Enhanced Liver Fibrosis Score for Diagnosing Liver Fibrosis in Chronic Hepatitis
Abstract
:1. Introduction
2. Methods
2.1. Study Protocol
2.2. Liver Biopsy Assessment
2.3. ELF Score and FIB-4
2.4. Statistical Analyses
3. Results
3.1. Patient Characteristics
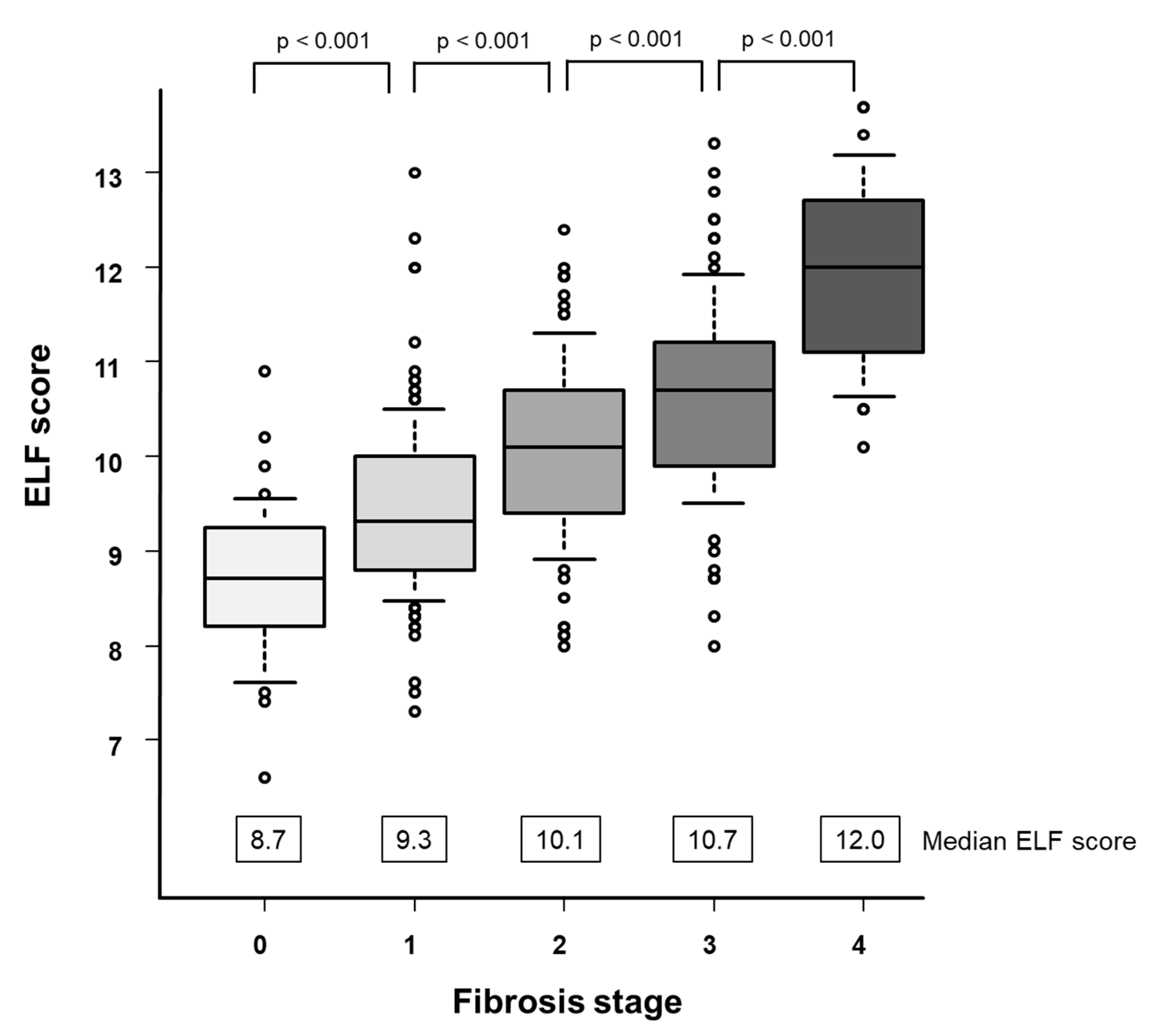
3.2. ELF Score and Fibrosis Stage
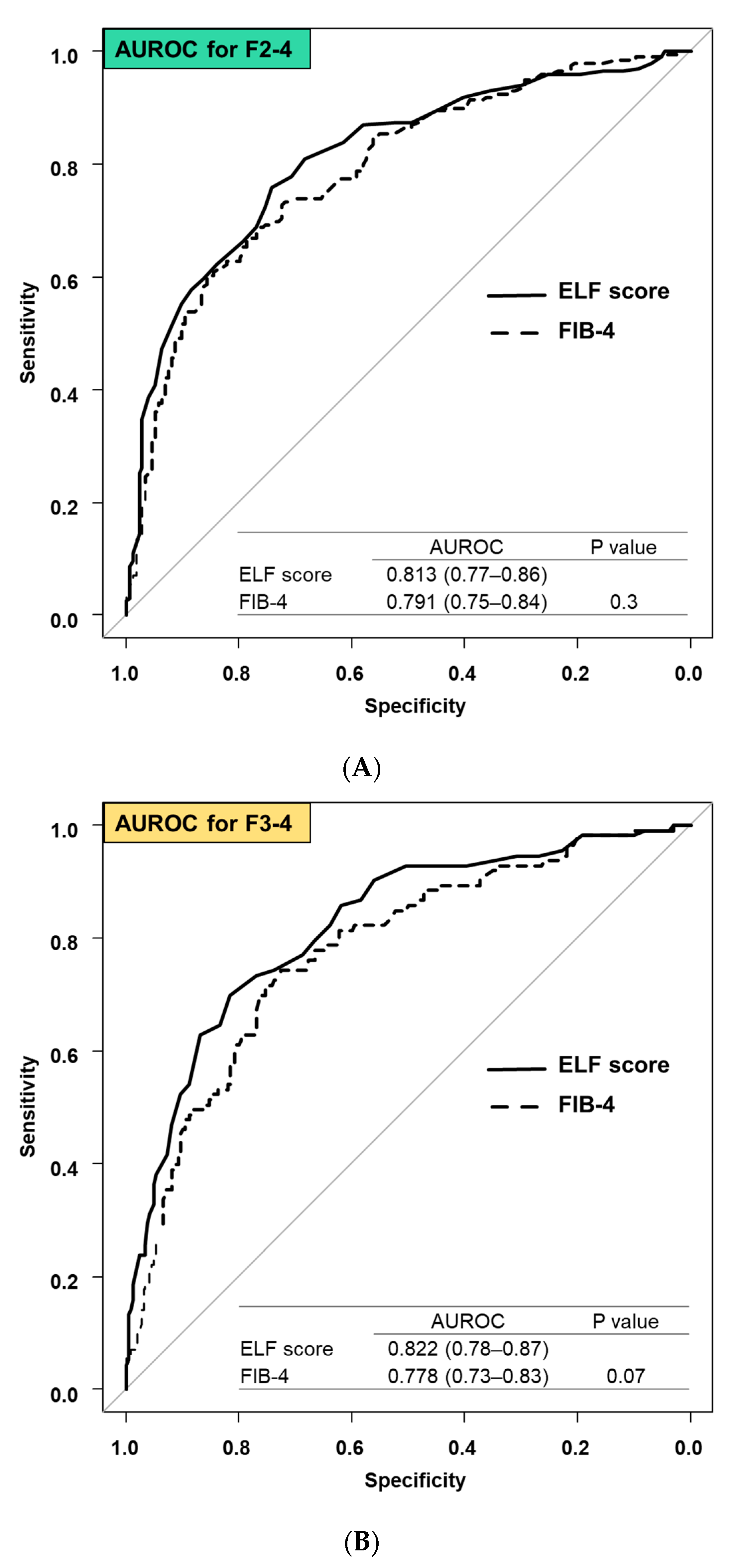
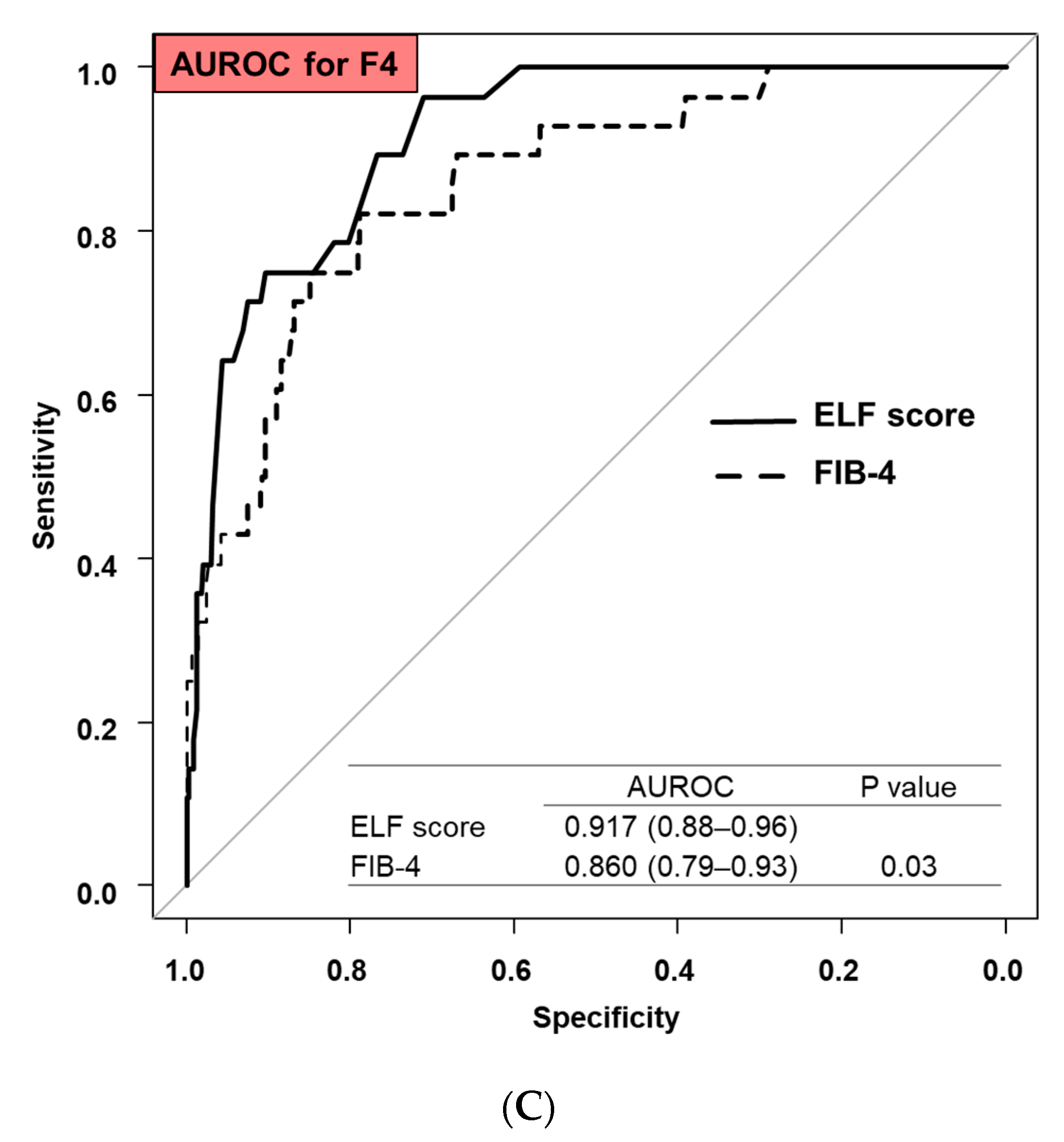
3.3. Comparison Diagnostic Accuracy between ELF Score and FIB-4
3.4. Effect of Age on ELF Score and FIB-4
4. Discussions
4.1. Main Findings
4.2. In Context with Published Literature
4.3. Strengths and Limitations
4.4. Future Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Higuchi, M.; Tamaki, N.; Kurosaki, M.; Inada, K.; Kirino, S.; Yamashita, K.; Hayakawa, Y.; Osawa, L.; Takaura, K.; Maeyashiki, C.; et al. Longitudinal association of magnetic resonance elastography-associated liver stiffness with complications and mortality. Aliment. Pharmacol. Ther. 2022, 55, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.C.; Huang, D.Q.; Nguyen, M.H. Global burden of hepatitis B virus: Current status, missed opportunities and a call for action. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Pham, Y.T.; Huang, D.Q.; Zhang, Z.; Ng, C.H.; Tan, D.J.H.; Nguyen, H.C.; Nguyen, T.C.; Behari, J.; Yuan, J.M.; Luu, H.N. Changing global epidemiology of chronic hepatitis C virus-related outcomes from 2010 to 2019: Cirrhosis is the growing burden of hepatitis C virus-related disease. Eur. J. Cancer Prev. 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Dulai, P.S.; Singh, S.; Patel, J.; Soni, M.; Prokop, L.J.; Younossi, Z.; Sebastiani, G.; Ekstedt, M.; Hagstrom, H.; Nasr, P.; et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017, 65, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Bergstrom, J.; Loomba, R.; Tamaki, N.; Izumi, N.; Nakajima, A.; Idilman, R.; Gumussoy, M.; Oz, D.K.; Erden, A.; et al. Magnetic resonance elastography-based prediction model for hepatic decompensation in NAFLD: A multicenter cohort study. Hepatology 2023, 78, 1858–1866. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, N.; Higuchi, M.; Kurosaki, M.; Loomba, R.; Izumi, N. Risk Difference of Liver-Related and Cardiovascular Events by Liver Fibrosis Status in Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2022, 20, 1171–1173.e2. [Google Scholar] [CrossRef] [PubMed]
- Bravo, A.A.; Sheth, S.G.; Chopra, S. Liver biopsy. N. Engl. J. Med. 2001, 344, 495–500. [Google Scholar] [CrossRef]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive Assessment of Liver Disease in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1264–1281.e4. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, N.; Ajmera, V.; Loomba, R. Non-invasive methods for imaging hepatic steatosis and their clinical importance in NAFLD. Nat. Rev. Endocrinol. 2022, 18, 55–66. [Google Scholar] [CrossRef]
- Tamaki, N.; Kurosaki, M.; Huang, D.Q.; Loomba, R. Noninvasive assessment of liver fibrosis and its clinical significance in nonalcoholic fatty liver disease. Hepatol. Res. 2022, 52, 497–507. [Google Scholar] [CrossRef]
- Tamaki, N.; Imajo, K.; Sharpton, S.R.; Jung, J.; Sutter, N.; Kawamura, N.; Yoneda, M.; Valasek, M.A.; Behling, C.; Sirlin, C.B.; et al. Two-Step Strategy, FIB-4 Followed by Magnetic Resonance Elastography, for Detecting Advanced Fibrosis in NAFLD. Clin. Gastroenterol. Hepatol. 2023, 21, 380–387.e3. [Google Scholar] [CrossRef]
- Lichtinghagen, R.; Pietsch, D.; Bantel, H.; Manns, M.P.; Brand, K.; Bahr, M.J. The Enhanced Liver Fibrosis (ELF) score: Normal values, influence factors and proposed cut-off values. J. Hepatol. 2013, 59, 236–242. [Google Scholar] [CrossRef]
- Asano, H. Possibility of Using ELF Score as Index for Hepatic Fibrosis Evaluation. Rinsho Byori 2015, 63, 78–83. [Google Scholar]
- Kjaergaard, M.; Lindvig, K.P.; Thorhauge, K.H.; Andersen, P.; Hansen, J.K.; Kastrup, N.; Jensen, J.M.; Hansen, C.D.; Johansen, S.; Israelsen, M.; et al. Using the ELF test, FIB-4 and NAFLD fibrosis score to screen the population for liver disease. J. Hepatol. 2023, 79, 277–286. [Google Scholar] [CrossRef]
- Are, V.S.; Vuppalanchi, R.; Vilar-Gomez, E.; Chalasani, N. Enhanced Liver Fibrosis Score Can Be Used to Predict Liver-Related Events in Patients with Nonalcoholic Steatohepatitis and Compensated Cirrhosis. Clin. Gastroenterol. Hepatol. 2021, 19, 1292–1293.e3. [Google Scholar] [CrossRef] [PubMed]
- Seko, Y.; Takahashi, H.; Toyoda, H.; Hayashi, H.; Yamaguchi, K.; Iwaki, M.; Yoneda, M.; Arai, T.; Shima, T.; Fujii, H.; et al. Diagnostic accuracy of enhanced liver fibrosis test for nonalcoholic steatohepatitis-related fibrosis: Multicenter study. Hepatol. Res. 2023, 53, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Inadomi, C.; Takahashi, H.; Ogawa, Y.; Oeda, S.; Imajo, K.; Kubotsu, Y.; Tanaka, K.; Kessoku, T.; Okada, M.; Isoda, H.; et al. Accuracy of the Enhanced Liver Fibrosis test, and combination of the Enhanced Liver Fibrosis and non-invasive tests for the diagnosis of advanced liver fibrosis in patients with non-alcoholic fatty liver disease. Hepatol. Res. 2020, 50, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef]
- Tamaki, N.; Kurosaki, M.; Yasui, Y.; Mori, N.; Tsuji, K.; Hasebe, C.; Joko, K.; Akahane, T.; Furuta, K.; Kobashi, H.; et al. Change in Fibrosis 4 Index as Predictor of High Risk of Incident Hepatocellular Carcinoma After Eradication of Hepatitis C Virus. Clin. Infect. Dis. 2021, 73, e3349–e3354. [Google Scholar] [CrossRef]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef]
- European Association for the Study of The Liver; European Association for the Study of Diabetes. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Arai, T.; Takahashi, H.; Seko, Y.; Toyoda, H.; Hayashi, H.; Yamaguchi, K.; Iwaki, M.; Yoneda, M.; Shima, T.; Fujii, H.; et al. Accuracy of the Enhanced Liver Fibrosis Test in Patients With Type 2 Diabetes Mellitus and Its Clinical Implications. Clin. Gastroenterol. Hepatol. 2024, 22, 789–797.e8. [Google Scholar] [CrossRef]
- Fujinaga, Y.; Namisaki, T.; Takaya, H.; Tsuji, Y.; Suzuki, J.; Shibamoto, A.; Kubo, T.; Iwai, S.; Tomooka, F.; Takeda, S.; et al. Enhanced liver fibrosis score as a surrogate of liver-related complications and mortality in primary biliary cholangitis. Medicine 2021, 100, e27403. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Ide, T.; Amano, K.; Arinaga-Hino, T.; Kuwahara, R.; Sano, T.; Miki, S.; Ono, N.; Torimura, T. Enhanced liver fibrosis score as a predictive marker for hepatocellular carcinoma development after hepatitis C virus eradication. Mol. Clin. Oncol. 2021, 15, 215. [Google Scholar] [CrossRef]
- Tokushige, K.; Ikejima, K.; Ono, M.; Eguchi, Y.; Kamada, Y.; Itoh, Y.; Akuta, N.; Yoneda, M.; Iwasa, M.; Yoneda, M.; et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020. J. Gastroenterol. 2021, 56, 951–963. [Google Scholar] [CrossRef]
- Ishiba, H.; Sumida, Y.; Tanaka, S.; Yoneda, M.; Hyogo, H.; Ono, M.; Fujii, H.; Eguchi, Y.; Suzuki, Y.; Yoneda, M.; et al. The novel cutoff points for the FIB4 index categorized by age increase the diagnostic accuracy in NAFLD: A multi-center study. J. Gastroenterol. 2018, 53, 1216–1224. [Google Scholar] [CrossRef]
- Tamaki, N.; Higuchi, M.; Kurosaki, M.; Kirino, S.; Osawa, L.; Watakabe, K.; Wang, W.; Okada, M.; Shimizu, T.; Takaura, K.; et al. Wisteria floribunda agglutinin-positive mac-2 binding protein as an age-independent fibrosis marker in nonalcoholic fatty liver disease. Sci. Rep. 2019, 9, 10109. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Magnanensi, J.; Hajji, Y.; Caron, A.; Majd, Z.; Rosenquist, C.; Hum, D.W.; Staels, B.; Connelly, M.A.; Loomba, R.; et al. Impact of age on NIS2+™ and other non-invasive blood tests for the evaluation of liver disease and detection of at-risk MASH. JHEP Rep. 2024, 6, 101011. [Google Scholar] [CrossRef]
- Kirino, S.; Tamaki, N.; Kurosaki, M.; Takahashi, Y.; Higuchi, M.; Itakura, Y.; Tanaka, Y.; Inada, K.; Ishido, S.; Yamashita, K.; et al. Detecting advanced liver fibrosis using ultrasound shear wave velocity measurement in the general population. Quant. Imaging Med. Surg. 2023, 13, 6493–6502. [Google Scholar] [CrossRef]

| n = 373 | ||
|---|---|---|
| Age, years | 60 (48–66) | |
| Males | 175 (47%) | |
| Etiology | HCV | 208 (55.8%) |
| HBV | 84 (22.5%) | |
| Non-viral | 81 (21.7%) | |
| Fibrosis stage | 0 | 36 (9.7%) |
| 1 | 138 (37.0%) | |
| 2 | 86 (23.1%) | |
| 3 | 85 (22.7%) | |
| 4 | 28 (7.5%) | |
| Albumin, g/dL | 4.2 (3.9–4.4) | |
| AST, IU/L | 43 (31–68) | |
| ALT, IU/L | 49 (31–86) | |
| Platelet counts, 109/L | 175 (135–214) | |
| FIB-4 | 2.09 (1.35–3.49) | |
| ELF score | 9.9 (9.0–10.7) | |
| Continuous values are shown in median (interquartile range). | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamaki, N.; Takaura, K.; Higuchi, M.; Yasui, Y.; Itakura, J.; Tsuchiya, K.; Nakanishi, H.; Izumi, N.; Kurosaki, M. Enhanced Liver Fibrosis Score for Diagnosing Liver Fibrosis in Chronic Hepatitis. Diagnostics 2024, 14, 1317. https://doi.org/10.3390/diagnostics14131317
Tamaki N, Takaura K, Higuchi M, Yasui Y, Itakura J, Tsuchiya K, Nakanishi H, Izumi N, Kurosaki M. Enhanced Liver Fibrosis Score for Diagnosing Liver Fibrosis in Chronic Hepatitis. Diagnostics. 2024; 14(13):1317. https://doi.org/10.3390/diagnostics14131317
Chicago/Turabian StyleTamaki, Nobuharu, Kenta Takaura, Mayu Higuchi, Yutaka Yasui, Jun Itakura, Kaoru Tsuchiya, Hiroyuki Nakanishi, Namiki Izumi, and Masayuki Kurosaki. 2024. "Enhanced Liver Fibrosis Score for Diagnosing Liver Fibrosis in Chronic Hepatitis" Diagnostics 14, no. 13: 1317. https://doi.org/10.3390/diagnostics14131317





