Direct Single-Operator Cholangioscopy and Intraductal Ultrasonography in Patients with Indeterminate Biliary Strictures: A Single Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Definitions
2.2. Statistical Analysis
3. Results
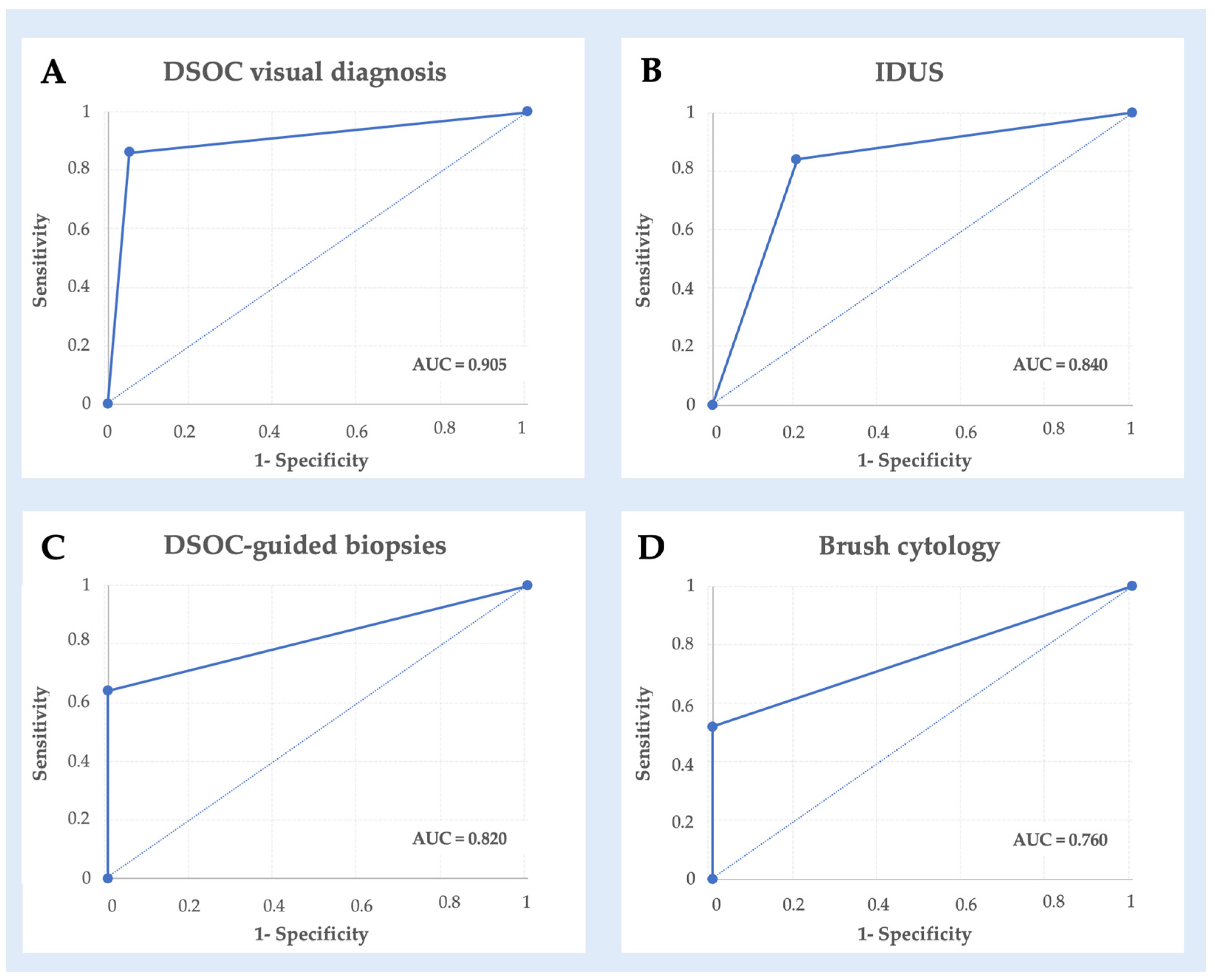
3.1. Diagnostic Performance
3.2. Secondary Aim: Effect of Previous Stenting
3.3. Secondary Aim: Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, C.; Aloreidi, K.; Patel, B.; Ridgway, T.; Thambi-Pillai, T.; Timmerman, G.; Khan, A.; Atiq, M. Indeterminate biliary strictures: A simplified approach. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 189–199. [Google Scholar] [CrossRef]
- Gerhards, M.F.; Vos, P.; van Gulik, T.M.; Rauws, E.A.J.; Bosma, A.; Gouma, D.J. Incidence of benign lesions in patients resected for suspicious hilar obstruction. Br. J. Surg. 2002, 88, 48–51. [Google Scholar] [CrossRef]
- Dalal, A.; Gandhi, C.; Patil, G.; Kamat, N.; Vora, S.; Maydeo, A. Safety and efficacy of different techniques in difficult biliary cannulation at endoscopic retrograde cholangiopancreatography. Hosp. Pract. 1995, 50, 61–67. [Google Scholar] [CrossRef]
- de Clemente Junior, C.C.; Bernardo, W.M.; Franzini, T.P.; Luz, G.O.; Dos Santos, M.E.L.; Cohen, J.M.; de Moura, D.T.H.; Marinho, F.R.T.; Coronel, M.; Sakai, P.; et al. Comparison between endoscopic sphincterotomy vs endoscopic sphincterotomy associated with balloon dilation for removal of bile duct stones: A systematic review and meta-analysis based on randomized controlled trials. World J. Gastrointest. Endosc. 2018, 10, 130–144. [Google Scholar] [CrossRef]
- Goyal, H.; Sachdeva, S.; Sherazi, S.A.A.; Gupta, S.; Perisetti, A.; Ali, A.; Chandan, S.; Tharian, B.; Sharma, N.; Thosani, N. Early prediction of post-ERCP pancreatitis by post-procedure amylase and lipase levels: A systematic review and meta-analysis. Endosc. Int. Open 2022, 10, E952–E970. [Google Scholar] [CrossRef] [PubMed]
- Cirocchi, R.; Kelly, M.D.; Griffiths, E.A.; Tabola, R.; Sartelli, M.; Carlini, L.; Ghersi, S.; Di Saverio, S. A systematic review of the management and outcome of ERCP related duodenal perforations using a standardized classification system. Surgeon 2017, 15, 379–387. [Google Scholar] [CrossRef]
- Almadi, M.A.; Itoi, T.; Moon, J.H.; Goenka, M.K.; Seo, D.W.; Rerknimitr, R.; Lau, J.Y.; Maydeo, A.P.; Lee, J.K.; Nguyen, N.Q.; et al. Using single-operator cholangioscopy for endoscopic evaluation of indeterminate biliary strictures: Results from a large multinational registry. Endoscopy 2020, 52, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Hu, B. The role of intraductal ultrasonography in pancreatobiliary diseases. Endosc. Ultrasound. 2016, 5, 291. [Google Scholar] [CrossRef]
- Farrell, R.J.; Agarwal, B.; Brandwein, S.L.; Underhill, J.; Chuttani, R.; Pleskow, D.K. Intraductal US is a useful adjunct to ERCP for distinguishing malignant from benign biliary strictures. Gastrointest. Endosc. 2002, 56, a128918. [Google Scholar] [CrossRef]
- De Angelis, C.G.; Dall’Amico, E.; Staiano, M.T.; Gesualdo, M.; Bruno, M.; Gaia, S.; Sacco, M.; Fimiano, F.; Mauriello, A.; Dibitetto, S.; et al. The Endoscopic Retrograde Cholangiopancreatography and Endoscopic Ultrasound Connection: Unity Is Strength, or the Endoscopic Ultrasonography Retrograde Cholangiopancreatography Concept. Diagnostics 2023, 13, 3265. [Google Scholar] [CrossRef]
- Lenze, F.; Bokemeyer, A.; Gross, D.; Nowacki, T.; Bettenworth, D.; Ullerich, H. Safety, diagnostic accuracy and therapeutic efficacy of digital single-operator cholangioscopy. United Eur. Gastroenterol. J. 2018, 6, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Urban, O.; Evinová, E.; Fojtík, P.; Loveček, M.; Kliment, M.; Zoundjiekpon, V.; Falt, P. Digital cholangioscopy: The diagnostic yield and impact on management of patients with biliary stricture. Scand. J. Gastroenterol. 2018, 53, 1364–1367. [Google Scholar] [CrossRef] [PubMed]
- Cotton, P.B.; Eisen, G.M.; Aabakken, L.; Baron, T.H.; Hutter, M.M.; Jacobson, B.C.; Mergener, K.; Nemcek, A.; Petersen, B.T.; Petrini, J.L.; et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest. Endosc. 2010, 71, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Novikov, A.; Kowalski, T.E.; Loren, D.E. Practical Management of Indeterminate Biliary Strictures. Gastrointest. Endosc. Clin. N. Am. 2019, 29, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Angsuwatcharakon, P.; Kulpatcharapong, S.; Moon, J.H.; Ramchandani, M.; Lau, J.; Isayama, H.; Seo, D.W.; Maydeo, A.; Wang, H.P.; Nakai, Y.; et al. Consensus guidelines on the role of cholangioscopy to diagnose indeterminate biliary stricture. HPB 2022, 24, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Korc, P.; Sherman, S. ERCP tissue sampling. Gastrointest. Endosc. 2016, 84, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Fugazza, A.; Gabbiadini, R.; Tringali, A.; De Angelis, C.G.; Mosca, P.; Maurano, A.; Di Mitri, R.; Manno, M.; Mariani, A.; Cereatti, F.; et al. Digital single-operator cholangioscopy in diagnostic and therapeutic bilio-pancreatic diseases: A prospective, multicenter study. Dig. Liver Dis. 2022, 54, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Onoyama, T.; Hamamoto, W.; Sakamoto, Y.; Kawahara, S.; Yamashita, T.; Koda, H.; Kawata, S.; Takeda, Y.; Matsumoto, K.; Isomoto, H.; et al. Peroral cholangioscopy-guided forceps biopsy versus fluoroscopy-guided forceps biopsy for extrahepatic biliary lesions. JGH Open 2020, 4, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Baars, J.E.; Keegan, M.; Bonnichsen, M.H.; Aepli, P.; Theyventhiran, R.; Farrell, E.; Kench, J.G.; Saxena, P.; Kaffes, A.J. The ideal technique for processing SpyBite tissue specimens: A prospective, single-blinded, pilot-study of histology and cytology techniques. Endosc. Int. Open 2019, 7, E1241–E1247. [Google Scholar] [CrossRef]
- Polkowski, M.; Jenssen, C.; Kaye, P.; Carrara, S.; Deprez, P.; Gines, A.; Fernández-Esparrach, G.; Eisendrath, P.; Aithal, G.P.; Arcidiacono, P.; et al. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline—March 2017. Endoscopy 2017, 49, 989–1006. [Google Scholar] [CrossRef]
- Bang, J.Y.; Navaneethan, U.; Hasan, M.; Sutton, B.; Hawes, R.; Varadarajulu, S. Optimizing Outcomes of Single-Operator Cholangioscopy–Guided Biopsies Based on a Randomized Trial. Clin. Gastroenterol. Hepatol. 2020, 18, 441–448.e1. [Google Scholar] [CrossRef] [PubMed]
- Varadarajulu, S.; Bang, J.Y.; Hasan, M.K.; Navaneethan, U.; Hawes, R.; Hebert-Magee, S. Improving the diagnostic yield of single-operator cholangioscopy-guided biopsy of indeterminate biliary strictures: ROSE to the rescue? (with video). Gastrointest. Endosc. 2016, 84, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Parsa, N.; Khashab, M.A. The Role of Peroral Cholangioscopy in Evaluating Indeterminate Biliary Strictures. Clin. Endosc. 2019, 52, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Sethi, A.; Widmer, J.; Shah, N.L.; Pleskow, D.K.; Edmundowicz, S.A.; Sejpal, D.V.; Gress, F.G.; Pop, G.H.; Gaidhane, M.; Sauer, B.G.; et al. Interobserver agreement for evaluation of imaging with single operator choledochoscopy: What are we looking at? Dig. Liver Dis. 2014, 46, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Tsuyuguchi, T.; Sakai, Y.; Sugiyama, H.; Miyazaki, M.; Yokosuka, O. Comparison of the diagnostic accuracy of peroral video-cholangioscopic visual findings and cholangioscopy-guided forceps biopsy findings for indeterminate biliary lesions: A prospective study. Gastrointest. Endosc. 2013, 77, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Ramchandani, M.; Reddy, D.N.; Gupta, R.; Lakhtakia, S.; Tandan, M.; Darisetty, S.; Sekaran, A.; Rao, G.V. Role of single-operator peroral cholangioscopy in the diagnosis of indeterminate biliary lesions: A single-center, prospective study. Gastrointest. Endosc. 2011, 74, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Robles-Medranda, C.; Valero, M.; Soria-Alcivar, M.; Puga-Tejada, M.; Oleas, R.; Ospina-Arboleda, J.; Alvarado-Escobar, H.; Baquerizo-Burgos, J.; Robles-Jara, C.; Pitanga-Lukashok, H.; et al. Reliability and accuracy of a novel classification system using peroral cholangioscopy for the diagnosis of bile duct lesions. Endoscopy 2018, 50, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Sethi, A.; Tyberg, A.; Slivka, A.; Adler, D.G.; Desai, A.P.; Sejpal, D.V.; Pleskow, D.K.; Bertani, H.; Gan, S.I.; Shah, R.; et al. Digital Single-operator Cholangioscopy (DSOC) Improves Interobserver Agreement (IOA) and Accuracy for Evaluation of Indeterminate Biliary Strictures: The Monaco Classification. J. Clin. Gastroenterol. 2022, 56, e94–e97. [Google Scholar] [CrossRef] [PubMed]
- Kahaleh, M.; Raijman, I.; Gaidhane, M.; Tyberg, A.; Sethi, A.; Slivka, A.; Adler, D.G.; Sejpal, D.; Shahid, H.; Sarkar, A.; et al. Digital Cholangioscopic Interpretation: When North Meets the South. Dig. Dis. Sci. 2022, 67, 1345–1351. [Google Scholar] [CrossRef]
- Venezia, L.; Rizza, S.; Pablo, C.V.; De Angelis, C.G. Intraductal ultrasound (IDUS) for second-level evaluation of biliary and ampullary stenosis: Experience from the Turin center. Dig. Liver Dis. 2018, 50, e214–e215. [Google Scholar] [CrossRef]
- Kim, D.C.; Moon, J.H.; Choi, H.J.; Chun, A.R.; Lee, Y.N.; Lee, M.H.; Lee, T.H.; Cha, S.W.; Kim, S.G.; Kim, Y.S.; et al. Usefulness of Intraductal Ultrasonography in Icteric Patients with Highly Suspected Choledocholithiasis Showing Normal Endoscopic Retrograde Cholangiopancreatography. Dig. Dis. Sci. 2014, 59, 1902–1908. [Google Scholar] [CrossRef] [PubMed]
- Fusaroli, P.; Caletti, G. Intraductal Ultrasound for High-Risk Patients: When Will the Last Be First? Dig. Dis. Sci. 2014, 59, 1676–1678. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tejido, C.; Puga, M.; Regueiro, C.; Francisco, M.; Rivas, L.; Sánchez, E. Evaluation of the effectiveness and safety of single-operator cholangiopancreatoscopy with the SpyGlass™ system. Gastroenterol. Hepatol. 2024, 47, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Laleman, W.; Verraes, K.; Van Steenbergen, W.; Cassiman, D.; Nevens, F.; Van der Merwe, S.; Verslype, C. Usefulness of the single-operator cholangioscopy system SpyGlass in biliary disease: A single-center prospective cohort study and aggregated review. Surg. Endosc. 2017, 31, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Yodice, M.; Choma, J.; Tadros, M. The Expansion of Cholangioscopy: Established and Investigational Uses of SpyGlass in Biliary and Pancreatic Disorders. Diagnostics 2020, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Dumonceau, J.M.; Kapral, C.; Aabakken, L.; Papanikolaou, I.S.; Tringali, A.; Vanbiervliet, G.; Beyna, T.; Dinis-Ribeiro, M.; Hritz, I.; Mariani, A.; et al. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2020, 52, 127–149. [Google Scholar] [CrossRef] [PubMed]
- Andriulli, A.; Loperfido, S.; Napolitano, G.; Niro, G.; Valvano, M.R.; Spirito, F.; Pilotto, A.; Forlano, R. Incidence Rates of Post-ERCP Complications: A Systematic Survey of Prospective Studies. Am. J. Gastroenterol. 2007, 102, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Meister, T.; Heinzow, H.; Heinecke, A.; Hoehr, R.; Domschke, W.; Domagk, D. Post-ERCP pancreatitis in 2364 ERCP procedures: Is intraductal ultrasonography another risk factor? Endoscopy 2011, 43, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Tringali, A.; Lemmers, A.; Meves, V.; Terheggen, G.; Pohl, J.; Manfredi, G.; Häfner, M.; Costamagna, G.; Devière, J.; Neuhaus, H.; et al. Intraductal biliopancreatic imaging: European Society of Gastrointestinal Endoscopy (ESGE) technology review. Endoscopy 2015, 47, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Pouw, R.E.; Barret, M.; Biermann, K.; Bisschops, R.; Czakó, L.; Gecse, K.B.; de Hertogh, G.; Hucl, T.; Iacucci, M.; Jansen, M.; et al. Endoscopic tissue sampling—Part 1: Upper gastrointestinal and hepatopancreatobiliary tractsEuropean Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2021, 53, 1174–1188. [Google Scholar] [CrossRef]
- Akoglu, H. User’s guide to sample size estimation in diagnostic accuracy studies. Turk. J. Emerg. Med. 2022, 22, 177–185. [Google Scholar] [CrossRef] [PubMed]
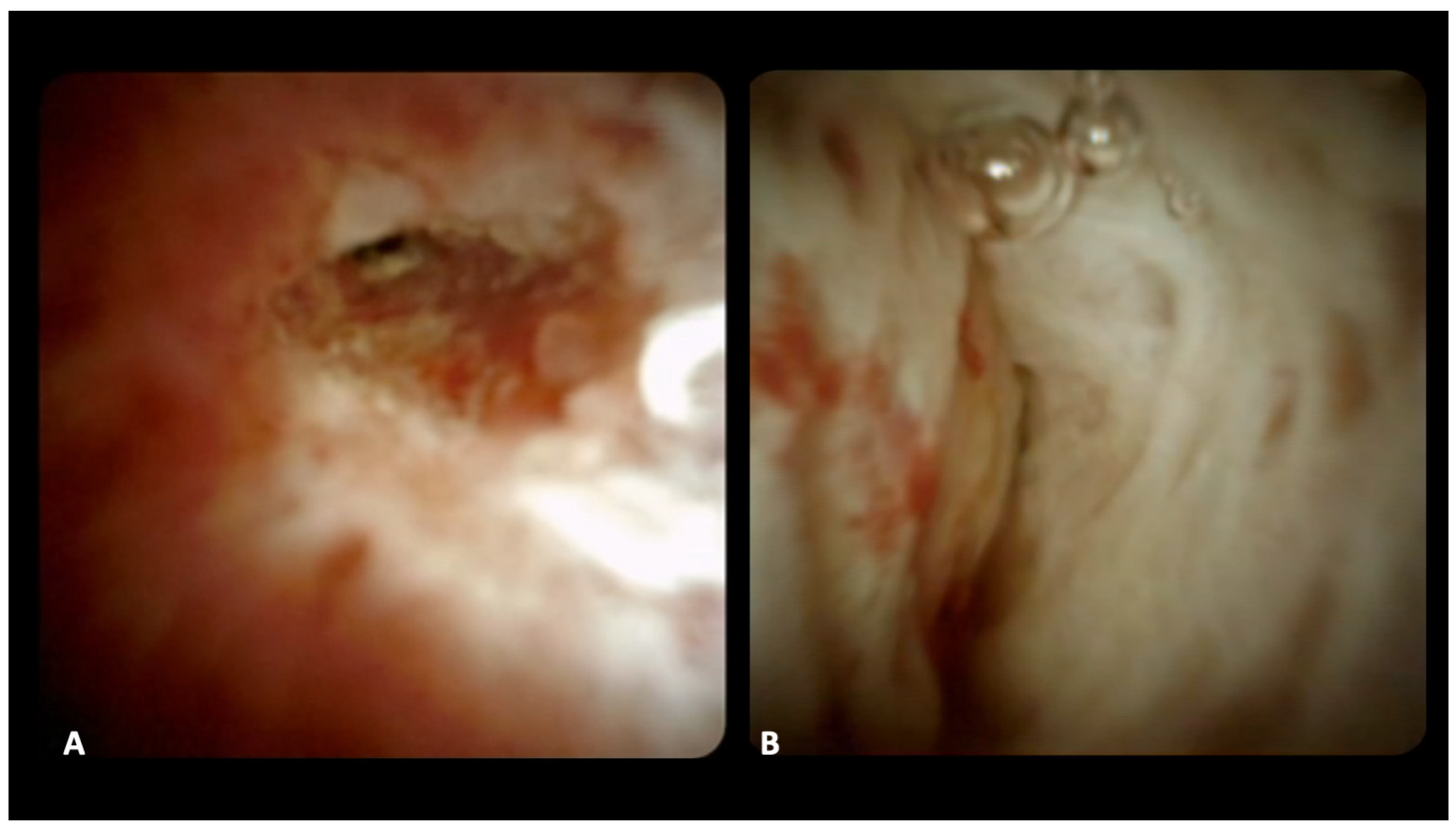
| Mean age (±SD), years | 67.2 (±10.0) |
| Male/Female, n (%)/n (%) | 39 (68.4%)/18 (31.6%) |
| Comorbidities, n (%) | 34 (59.6%) |
| Cardiovascular | 24 (42.1%) |
| Pulmonary | 6 (10.5%) |
| Liver diseases | 7 (12.3%) |
| Chronic kidney disease | 1 (1.7%) |
| Type II diabetes mellitus | 10 (17.5%) |
| Tobacco consumption | 11 (19.3%) |
| Patients with previous ERCP, n % | 39 (68.4%) |
| Mean previous ERCP (±SD) | 1.97 ± 1.42 |
| Patients with stent in place prior to DSOC, n (%) | 29 (50.9%) |
| Previous biliary sphincterotomy, n (%) | 38 (66.7%) |
| Previous cholecystectomy, n (%) | 21 (36.8%) |
| Stricture location, n (%) | |
| Common bile duct | 26 (45.6%) |
| Common hepatic duct | 13 (22.8%) |
| Cystic duct | 1 (1.7%) |
| Hepatic hilum | 12 (21.1%) |
| Intrahepatic ducts | 5 (8.8%) |
| Techniques | Sensitivity (CI 95%) | Specificity (CI 95%) | Accuracy (CI 95%) | NPV (CI 95%) | PPV (CI 95%) |
|---|---|---|---|---|---|
| DSOC visualization | 85.7% (76.6–94.8%) | 95.5% (90.0–100%) | 89.5% (81.5–97.4%) | 80.8% (70.5–91%) | 96.8% (92.2–100%) |
| IDUS | 84.4% (74.5–94.2%) | 80.0% (69.1–90.9%) | 82.7% (72.4–93.0%) | 76.2% (64.6–87.8%) | 87.1% (78.0–96.2%) |
| DSOC targeted biopsy | 63.6% (51.1–76.1%) | 100% (83.0–100%) | 76.9% (66.0–87.9%) | 61.3% (48.6–73.9%) | 100% (85.0–100%) |
| Brush cytology | 51.6% (38.6–64.6%) | 100% (78–100%) | 61.5% (48.9–74.2%) | 34.8% (22.4–47.1%) | 100% (82–100%) |
| Sensitivity | Specificity | Accuracy | NPV | PPV | |
|---|---|---|---|---|---|
| Comparison | p Value | ||||
| DSOC vs. IDUS | >0.99 | 0.17 | 0.41 | 0.73 | 0.35 |
| DSOC vs. Biopsy | 0.05 | >0.99 | 0.12 | 0.15 | >0.99 |
| DSOC vs. Cytology | <0.01 | >0.99 | <0.01 | <0.01 | >0.99 |
| IDUS vs. Biopsy | 0.09 | 0.11 | 0.63 | 0.73 | 0.11 |
| IDUS vs. Cytology | <0.01 | 0.29 | 0.03 | <0.01 | 0.23 |
| Biopsy vs. Cytology | 0.45 | >0.99 | 0.16 | 0.10 | >0.99 |
| Patient | Adverse Event | Severity Grade | Onset (Day after Procedure) | Management | Outcome |
|---|---|---|---|---|---|
| Patient 1 | Cholangitis | Moderate | 9 | Endoscopic (repeated ERCP with stent replacement) | Favorable |
| Patient 2 | Acute pancreatitis | Mild | 1 | Medical therapy | Favorable |
| Patient 2 | Cholangitis | Moderate | 2 | Medical therapy | Favorable |
| Patient 3 | Acute pancreatitis | Mild | 0 | Medical therapy | Favorable |
| Patient 4 | Acute pancreatitis | Mild | 0 | Medical therapy | Favorable |
| Patient 5 | Myocardial infarction | Fatal | 4 | Percutaneous transluminal coronary angioplasty | Fatal |
| Patient 6 | Aspiration pneumonia | Moderate | 0 | Medical therapy | Favorable |
| Patient 7 | Cholangitis | Moderate | 1 | Medical therapy | Favorable |
| Patient 8 | Cholangitis | Mild | 1 | Medical therapy | Favorable |
| Patient 9 | Cholangitis | Mild | 1 | Medical therapy | Favorable |
| Patient 10 | Acute pancreatitis | Moderate | 0 | Medical therapy | Favorable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacco, M.; Gesualdo, M.; Staiano, M.T.; Dall’Amico, E.; Caronna, S.; Dibitetto, S.; Canalis, C.; Caneglias, A.; Mediati, F.; Stasio, R.C.; et al. Direct Single-Operator Cholangioscopy and Intraductal Ultrasonography in Patients with Indeterminate Biliary Strictures: A Single Center Experience. Diagnostics 2024, 14, 1316. https://doi.org/10.3390/diagnostics14131316
Sacco M, Gesualdo M, Staiano MT, Dall’Amico E, Caronna S, Dibitetto S, Canalis C, Caneglias A, Mediati F, Stasio RC, et al. Direct Single-Operator Cholangioscopy and Intraductal Ultrasonography in Patients with Indeterminate Biliary Strictures: A Single Center Experience. Diagnostics. 2024; 14(13):1316. https://doi.org/10.3390/diagnostics14131316
Chicago/Turabian StyleSacco, Marco, Marcantonio Gesualdo, Maria Teresa Staiano, Eleonora Dall’Amico, Stefania Caronna, Simone Dibitetto, Chiara Canalis, Alessandro Caneglias, Federica Mediati, Rosa Claudia Stasio, and et al. 2024. "Direct Single-Operator Cholangioscopy and Intraductal Ultrasonography in Patients with Indeterminate Biliary Strictures: A Single Center Experience" Diagnostics 14, no. 13: 1316. https://doi.org/10.3390/diagnostics14131316
APA StyleSacco, M., Gesualdo, M., Staiano, M. T., Dall’Amico, E., Caronna, S., Dibitetto, S., Canalis, C., Caneglias, A., Mediati, F., Stasio, R. C., Gaia, S., Saracco, G. M., Bruno, M., & De Angelis, C. G. (2024). Direct Single-Operator Cholangioscopy and Intraductal Ultrasonography in Patients with Indeterminate Biliary Strictures: A Single Center Experience. Diagnostics, 14(13), 1316. https://doi.org/10.3390/diagnostics14131316







