Left Ventricular Papillary Muscle: Anatomy, Pathophysiology, and Multimodal Evaluation
Abstract
:1. Introduction
2. Normal Anatomy of the PM
2.1. PM Structure and Variation
2.2. Blood Supply of the PM
3. Physiologic Functions of the Papillary Muscle
4. Pathophysiology of Papillary Muscle Dysfunction
4.1. Ischemic Papillary Muscle Dysfunction
4.2. Nonischemic Papillary Muscle Dysfunction
5. Multimodal Image Evaluation of PM
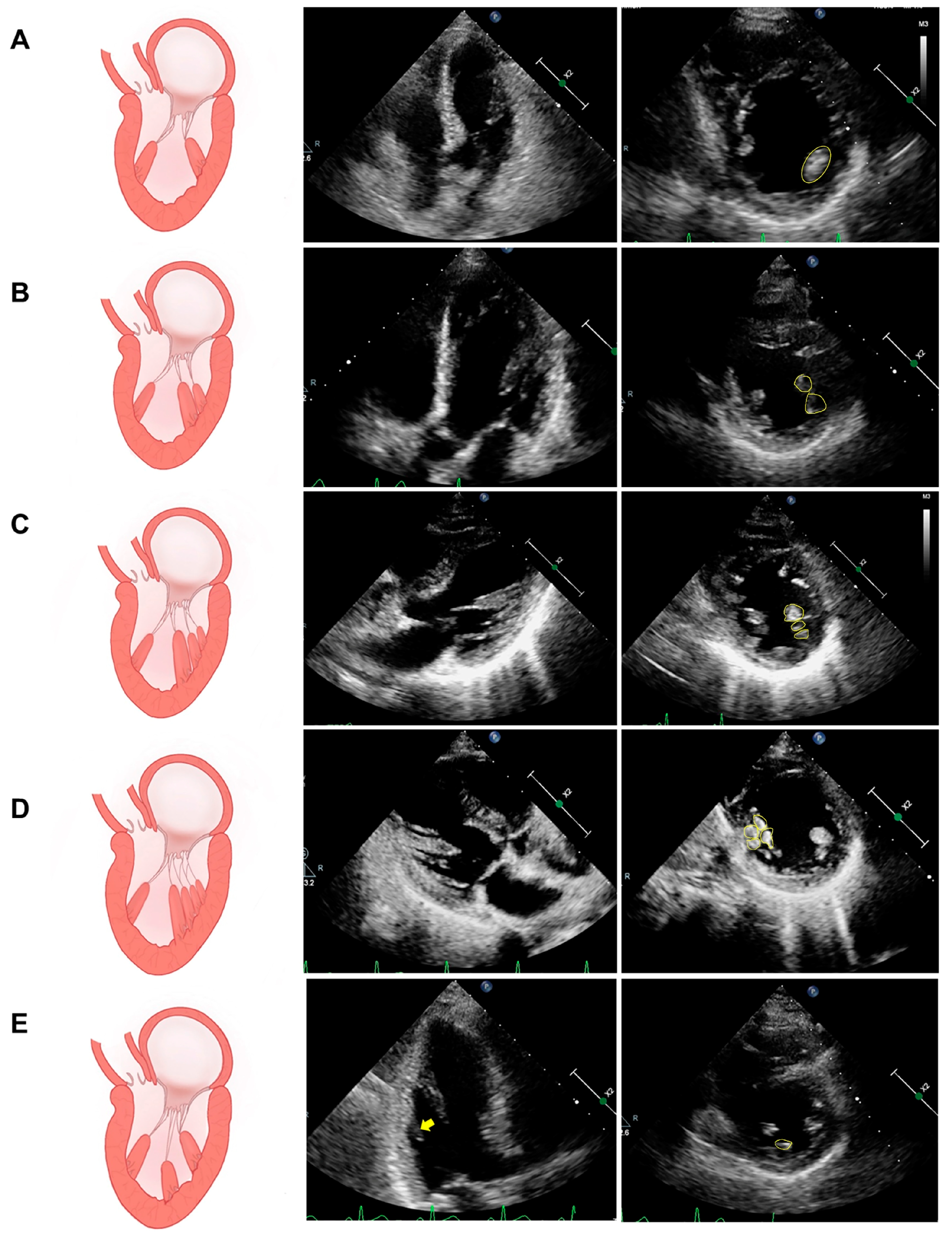
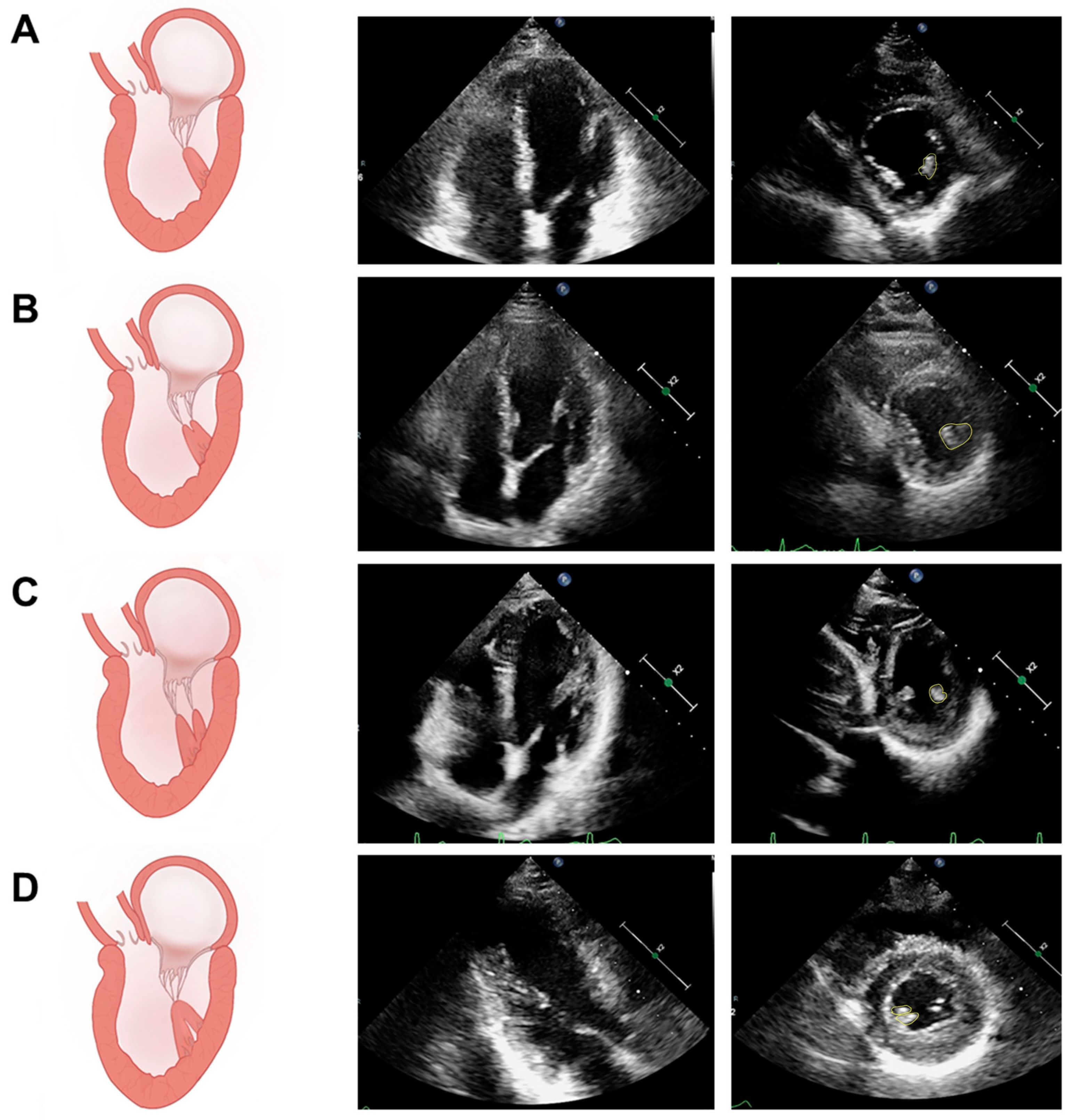
5.1. Assessment of PM Structure
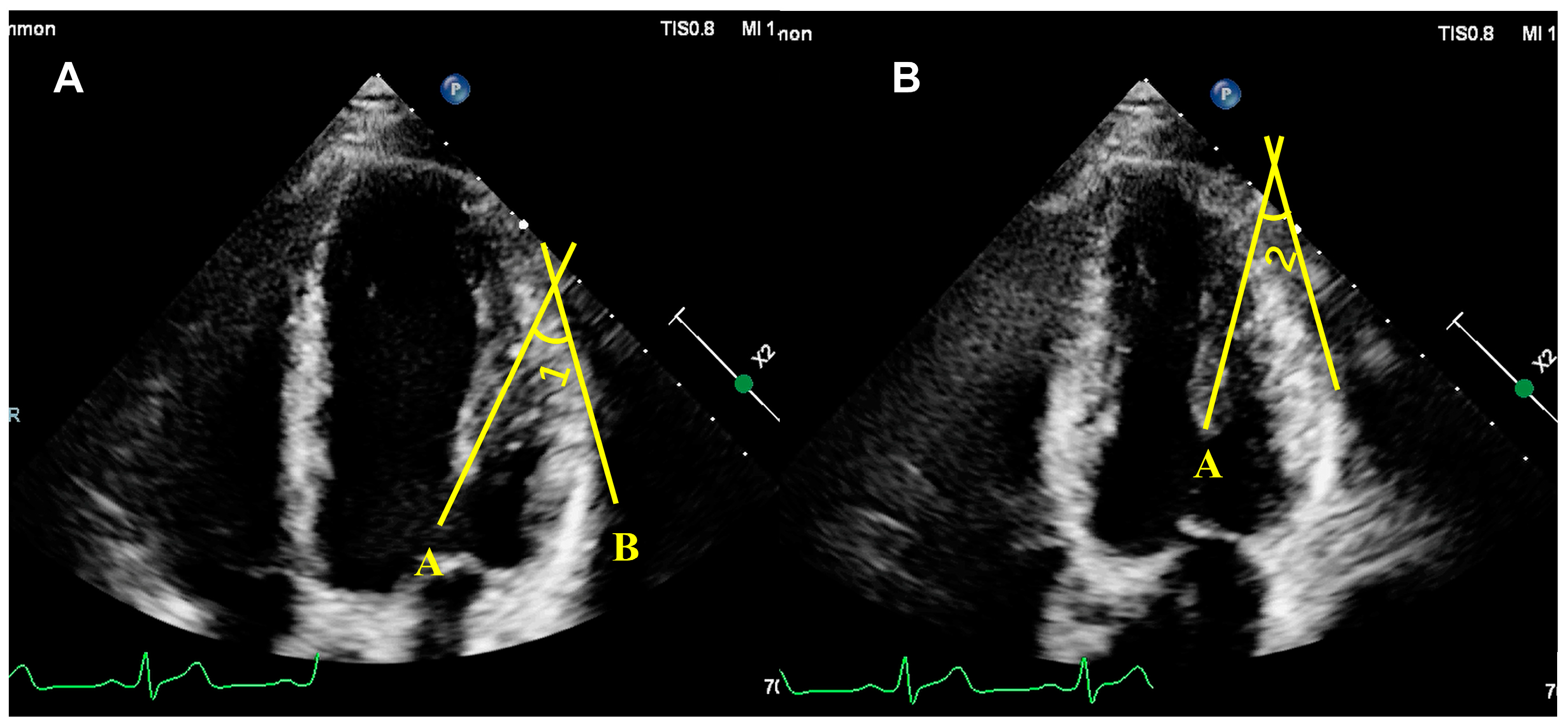
5.2. Assessment of PM Function
5.2.1. Qualitative Assessment of PM Function
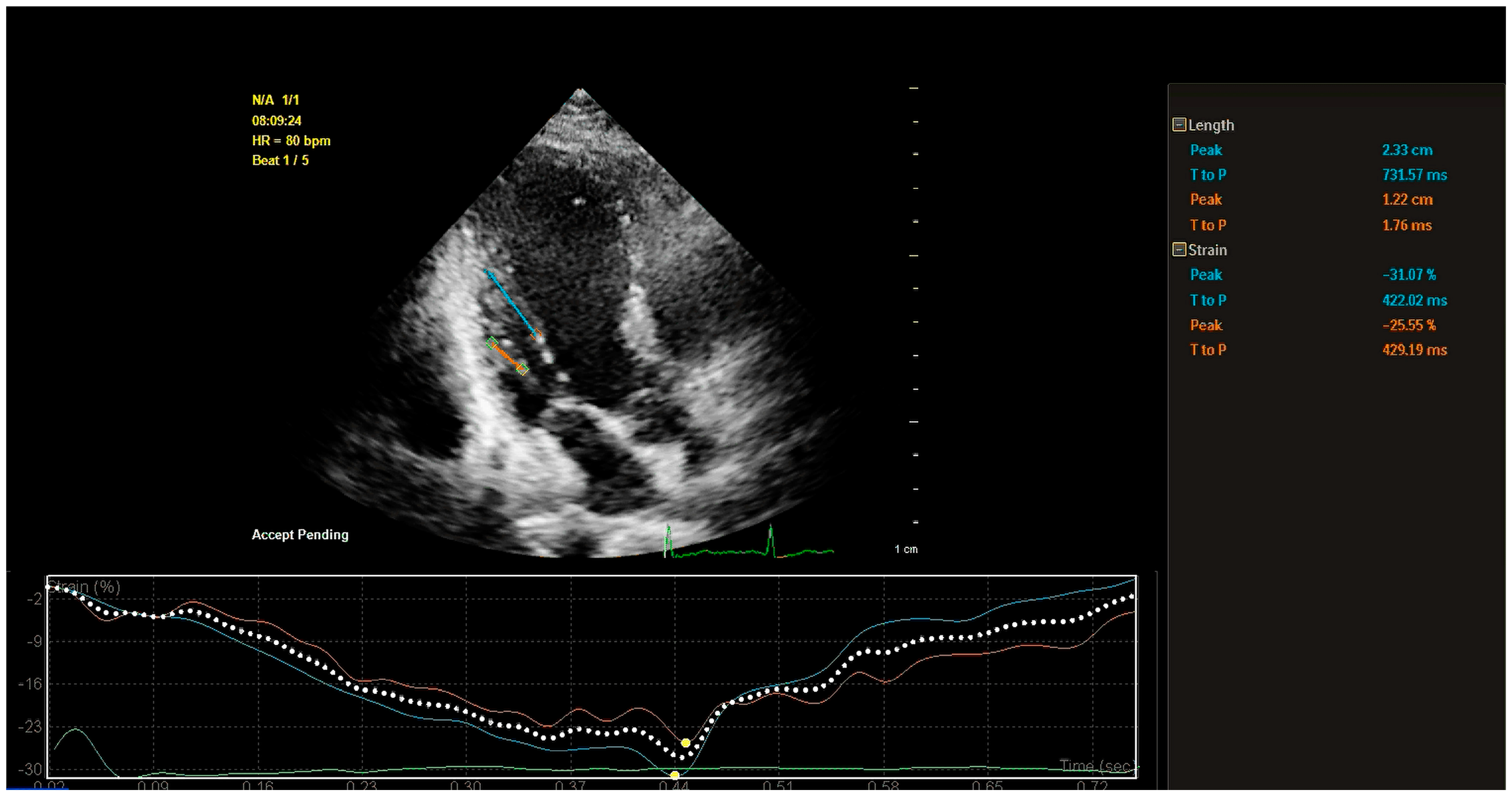
5.2.2. Quantitative Assessment of PM Function
| Aim of Study | Population | Groups | Index | Imaging Modality | Papers |
|---|---|---|---|---|---|
| assess the diagnostic performance of nT1 and PMls in the identification of iPPM. | 46 STEMI patients |
| PMls, nT1 | CMR | Pambianchi, G. et al., 2023 [40] |
| investigate whether IPMD can affect MR severity independently of PM tethering distance in patients with LV dysfunction | 83 patients with LV dysfunction (LVEF < 50%) |
| IPMD, PM tethering distance | volumetric multislice computed tomography | Kim, K. et al., 2014 [41] |
| test whether PM dysfunction attenuates ischemic MR in patients with LV remodeling | 40 patients with a piMI but without other lesions |
| PM tethering distance, PMls | 2D echocardiography + Tissue Doppler strain imaging | Uemura, T. et al. 2005 [54] |
| evaluate PMls in HCM and to find whether it has a value for prediction of SCD risk score | 55 HCM patients |
| PMls | 2D speckle tracking echocardiography | Koyuncu, A. et al., 2023 [55] |
| evaluate and compare PM function in and between patients with severe DMR and FMR | 64 patients with severe MR |
| PMls, circumferential strain | 2D speckle tracking echocardiography | Kilicgedik, A. et al., 2017 [56] |
| assess the PMls as a contributor to recurrent MR after mitral valve repair for fibroelastic deficiency | 64 patients with isolated posterior MVP and severe MR referred for surgery between 2008 and 2012 |
| PMls | 2D speckle tracking echocardiography | Grapsa, J. et al., 2015 [57] |
| assess PM and mitral valve structure and function in children and young adults with mild and moderate HCM | 20 HCM patients |
| Apical PM displacement index | 3D chocardiography | Joseph, N. et al., 2021 [46] |
| 524 consecutive patients who survived CABG and restrictive annuloplasty between 2001 and 2010 |
| DYS-PAP, peak times | 2D speckle tracking echocardiography | Van Garsse, L. et al., 2013 [58] | |
| find a relationship between the level of PM asynchrony and the degree of MR in patients with ICM and nICM | 31 patients underwent CAA with clinically advanced HF |
| DYS-PAP | Tissue Doppler strain imaging | Kordybach, M. et al., 2011 [59] |
| investigate the impact of impaired lateral shortening in the IPMD on mitral valve geometry and function in ischemic heart disease | 67 patients with ischemic heart disease confirmed by cardiac catheterization underwent CMR |
| IPMD | CMR | Kalra, K. et al., 2014 [60] |
| evaluate the relationship between papillary muscle T1 time and MR in DCM patients | 40 DCM patients and 20 healthy adults |
| nT1 | CMR+ | Kato, S. et al., 2016 [30] |
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- De Busk, R.F.; Harrison, D.C. The clinical spectrum of papillary-muscle disease. N. Engl. J. Med. 1969, 281, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.F.; Vaitenas, I.; Malaisrie, S.C.; Maganti, K. Mechanical Complications of Acute Myocardial Infarction: A Review. JAMA Cardiol. 2021, 6, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Lentz Carvalho, J.; Schaff, H.V.; Morris, C.S.; Nishimura, R.A.; Ommen, S.R.; Maleszewski, J.J.; Dearani, J.A. Anomalous papillary muscles-Implications in the surgical treatment of hypertrophic obstructive cardiomyopathy. J. Thorac. Cardiovasc. Surg. 2022, 163, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Roy, S. Papillary muscles of left ventricle-Morphological variations and it’s clinical relevance. Indian. Heart J. 2018, 70, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Rajiah, P.; Fulton, N.L.; Bolen, M. Magnetic resonance imaging of the papillary muscles of the left ventricle: Normal anatomy, variants, and abnormalities. Insights Imaging 2019, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Filomena, D.A.-O.X.; Vandenberk, B.; Dresselaers, T.; Willems, R.A.-O.; Van Cleemput, J.A.-O.; Olivotto, I.A.-O.; Robyns, T.A.-O.; Bogaert, J.A.-O. Apical papillary muscle displacement is a prevalent feature and a phenotypic precursor of apical hypertrophic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Kozor, R.; Nordin, S.; Treibel, T.A.; Rosmini, S.; Castelletti, S.; Fontana, M.; Captur, G.; Baig, S.; Steeds, R.P.; Hughes, D.; et al. Insight into hypertrophied hearts: A cardiovascular magnetic resonance study of papillary muscle mass and T1 mapping. Eur. Heart J. Cardiovasc. Imaging. 2017, 18, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Axel, L. Papillary muscles do not attach directly to the solid heart wall. Circulation 2004, 109, 3145–3148. [Google Scholar] [CrossRef] [PubMed]
- Gunnal, S.A.; Wabale, R.N.; Farooqui, M.S. Morphological variations of papillary muscles in the mitral valve complex in human cadaveric hearts. Singap. Med. J. 2013, 54, 44–48. [Google Scholar]
- Sato, T.; Moussa, I.D.; Hanna, P.A.-O.; Shivkumar, K.A.-O.; Mori, S.A.-O. Intermediate Accessory Papillary Muscle: An Anatomic Variant of Interest for Transcatheter Edge-to-Edge Mitral Valve Repair. Circ. Cardiovasc. Imaging 2023, 16, e015151. [Google Scholar] [CrossRef]
- Harvey Estes, E.H., Jr.; Dalton, F.M.; Entman, M.L.; Dixon, H.B., 2nd; Hackel, D.B. The anatomy and blood supply of the papillary muscles of the left ventricle. Am. Heart J. 1996, 71, 356–362. [Google Scholar] [CrossRef]
- Massimi, G.; Matteucci, M.; Kowalewski, M.; Ronco, D.; Jiritano, F.; Beghi, C.; Severgnini, P.; Lorusso, R. Surgical treatment of post-infarction papillary muscle rupture: Systematic review and meta-analysis. Ann. Cardiothorac. Surg. 2021, 11, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Wendell, D.A.-O.; Jenista, E.A.-O.; Kim, H.W.; Chen, E.L.; Azevedo, C.A.-O.; Kaolawanich, Y.A.-O.; Alenezi, F.A.-O.; Rehwald, W.A.-O.; Darty, S.; Parker, M.A.-O.; et al. Assessment of Papillary Muscle Infarction with Dark-Blood Delayed Enhancement Cardiac MRI in Canines and Humans. Radiology 2022, 305, 329–338. [Google Scholar] [CrossRef]
- Ranganathan, N.; Burch, G.E.; Burch, G.E. Gross morphology and arterial supply of the papillary muscles of the left ventricle of man. Am. Heart J. 1969, 71, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Levine, R.A.; Flachskampf, F.; Grayburn, P.; Gillam, L.; Leipsic, J.; Thomas, J.D.; Kwong, R.Y.; Vandervoort, P.; Chandrashekhar, Y. Atrial Functional Mitral Regurgitation: A JACC: Cardiovascular Imaging Expert Panel Viewpoint. JACC Cardiovasc. Imaging 2022, 15, 1870–1882. [Google Scholar] [CrossRef] [PubMed]
- Kagiyama, N.; Mondillo, S.; Yoshida, K.; Mandoli, G.E.; Cameli, M. Subtypes of Atrial Functional Mitral Regurgitation: Imaging Insights Into Their Mechanisms and Therapeutic Implications. JACC Cardiovasc. Imaging 2020, 13, 820–835. [Google Scholar] [CrossRef]
- Kim, D.H.; Heo, R.; Handschumacher, M.D.; Lee, S.; Choi, Y.S.; Kim, K.R.; Shin, Y.; Park, H.K.; Bischoff, J.; Aikawa, E.; et al. Mitral Valve Adaptation to Isolated Annular Dilation: Insights Into the Mechanism of Atrial Functional Mitral Regurgitation. JACC Cardiovasc. Imaging 2019, 12, 665–677. [Google Scholar] [CrossRef]
- Deferm, S.; Bertrand, P.B.; Verhaert, D.; Verbrugge, F.H.; Dauw, J.; Thoelen, K.; Giesen, A.; Bruckers, L.; Rega, F.; Thomas, J.D.; et al. Mitral Annular Dynamics in AF Versus Sinus Rhythm: Novel Insights Into the Mechanism of AFMR. JACC Cardiovasc. Imaging 2022, 15, 1–13. [Google Scholar] [CrossRef]
- Chuang, M.L.; Gona, P.; Hautvast, G.L.T.F.; Hautvast, G.L.T.F.; Salton, C.J.; Salton, C.J.; Blease, S.J.; Blease, S.J.; Yeon, S.B.; Yeon, S.B.; et al. Correlation of trabeculae and papillary muscles with clinical and cardiac characteristics and impact on CMR measures of LV anatomy and function. JACC Cardiovasc. Imaging 2012, 5, 1115–1123. [Google Scholar] [CrossRef]
- Kozor, R.; Callaghan, F.; Tchan, M.; Hamilton-Craig, C.; Figtree, G.A.; Grieve, S.M. A disproportionate contribution of papillary muscles and trabeculations to total left ventricular mass makes choice of cardiovascular magnetic resonance analysis technique critical in Fabry disease. J. Cardiovasc. Magn. Reson. 2015, 17, 22. [Google Scholar] [CrossRef]
- Watanabe, N. Acute mitral regurgitation. Heart 2019, 105, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Hennessey, B.; Sabatovicz, N., Jr.; Del Trigo, M. Acute Ischaemic Mitral Valve Regurgitation. J. Clin. Med. 2022, 11, 5526. [Google Scholar] [CrossRef] [PubMed]
- Eitel, I.; Gehmlich, D.; Amer, O.; Wohrle, J.; Kerber, S.; Lauer, B.; Pauschinger, M.; Schwab, J.; Birkemeyer, R.; Zimmermann, R.; et al. Prognostic relevance of papillary muscle infarction in reperfused infarction as visualized by cardiovascular magnetic resonance. Circ. Cardiovasc. Imaging 2013, 6, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, T.; Imanishi, T.; Kitabata, H.; Nakamura, N.; Kimura, K.; Yamano, T.; Ishibashi, K.; Komukai, K.; Ino, Y.; Takarada, S.; et al. Prevalence and Clinical Significance of Papillary Muscle Infarction Detected by Late Gadolinium-Enhanced Magnetic Resonance Imaging in Patients With ST-Segment Elevation Myocardial Infarction. Circulation 2010, 122, 2281–2287. [Google Scholar] [CrossRef] [PubMed]
- Kochav, J.D.; Kim, J.; Judd, R.; Kim, H.W.; Klem, I.; Heitner, J.; Shah, D.; Shenoy, C.; Farzaneh-Far, A.; Polsani, V.; et al. Ischemia-Mediated Dysfunction in Subpapillary Myocardium as a Marker of Functional Mitral Regurgitation. JACC Cardiovasc. Imaging 2021, 14, 826–839. [Google Scholar] [CrossRef] [PubMed]
- Messas, E.; Guerrero, J.L.; Handschumacher, M.D.; Chow, C.M.; Sullivan, S.; Schwammenthal, E.; Levine, R.A. Paradoxic Decrease in Ischemic Mitral Regurgitation With Papillary Muscle Dysfunction. Circulation 2001, 104, 1952–1957. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fang, L.; Wu, W.; Zhang, Z.; Ji, L.; Sun, Z.; He, L.; Wang, Z.; Fu, W.; Li, F.; et al. Echocardiographic diagnosis of rupture of mitral valve papillary muscle. Int. J. Cardiol. 2023, 391, 131273. [Google Scholar] [CrossRef]
- Damluji, A.A.; van Diepen, S.; Katz, J.N.; Menon, V.; Tamis-Holland, J.E.; Bakitas, M.; Cohen, M.G.; Balsam, L.B.; Chikwe, J. Mechanical Complications of Acute Myocardial Infarction: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e16–e35. [Google Scholar] [CrossRef]
- Hamid, U.I.; Aksoy, R.; Sardari Nia, P. Mitral valve repair in papillary muscle rupture. Ann. Cardiothorac. Surg. 2022, 11, 281–289. [Google Scholar] [CrossRef]
- Kato, S.; Nakamori, S.; Roujol, S.; Delling, F.N.; Akhtari, S.; Jang, J.; Basha, T.; Berg, S.; Kissinger, K.V.; Goddu, B.; et al. Relationship between native papillary muscle T(1) time and severity of functional mitral regurgitation in patients with non-ischemic dilated cardiomyopathy. J. Cardiovasc. Magn. Reson. 2016, 18, 79. [Google Scholar] [CrossRef]
- Junker, A.; Thayssen, P.; Nielsen, B.; Andersen, P.E. The Hemodynamic and Prognostic Significance of Echo-Doppler-Proven Mitral Regurgitation in Patients with Dilated Cardiomyopathy. Cardiology 2008, 83, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Otsuji, Y.; Handschumacher, M.D.; Schwammenthal, E.; Jiang, L.; Song, J.-K.; Guerrero, J.L.; Vlahakes, G.J.; Levine, R.A. Insights From Three-Dimensional Echocardiography Into the Mechanism of Functional Mitral Regurgitation. Circulation 1997, 96, 1999–2008. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Desai, M.Y.; Nishimura, R.A.; Spirito, P.; Rakowski, H.; Towbin, J.A.; Rowin, E.J.; Maron, M.S.; Sherrid, M.V. Diagnosis and Evaluation of Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2022, 79, 372–389. [Google Scholar] [CrossRef] [PubMed]
- Silbiger, J.J. Abnormalities of the Mitral Apparatus in Hypertrophic Cardiomyopathy: Echocardiographic, Pathophysiologic, and Surgical Insights. J. Am. Soc. Echocardiogr. 2016, 29, 622–639. [Google Scholar] [CrossRef] [PubMed]
- Halpern, D.G.; Swistel, D.G.; Po, J.R.; Joshi, R.; Winson, G.; Arabadjian, M.; Lopresto, C.; Kushner, J.; Kim, B.; Balaram, S.K.; et al. Echocardiography before and after resect-plicate-release surgical myectomy for obstructive hypertrophic cardiomyopathy. J. Am. Soc. Echocardiogr. 2015, 28, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Nampiaparampil, R.G.; Swistel, D.G.; Schlame, M.; Saric, M.; Sherrid, M.V. Intraoperative Two- and Three-Dimensional Transesophageal Echocardiography in Combined Myectomy-Mitral Operations for Hypertrophic Cardiomyopathy. J. Am. Soc. Echocardiogr. 2018, 31, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Bartko, P.E.; Arfsten, H.; Heitzinger, G.; Pavo, N.; Strunk, G.; Gwechenberger, M.; Hengstenberg, C.; Binder, T.; H Hülsmann, M.; Goliasch, G. Papillary Muscle Dyssynchrony-Mediated Functional Mitral Regurgitation: Mechanistic Insights and Modulation by Cardiac Resynchronization. JACC Cardiovasc. Imaging 2019, 12, 1728–1737. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, H.; Bazaz, R.; Schwartzman, D.; Dohi, K.; Sade, L.E.; Gorcsan, J. A mechanism for immediate reduction in mitral regurgitation after cardiac resynchronization therapy: Insights from mechanical activation strain mapping. J. Am. Coll. Cardiol. 2004, 44, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Ypenburg, C.; Lancellotti, P.; Tops, L.F.; Bleeker, G.B.; Holman, E.R.; Pirard, L.A.; Schalij, M.J.; Bax, J.J. Acute Effects of Initiation and Withdrawal of Cardiac Resynchronization Therapy on Papillary Muscle Dyssynchrony and Mitral Regurgitation. J. Am. Coll. Cardiol. 2007, 50, 2071–2077. [Google Scholar] [CrossRef]
- Pambianchi, G.; Giannetti, M.; Marchitelli, L.; Cundari, G.; Maestrini, V.; Mancone, M.; Francone, M.; Catalano, C.; Galea, N. Papillary Muscle Involvement during Acute Myocardial Infarction: Detection by Cardiovascular Magnetic Resonance Using T1 Mapping Technique and Papillary Longitudinal Strain. J. Clin. Med. 2023, 12, 1497. [Google Scholar] [CrossRef]
- Kim, K.; Kaji, S.; An, Y.; Nishino, T.; Tani, T.; Kitai, T.; Furukawa, Y. Interpapillary muscle distance independently affects severity of functional mitral regurgitation in patients with systolic left ventricular dysfunction. J. Thorac. Cardiovasc. Surg. 2014, 148, 434–440.e1. [Google Scholar] [CrossRef] [PubMed]
- Shudo, Y.; Matsumiya, G.; Sakaguchi, T.; Miyagawa, S.; Yoshikawa, Y.; Yamauchi, T.; Takeda, K.; Saito, S.; Nakatani, S.; Taniguchi, K.; et al. Assessment of Changes in Mitral Valve Configuration With Multidetector Computed Tomography. Circulation 2010, 122 (Suppl. S11), S29–S36. [Google Scholar] [CrossRef] [PubMed]
- Nakao, R.; Nagao, M.; Yamamoto, A.; Fukushima, K.; Watanabe, E.; Sakai, S.; Hagiwara, N. Papillary muscle ischemia on high-resolution cine imaging of nitrogen-13 ammonia positron emission tomography: Association with myocardial flow reserve and prognosis in coronary artery disease. J. Nucl. Cardiol. 2022, 29, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Sonny, A.; Sale, S.; Smedira, N.G. Abnormalities of Mitral Subvalvular Apparatus in Hypertrophic Cardiomyopathy: Role of Intraoperative 3D Transesophageal Echocardiography. Anesth. Analg. 2016, 123, 1094–1097. [Google Scholar] [CrossRef] [PubMed]
- Bothe, W.; Timek, T.A.; Tibayan, F.A.; Walther, M.; Daughters, G.T.; Ingels, N.B.; Miller, D.C. Characterization of 3-dimensional papillary muscle displacement in in vivo ovine models of ischemic/functional mitral regurgitation. J. Thorac. Cardiovasc. Surgery. 2019, 157, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Joseph, N.; Craft, M.; Mill, L.; Erickson, C.C.; Danford, D.A.; Kutty, S.; Li, L. Assessment of mitral valve function in children and young adults with hypertrophic cardiomyopathy using three-dimensional echocardiography. Int. J. Cardiol. 2021, 332, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Vogel-Claussen, J.; Finn, J.P.; Gomes, A.S.; Gomes, A.S.; Hundley, G.W.; Hundley, G.W.; Jerosch-Herold, M.; Pearson, G.; Sinha, S.; Lima, J.A.C.; et al. Left ventricular papillary muscle mass: Relationship to left ventricular mass and volumes by magnetic resonance imaging. J. Comput. Assist. Tomogr. 2006, 30, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Moura-Ferreira, S.; Vandenberk, B.; Masci, P.G.; Dresselaers, T.; Garweg, C.; Symons, R.; Willems, R.; Bogaert, J. Left ventricular remodelling in mitral valve prolapse patients: Implications of apical papillary muscle insertion. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Dhillon, A.; Popovic, Z.B.; Smedira, N.G.; Rizzo, J.; Thamilarasan, M.; Agler, D.; Lytle, B.W.; Lever, H.M.; Desai, M.Y. Left Ventricular Outflow Tract Obstruction in Hypertrophic Cardiomyopathy Patients Without Severe Septal Hypertrophy. Circ. Cardiovasc. Imaging 2015, 8, e003132. [Google Scholar] [CrossRef]
- Wagner, A.; Mahrholdt, H.; Holly, T.A.; Elliott, M.D.; Regenfus, M.; Parker, M.; Klocke, F.J.; Bonow, R.O.; Kim, R.J.; Judd, R.M. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: An imaging study. Lancet 2003, 361, 374–379. [Google Scholar] [CrossRef]
- Holtackers, R.J.; Van De Heyning, C.M.; Chiribiri, A.; Wildberger, J.E.; Botnar, R.M.; Kooi, M.E. Dark-blood late gadolinium enhancement cardiovascular magnetic resonance for improved detection of subendocardial scar: A review of current techniques. J. Cardiovasc. Magn. Reson. 2021, 23, 96. [Google Scholar] [CrossRef]
- Kim, H.W.; Rehwald, W.G.; Jenista, E.R.; Wendell, D.C.; Filev, P.; van Assche, L.; Jensen, C.J.; Parker, M.A.; Chen, E.-l.; Crowley, A.L.C.; et al. Dark-Blood Delayed Enhancement Cardiac Magnetic Resonance of Myocardial Infarction. JACC Cardiovasc. Imaging 2018, 11, 1758–1769. [Google Scholar] [CrossRef] [PubMed]
- Smiseth, O.A.; Torp, H.; Opdahl, A.; Haugaa, K.H.; Urheim, S. Myocardial strain imaging: How useful is it in clinical decision making? Eur. Heart J. 2016, 37, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Otsuji, Y.; Nakashiki, K.; Yoshifuku, S.; Maki, Y.; Yu, B.; Mizukami, N.; Kuwahara, E.; Hamasaki, S.; Biro, S.; et al. Papillary muscle dysfunction attenuates ischemic mitral regurgitation in patients with localized basal inferior left ventricular remodeling: Insights from tissue Doppler strain imaging. J. Am. Coll. Cardiol. 2005, 46, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Koyuncu, A.; Yildiz, C.; Ocal, L.; Kalkan, S.; Kilicgedik, A.; Gursoy, M.O.; Oflar, E.; Kahveci, G. Does papillary muscle free strain has predictive value in risk stratification of patients with hypertrophic cardiomyopathy? PLoS ONE 2023, 18, e0282054. [Google Scholar] [CrossRef]
- Kilicgedik, A.; Kahveci, G.; Gurbuz, A.S.; Karabay, C.Y.; Guler, A.; Efe, S.C.; Aung, S.M.; Arslantas, U.; Demir, S.; Izgi, I.A.; et al. Papillary Muscle Free Strain in Patients with Severe Degenerative and Functional Mitral Regurgitation. Arq. Bras. Cardiol. 2017, 108, 339–346. [Google Scholar]
- Grapsa, J.; Zimbarra Cabrita, I.; Jakaj, G.; Ntalarizou, E.; Serapheim, A.; Demir, O.M.; Smith, B.; Dawson, D.; Momin, A.; Punjabi, P.P.; et al. Strain balance of papillary muscles as a prerequisite for successful mitral valve repair in patients with mitral valve prolapse due to fibroelastic deficiency. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 53–61. [Google Scholar] [CrossRef]
- van Garsse, L.; Gelsomino, S.; Lucà, F.; Parise, O.; Lorusso, R.; Cheriex, E.; Caciolli, S.; Vizzardi, E.; Rao, C.M.; Carella, R.; et al. Left ventricular dyssynchrony is associated with recurrence of ischemic mitral regurgitation after restrictive annuloplasty. Int. J. Cardiol. 2013, 168, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Kordybach, M.; Kowalski, M.; Kowalik, E.; Hoffman, P. Papillary muscle dyssynchrony in patients with systolic left ventricular dysfunction. Scand. Cardiovasc. J. 2011, 46, 16–22. [Google Scholar] [CrossRef]
- Kalra, K.; Wang, Q.; McIver, B.V.; Shi, W.; Guyton, R.A.; Sun, W.; Sarin, E.L.; Thourani, V.H.; Padala, M. Temporal changes in interpapillary muscle dynamics as an active indicator of mitral valve and left ventricular interaction in ischemic mitral regurgitation. J. Am. Coll. Cardiol. 2014, 64, 1867–1879. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Wang, Z.; Fu, W.; Li, F.; Gu, H.; Cui, N.; Lin, Y.; Xie, M.; Yang, Y. Left Ventricular Papillary Muscle: Anatomy, Pathophysiology, and Multimodal Evaluation. Diagnostics 2024, 14, 1270. https://doi.org/10.3390/diagnostics14121270
Li S, Wang Z, Fu W, Li F, Gu H, Cui N, Lin Y, Xie M, Yang Y. Left Ventricular Papillary Muscle: Anatomy, Pathophysiology, and Multimodal Evaluation. Diagnostics. 2024; 14(12):1270. https://doi.org/10.3390/diagnostics14121270
Chicago/Turabian StyleLi, Shiying, Zhen Wang, Wenpei Fu, Fangya Li, Hui Gu, Nan Cui, Yixia Lin, Mingxing Xie, and Yali Yang. 2024. "Left Ventricular Papillary Muscle: Anatomy, Pathophysiology, and Multimodal Evaluation" Diagnostics 14, no. 12: 1270. https://doi.org/10.3390/diagnostics14121270





