MRI-Derived Dural Sac and Lumbar Vertebrae 3D Volumetry Has Potential for Detection of Marfan Syndrome
Abstract
:1. Introduction
2. Materials and Methods
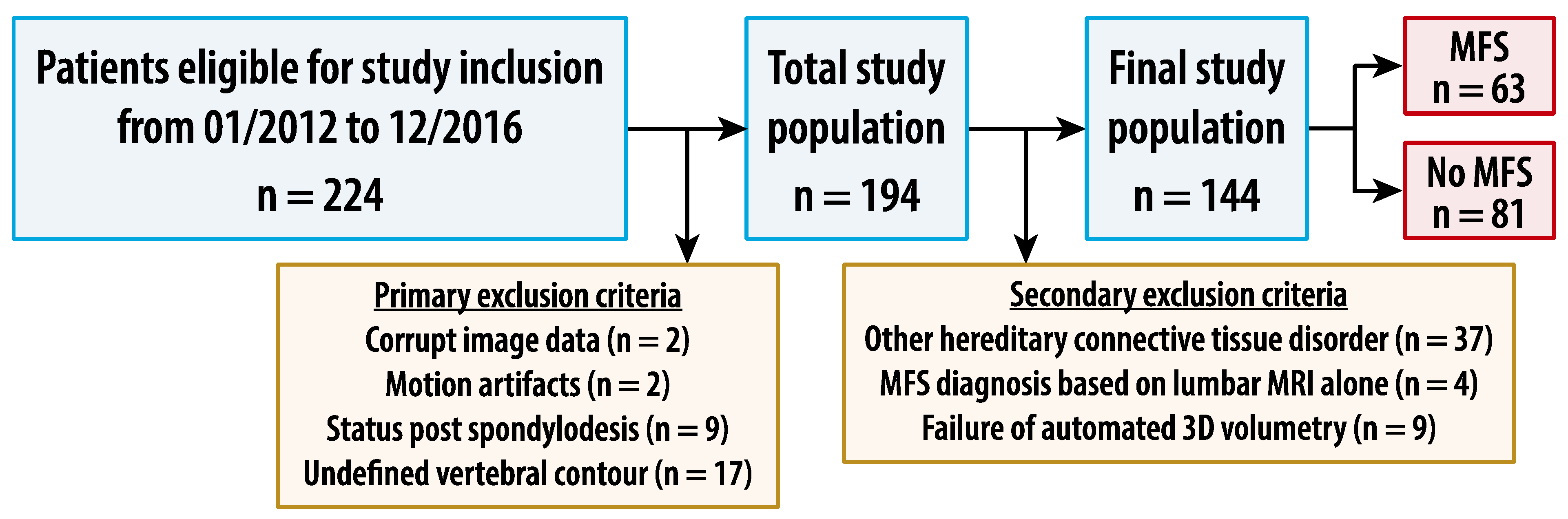
2.1. Patients
2.2. Image Acquisition
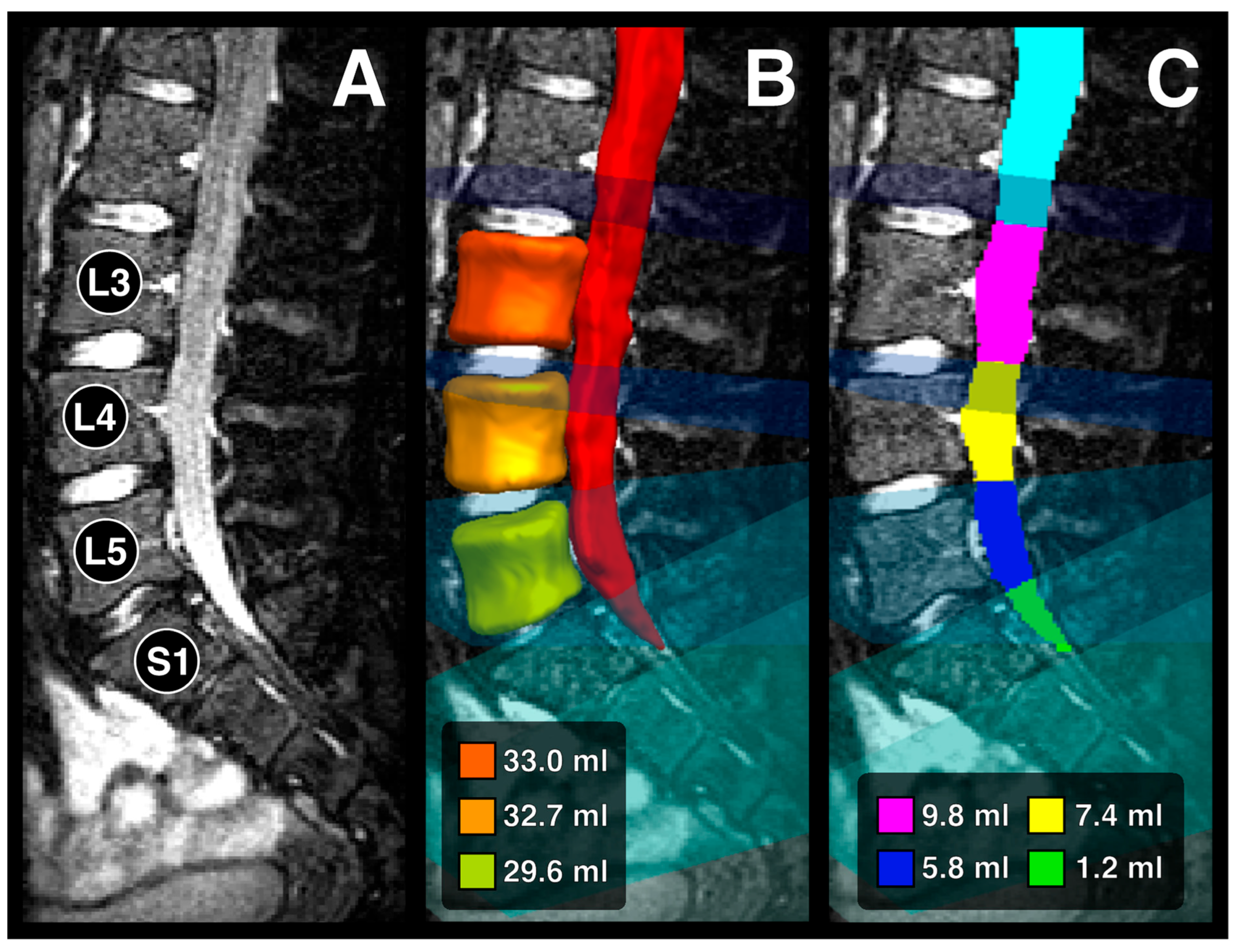
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Study Sample
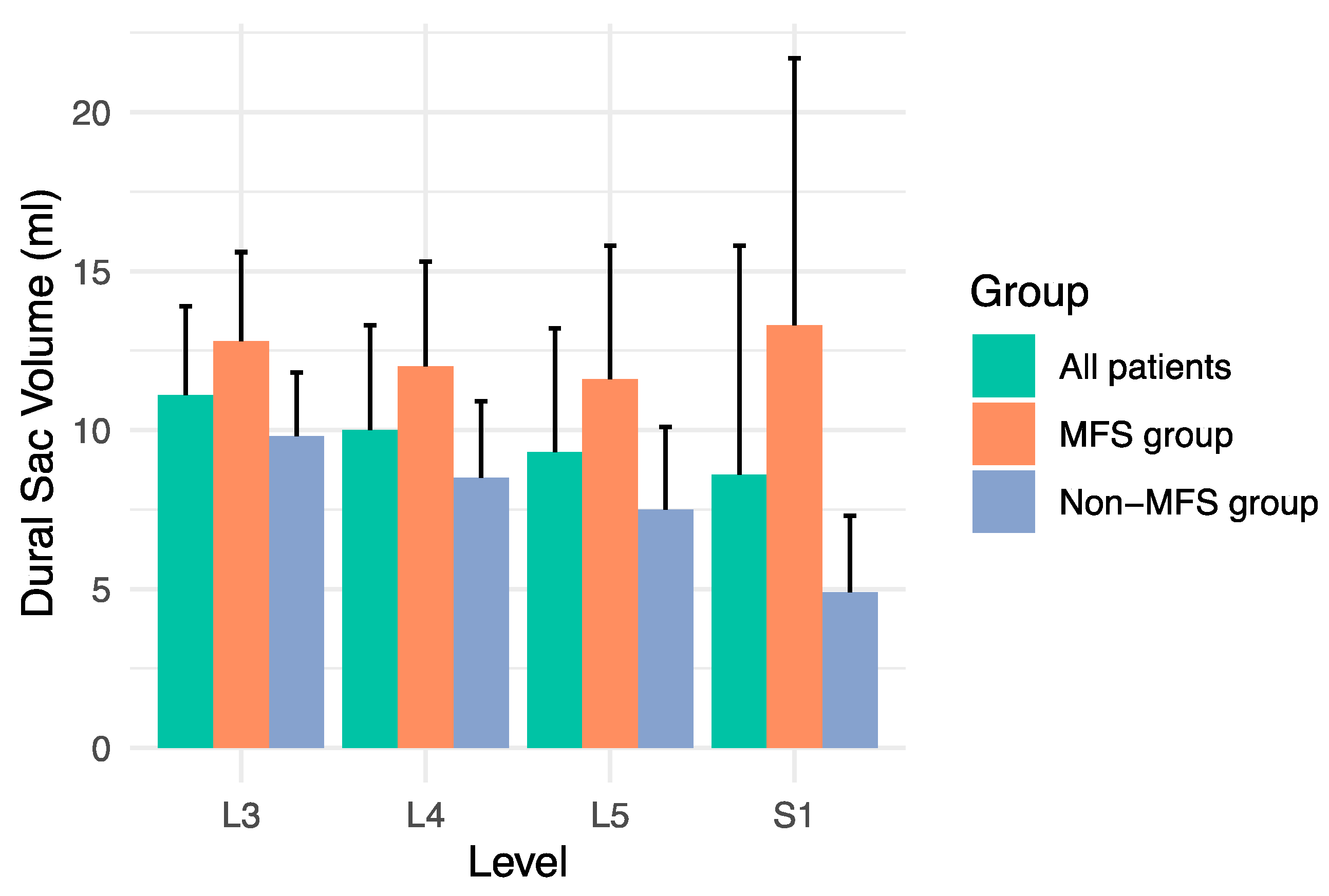
3.2. Volume Measurements
3.3. Diameter Measurements
3.4. Identification of Marfan Syndrome Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McKusick, V.A. The cardiovascular aspects of Marfan’s syndrome: A heritable disorder of connective tissue. Circulation 1955, 11, 321–342. [Google Scholar] [CrossRef] [PubMed]
- Weve, H. Über Arachnodaktylie (Dystrophia mesodermalis congenita, Typus Marfan). Arch. Augenheilk. 1931, 104, 1–46. [Google Scholar]
- Gray, J.R.; Bridges, A.B.; Faed, M.J.; Pringle, T.; Baines, P.; Dean, J.; Boxer, M. Ascertainment and severity of Marfan syndrome in a Scottish population. J. Med. Genet. 1994, 31, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Pyeritz, R.E. The Marfan syndrome. Am. Fam. Physician 1986, 34, 83–94. [Google Scholar] [PubMed]
- Marfan, A.B.J. Un Cas de Déformation Congénitale des Quatre Membres, Plus Prononcée aux Extrémités, Caractérisée par L’allongement des Os avec un Certain Degré D’amincissement; Impr. Maretheux: Paris, France, 1896. [Google Scholar]
- Dietz, H.C.; Cutting, G.R.; Pyeritz, R.E.; Maslen, C.L.; Sakai, L.Y.; Corson, G.M.; Puffenberger, E.G.; Hamosh, A.; Nanthakumar, E.J.; Curristin, S.M.; et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature 1991, 352, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Dietz, H.C.; Pyeritz, R.E.; Hall, B.D.; Cadle, R.G.; Hamosh, A.; Schwartz, J.; Meyers, D.A.; Francomano, C.A. The Marfan syndrome locus: Confirmation of assignment to chromosome 15 and identification of tightly linked markers at 15q15-q21.3. Genomics 1991, 9, 355–361. [Google Scholar] [CrossRef]
- Sakai, L.Y.; Keene, D.R.; Engvall, E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J. Cell Biol. 1986, 103, 2499–2509. [Google Scholar] [CrossRef] [PubMed]
- Pyeritz, R.E. Pleiotropy revisited: Molecular explanations of a classic concept. Am. J. Med. Genet. 1989, 34, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Lipscomb, K.J.; Clayton-Smith, J.; Harris, R. Evolving phenotype of Marfan’s syndrome. Arch. Dis. Child. 1997, 76, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, J.L.; Walker, B.A.; Halpern, B.L.; Kuzma, J.W.; McKusick, V.A. Life expectancy and causes of death in the Marfan syndrome. N. Engl. J. Med. 1972, 286, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Pyeritz, R.E. Etiology and pathogenesis of the Marfan syndrome: Current understanding. Ann. Cardiothorac. Surg. 2017, 6, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Kallenbach, K.; Remes, A.; Müller, O.J.; Arif, R.; Zaradzki, M.; Wagner, A.H. Translational Medicine: Towards Gene Therapy of Marfan Syndrome. J. Clin. Med. 2022, 11, 3934. [Google Scholar] [CrossRef] [PubMed]
- Gott, V.L.; Cameron, D.E.; Pyeritz, R.E.; Gillinov, A.M.; Greene, P.S.; Stone, C.D.; Alejo, D.E.; McKusick, V.A. Composite graft repair of Marfan aneurysm of the ascending aorta: Results in 150 patients. J. Card. Surg. 1994, 9, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Svensson, L.G.; Crawford, E.S.; Coselli, J.S.; Safi, H.J.; Hess, K.R. Impact of cardiovascular operation on survival in the Marfan patient. Circulation 1989, 80, I233–I242. [Google Scholar] [PubMed]
- Child, A.; Stuart, A.G.; Aragon-Martin, J.A.; Yuan, L.; Hu, J.; Van Dyck, L.; Knight, R.; Clayton, T.; Flather, M.; Dean, J.; et al. Irbesartan in Marfan syndrome (AIMS): A double-blind, placebo-controlled randomised trial. Lancet (Br. Ed.) 2019, 394, 2263–2270. [Google Scholar]
- Shores, J.; Berger, K.R.; Murphy, E.A.; Pyeritz, R.E. Progression of aortic dilatation and the benefit of long-term beta-adrenergic blockade in Marfan’s syndrome. N. Engl. J. Med. 1994, 330, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Loeys, B.L.; Dietz, H.C.; Braverman, A.C.; Callewaert, B.L.; De Backer, J.; Devereux, R.B.; Hilhorst-Hofstee, Y.; Jondeau, G.; Faivre, L.; Milewicz, D.M.; et al. The revised Ghent nosology for the Marfan syndrome. J. Med. Genet. 2010, 47, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Baer, R.W.; Taussig, H.B.; Oppenheimer, E.H. Congenital aneurysmal dilatation of the aorta associated with arachnodactyly. Bull. Johns Hopkins Hosp. 1943, 72, 309–331. [Google Scholar]
- Etter, L.E.; Glover, L.P. Arachnodactyly complicated by dislocated lens and death from rupture of dissecting aneurysm of aorta. JAMA 1943, 123, 88–89. [Google Scholar] [CrossRef]
- Borger, F. Über zwei Fälle von Arachnodaktylie. Z. Kinderheilkd. 1914, 12, 161–184. [Google Scholar] [CrossRef]
- Devereux, R.B.; de Simone, G.; Arnett, D.K.; Best, L.G.; Boerwinkle, E.; Howard, B.V.; Kitzman, D.; Lee, E.T.; Mosley, T.H., Jr.; Weder, A.; et al. Normal limits in relation to age, body size and gender of two-dimensional echocardiographic aortic root dimensions in persons ≥15 years of age. Am. J. Cardiol. 2012, 110, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Sheikhzadeh, S.; Sondermann, C.; Rybczynski, M.; Habermann, C.R.; Brockstaedt, L.; Keyser, B.; Kaemmerer, H.; Mir, T.; Staebler, A.; Robinson, P.N.; et al. Comprehensive analysis of dural ectasia in 150 patients with a causative FBN1 mutation. Clin. Genet. 2014, 86, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Mesfin, A.; Ahn, N.U.; Carrino, J.A.; Sponseller, P.D. Ten-year clinical and imaging follow-up of dural ectasia in adults with Marfan syndrome. Spine J. 2013, 13, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Pyeritz, R.E.; Fishman, E.K.; Bernhardt, B.A.; Siegelman, S.S. Dural ectasia is a common feature of the Marfan syndrome. Am. J. Hum. Genet. 1988, 43, 726–732. [Google Scholar] [PubMed]
- Ahn, N.U.; Sponseller, P.D.; Ahn, U.M.; Nallamshetty, L.; Kuszyk, B.S.; Zinreich, S.J. Dural ectasia is associated with back pain in Marfan syndrome. Spine 2000, 25, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Foran, J.R.; Pyeritz, R.E.; Dietz, H.C.; Sponseller, P.D. Characterization of the symptoms associated with dural ectasia in the Marfan patient. Am. J. Med. Genet. A 2005, 134A, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Begley, K.; Sergides, Y. Giant sacral dural ectasia causing ureteric obstruction in Marfan syndrome. ANZ J. Surg. 2022, 92, 1930. [Google Scholar] [CrossRef] [PubMed]
- Fattori, R.; Nienaber, C.A.; Descovich, B.; Ambrosetto, P.; Reggiani, L.B.; Pepe, G.; Kaufmann, U.; Negrini, E.; von Kodolitsch, Y.; Gensini, G.F. Importance of dural ectasia in phenotypic assessment of Marfan’s syndrome. Lancet 1999, 354, 910–913. [Google Scholar] [CrossRef]
- Attanasio, M.; Pratelli, E.; Porciani, M.C.; Evangelisti, L.; Torricelli, E.; Pellicano, G.; Abbate, R.; Gensini, G.F.; Pepe, G. Dural ectasia and FBN1 mutation screening of 40 patients with Marfan syndrome and related disorders: Role of dural ectasia for the diagnosis. Eur. J. Med. Genet. 2013, 56, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Ahn, N.U.; Sponseller, P.D.; Ahn, U.M.; Nallamshetty, L.; Rose, P.S.; Buchowski, J.M.; Garrett, E.S.; Kuszyk, B.S.; Fishman, E.K.; Zinreich, S.J. Dural ectasia in the Marfan syndrome: MR and CT findings and criteria. Genet. Med. 2000, 2, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Habermann, C.R.; Weiss, F.; Schoder, V.; Cramer, M.C.; Kemper, J.; Wittkugel, O.; Adam, G. MR evaluation of dural ectasia in Marfan syndrome: Reassessment of the established criteria in children, adolescents, and young adults. Radiology 2005, 234, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Lundby, R.; Rand-Hendriksen, S.; Hald, J.K.; Lilleas, F.G.; Pripp, A.H.; Skaar, S.; Paus, B.; Geiran, O.; Smith, H.J. Dural ectasia in Marfan syndrome: A case control study. Am. J. Neuroradiol. 2009, 30, 1534–1540. [Google Scholar] [CrossRef] [PubMed]
- Oosterhof, T.; Groenink, M.; Hulsmans, F.J.; Mulder, B.J.; van der Wall, E.E.; Smit, R.; Hennekam, R.C. Quantitative assessment of dural ectasia as a marker for Marfan syndrome. Radiology 2001, 220, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Soylen, B.; Hinz, K.; Prokein, J.; Becker, H.; Schmidtke, J.; Arslan-Kirchner, M. Performance of a new quantitative method for assessing dural ectasia in patients with FBN1 mutations and clinical features of Marfan syndrome. Neuroradiology 2009, 51, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Villeirs, G.M.; Van Tongerloo, A.J.; Verstraete, K.L.; Kunnen, M.F.; De Paepe, A.M. Widening of the spinal canal and dural ectasia in Marfan’s syndrome: Assessment by CT. Neuroradiology 1999, 41, 850–854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.S.; Schelb, P.; Kohl, S.; Radtke, J.P.; Wiesenfarth, M.; Schimmoller, L.; Kuder, T.A.; Stenzinger, A.; Hohenfellner, M.; Schlemmer, H.-P.; et al. Improvement of PI-RADS-dependent prostate cancer classification by quantitative image assessment using radiomics or mean ADC. Magn. Reson. Imaging 2021, 82, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Wennmann, M.; Bauer, F.; Klein, A.; Chmelik, J.; Grözinger, M.; Rotkopf, L.T.; Neher, P.; Gnirs, R.; Kurz, F.T.; Nonnenmacher, T.; et al. In vivo repeatability and multiscanner reproducibility of MRI radiomics features in patients with monoclonal plasma cell disorders: A prospective bi-institutional study. Investig. Radiol. 2023, 58, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Heimann, T.; Meinzer, H.P. Statistical shape models for 3D medical image segmentation: A review. Med. Image Anal. 2009, 13, 543–563. [Google Scholar] [CrossRef] [PubMed]
- Norajitra, T.; Engelhardt, S.; Held, T.; Al-Maisary, S.; de Simone, R.; Meinzer, H.-P.; Maier-Hein, K. Statistische 3D-Formmodelle mit verteilter Erscheinungsmodellierung. In Bildverarbeitung für die Medizin 2016; Springer: Berlin/Heidelberg, Germany, 2016; pp. 56–61. [Google Scholar]
- Kainmüller, D.; Lange, T.; Lamecker, H. Shape constrained automatic segmentation of the liver based on a heuristic intensity model. In Proceedings of the MICCAI Workshop 3D Segmentation in the Clinic, Brisbane, Australia, 29 October 2007; pp. 109–116. [Google Scholar]
- Ginneken, B.V.; Frangi, A.F.; Staal, J.J. Active shape model segmentation with optimal features. IEEE Trans. Med. Imaging 2002, 21, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Norajitra, T.; Meinzer, H.P.; Maier-Hein, K.H. 3D statistical shape models incorporating random regression forest voting for multi-organ segmentation. In Proceedings of the MICCAI 2015, Munich, Germany, 5–9 October 2015. [Google Scholar]
- Norajitra, T.; Meinzer, H.-P.; Maier-Hein, K. 3D Regression Voting on CT-Volumes of the Human Liver for SSM Surface Appearance Modeling. In Proceedings of the Shape 2014—Symposium on Statistical Shape Models and Applications, Delémont Switzerland, 11–13 June 2014. [Google Scholar]
- Norajitra, T.; Meinzer, H.-P.; Maier-Hein, K. 3D statistical shape models incorporating 3D random forest regression voting for robust CT liver segmentation. In Proceedings of the SPIE Medical Imaging, Orlando, FL, USA, 21–26 February 2015; p. 941406. [Google Scholar]
- Liu, X.; Song, L.; Liu, S.; Zhang, Y. A Review of Deep-Learning-Based Medical Image Segmentation Methods. Sustainability 2021, 13, 1224. [Google Scholar] [CrossRef]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. ImageNet classification with deep convolutional neural networks. Commun. ACM 2012, 60, 84–90. [Google Scholar] [CrossRef]
- Long, J.; Shelhamer, E.; Darrell, T. Fully convolutional networks for semantic segmentation. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Boston, MA, USA, 7–12 June 2015; pp. 3431–3440. [Google Scholar]
- Ronneberger, O.; Fischer, P.; Brox, T. U-net: Convolutional networks for biomedical image segmentation. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention—MICCAI 2015: 18th International Conference, Munich, Germany, 5–9 October 2015; pp. 234–241. [Google Scholar]
- Isensee, F.; Jaeger, P.F.; Kohl, S.A.; Petersen, J.; Maier-Hein, K.H. nnU-Net: A self-configuring method for deep learning-based biomedical image segmentation. Nature methods 2021, 18, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Rayed, M.E.; Islam, S.S.; Niha, S.I.; Jim, J.R.; Kabir, M.M.; Mridha, M. Deep learning for medical image segmentation: State-of-the-art advancements and challenges. Inform. Med. Unlocked 2024, 47, 101504. [Google Scholar] [CrossRef]
- Isensee, F.; Petersen, J.; Klein, A.; Zimmerer, D.; Jaeger, P.F.; Kohl, S.; Wasserthal, J.; Koehler, G.; Norajitra, T.; Wirkert, S. nnu-net: Self-adapting framework for u-net-based medical image segmentation. arXiv 2018, arXiv:1809.10486. [Google Scholar]
- van der Graaf, J.W.; van Hooff, M.L.; Buckens, C.F.; Rutten, M.; van Susante, J.L.; Kroeze, R.J.; de Kleuver, M.; van Ginneken, B.; Lessmann, N. Lumbar spine segmentation in MR images: A dataset and a public benchmark. Sci. Data 2024, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Hohenhaus, M.; Klingler, J.-H.; Scholz, C.; Watzlawick, R.; Hubbe, U.; Beck, J.; Reisert, M.; Würtemberger, U.; Kremers, N.; Wolf, K. Quantification of cervical spinal stenosis by automated 3D MRI segmentation of spinal cord and cerebrospinal fluid space. Spinal Cord 2024, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Seo, J.; Lee, J.H.; Jeon, E.-T.; Jeong, D.; Chae, H.D.; Lee, E.; Kang, J.H.; Choi, Y.-H.; Kim, H.J. Automated Detection and Segmentation of Bone Metastases on Spine MRI Using U-Net: A Multicenter Study. Korean J. Radiol. 2024, 25, 363. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, Y.; Wang, K.; Li, J.; Zhang, X.; Zhang, Y.; Li, J.; Wang, X. A quantitative evaluation of the deep learning model of segmentation and measurement of cervical spine MRI in healthy adults. J. Appl. Clin. Med. Phys. 2024, 25, e14282. [Google Scholar] [CrossRef] [PubMed]
- Rak, M.; Steffen, J.; Meyer, A.; Hansen, C.; Tönnies, K.D. Combining convolutional neural networks and star convex cuts for fast whole spine vertebra segmentation in MRI. Comput. Methods Programs Biomed. 2019, 177, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Kolarik, M.; Burget, R.; Riha, K.; Bartusek, K. Suitability of CT and MRI Imaging for Automatic Spine Segmentation Using Deep Learning. In Proceedings of the 2021 44th International Conference on Telecommunications and Signal Processing (TSP), Brno, Czech Republic, 26–28 July 2021; pp. 390–393. [Google Scholar]
- Li, H.; Luo, H.; Huan, W.; Shi, Z.; Yan, C.; Wang, L.; Mu, Y.; Liu, Y. Automatic lumbar spinal MRI image segmentation with a multi-scale attention network. Neural Comput. Appl. 2021, 33, 11589–11602. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jiang, Z.; Yang, H.; Li, X.; Yang, Z. Automatic segmentation of lumbar spine MRI images based on improved attention U-net. Comput. Intell. Neurosci. 2022, 2022, 4259471. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Xu, Q.; Yang, C.; Lu, Y.; Ge, C.; Wang, Z.; Liu, K.; Qiu, X.; Chang, S. Spine MRI image segmentation method based on ASPP and U-Net network. Math. Biosci. Eng. 2023, 20, 15999–16014. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Li, Q.; Li, X.; Zhang, M. An optimized segmentation convolutional neural network with dynamic energy loss function for 3D reconstruction of lumbar spine MR images. Comput. Biol. Med. 2023, 160, 106839. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qin, J.; Lv, L.; Cheng, M.; Wang, B.; Xia, D.; Wang, S. ICUnet++: An Inception-CBAM network based on Unet++ for MR spine image segmentation. Int. J. Mach. Learn. Cybern. 2023, 14, 3671–3683. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, D.; Du Bois, E.F. Clinical Calorimetry: Tenth Paper a Formula to Estimate the Approximate Surface Area If Height and Weight Be Known. Arch. Intern. Med. 1916, XVII, 863–871. [Google Scholar] [CrossRef]
- Norajitra, T.; Maier-Hein, K.H. 3D Statistical Shape Models Incorporating Landmark-Wise Random Regression Forests for Omni-Directional Landmark Detection. IEEE Trans. Med. Imaging 2017, 36, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Böker, T.; Vanem, T.T.; Pripp, A.H.; Rand-Hendriksen, S.; Paus, B.; Smith, H.-J.; Lundby, R. Dural ectasia in Marfan syndrome and other hereditary connective tissue disorders: A 10-year follow-up study. Spine J. 2019, 19, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Rand-Hendriksen, S.; Lundby, R.; Tjeldhorn, L.; Andersen, K.; Offstad, J.; Semb, S.O.; Smith, H.J.; Paus, B.; Geiran, O. Prevalence data on all Ghent features in a cross-sectional study of 87 adults with proven Marfan syndrome. Eur. J. Hum. Genet. 2009, 17, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Sznajder, M.; Krug, P.; Taylor, M.; Moura, B.; Leparc, J.M.; Boileau, C.; Jondeau, G.; Chevallier, B.; Pelage, J.P.; Stheneur, C. Spinal imaging contributes to the diagnosis of Marfan syndrome. Jt. Bone Spine 2010, 77, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Weigang, E.; Ghanem, N.; Chang, X.C.; Richter, H.; Frydrychowicz, A.; Szabo, G.; Dudeck, O.; Knirsch, W.; von Samson, P.; Langer, M.; et al. Evaluation of three different measurement methods for dural ectasia in Marfan syndrome. Clin. Radiol. 2006, 61, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Knirsch, W.; Kurtz, C.; Haffner, N.; Binz, G.; Heim, P.; Winkler, P.; Baumgartner, D.; Freund-Unsinn, K.; Stern, H.; Kaemmerer, H.; et al. Dural ectasia in children with Marfan syndrome: A prospective, multicenter, patient-control study. Am. J. Med. Genet. A 2006, 140, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Huang, Y.; Chen, H.; Liu, Y.; Liang, R.; Zeng, Q. Computed Tomography-Based Radiomic Features for Diagnosis of Indeterminate Small Pulmonary Nodules. J. Comput. Assist. Tomogr. 2020, 44, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Dong, D.; Chen, B.; Fang, M.; Cheng, Y.; Gan, Y.; Zhang, R.; Zhang, L.; Zang, Y.; Liu, Z.; et al. Diagnosis of Distant Metastasis of Lung Cancer: Based on Clinical and Radiomic Features. Transl. Oncol. 2018, 11, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Debelee, T.G. Skin Lesion Classification and Detection Using Machine Learning Techniques: A Systematic Review. Diagnostics 2023, 13, 3147. [Google Scholar] [CrossRef] [PubMed]
- Stabile, A.M.; Pistilli, A.; Mariangela, R.; Rende, M.; Bartolini, D.; Di Sante, G. New Challenges for Anatomists in the Era of Omics. Diagnostics 2023, 13, 2963. [Google Scholar] [CrossRef] [PubMed]

| Non-MFS Group (n = 81) | MFS Group (n = 63) | p-Value | |
|---|---|---|---|
| Sex | 0.97 | ||
| Female | 46 (57%) | 36 (57%) | |
| Male | 35 (43%) | 27 (43%) | |
| Age (years) | 36 ± 16 | 35 ± 11 | 0.81 |
| Height (cm) | 182.0 ± 9.8 | 187.8 ± 10.6 | 0.002 |
| Weight (kg) | 70.8 ± 15.5 | 79.4 ± 13.7 | 0.002 |
| BSA (m2) | 1.90 ± 0.22 | 2.05 ± 0.21 | <0.001 |
| BMI (kg/m2) | 21.3 ± 4.1 | 22.5 ± 3.2 | 0.07 |
| Aortic root diameter (cm) | 3.3 ± 0.6 | 4.2 ± 0.7 | <0.001 |
| Z score | 1.6 ± 1.7 | 3.7 ± 2.5 | <0.001 |
| Volume Ratio | Diameter Ratio | p-Value | |
|---|---|---|---|
| L1 | - | 0.603 (0.506, 0.700) | - |
| L2 | - | 0.636 (0.545, 0.728) | - |
| L3 | 0.743 (0.659, 0.828) | 0.673 (0.582, 0.764) | <0.001 |
| L4 | 0.752 (0.670, 0.834) | 0.707 (0.619, 0.795) | 0.12 |
| L5 | 0.808 (0.730, 0.885) | 0.791 (0.713, 0.870) | 0.30 |
| S1 | 0.824 (0.746, 0.901) | 0.848 (0.775, 0.922) | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naas, O.; Norajitra, T.; Lückerath, C.; Fink, M.A.; Maier-Hein, K.; Kauczor, H.-U.; Rengier, F. MRI-Derived Dural Sac and Lumbar Vertebrae 3D Volumetry Has Potential for Detection of Marfan Syndrome. Diagnostics 2024, 14, 1301. https://doi.org/10.3390/diagnostics14121301
Naas O, Norajitra T, Lückerath C, Fink MA, Maier-Hein K, Kauczor H-U, Rengier F. MRI-Derived Dural Sac and Lumbar Vertebrae 3D Volumetry Has Potential for Detection of Marfan Syndrome. Diagnostics. 2024; 14(12):1301. https://doi.org/10.3390/diagnostics14121301
Chicago/Turabian StyleNaas, Omar, Tobias Norajitra, Christian Lückerath, Matthias A. Fink, Klaus Maier-Hein, Hans-Ulrich Kauczor, and Fabian Rengier. 2024. "MRI-Derived Dural Sac and Lumbar Vertebrae 3D Volumetry Has Potential for Detection of Marfan Syndrome" Diagnostics 14, no. 12: 1301. https://doi.org/10.3390/diagnostics14121301





