | Left | 21.7904 | 24.7852 | 22.2585 | 2.9948 | 2.5267 | 0.4681 | 45 |
| 5 | 19 | Male | sports trauma | Left | 31.149 | 32.1745 | 30.4723 | 1.0255 | 1.7022 | −0.6767 | 35 |
| Age | Sex | Volume cm3 Right Orbit | Volume cm3 Left Orbit | Volume cm3 Difference | |
|---|---|---|---|---|---|
| 1 | 23 | Male | 24.3835 | 25.5133 | 1.1298 |
| 2 | 55 | Male | 24.2495 | 24.7679 | 0.5184 |
| 3 | 39 | Male | 24.4433 | 24.5261 | 0.0828 |
| 4 | 47 | Male | 20.2659 | 20.5763 | 0.0828 |
| 5 | 53 | Male | 23.5792 | 22.8334 | 0.7458 |
| 6 | 28 | Male | 22.7471 | 23.3860 | 0.6389 |
| 7 | 19 | Male | 27.9404 | 27.2011 | 0.7393 |
| 8 | 38 | Male | 23.0517 | 21.9158 | 1.1359 |
| 9 | 46 | Male | 23.5677 | 24.1943 | 0.6266 |
| 10 | 57 | Male | 28.6717 | 29.1944 | 0.5227 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kallaverja, E.; Barca, I.; Ferragina, F.; Cristofaro, M.G. Classical Orbital Floor Post-Traumatic Reconstruction vs. Customized Reconstruction with the Support of “In-House” 3D-Printed Models: A Retrospective Study with an Analysis of Volumetric Measurement. Diagnostics 2024, 14, 1248. https://doi.org/10.3390/diagnostics14121248
Kallaverja E, Barca I, Ferragina F, Cristofaro MG. Classical Orbital Floor Post-Traumatic Reconstruction vs. Customized Reconstruction with the Support of “In-House” 3D-Printed Models: A Retrospective Study with an Analysis of Volumetric Measurement. Diagnostics. 2024; 14(12):1248. https://doi.org/10.3390/diagnostics14121248
Chicago/Turabian StyleKallaverja, Elvis, Ida Barca, Francesco Ferragina, and Maria Giulia Cristofaro. 2024. "Classical Orbital Floor Post-Traumatic Reconstruction vs. Customized Reconstruction with the Support of “In-House” 3D-Printed Models: A Retrospective Study with an Analysis of Volumetric Measurement" Diagnostics 14, no. 12: 1248. https://doi.org/10.3390/diagnostics14121248







