Recovery from Severe COVID-19 Is an Independent Predictor of Electrocardiographic Abnormal P-Wave Axis
Abstract
:1. Introduction
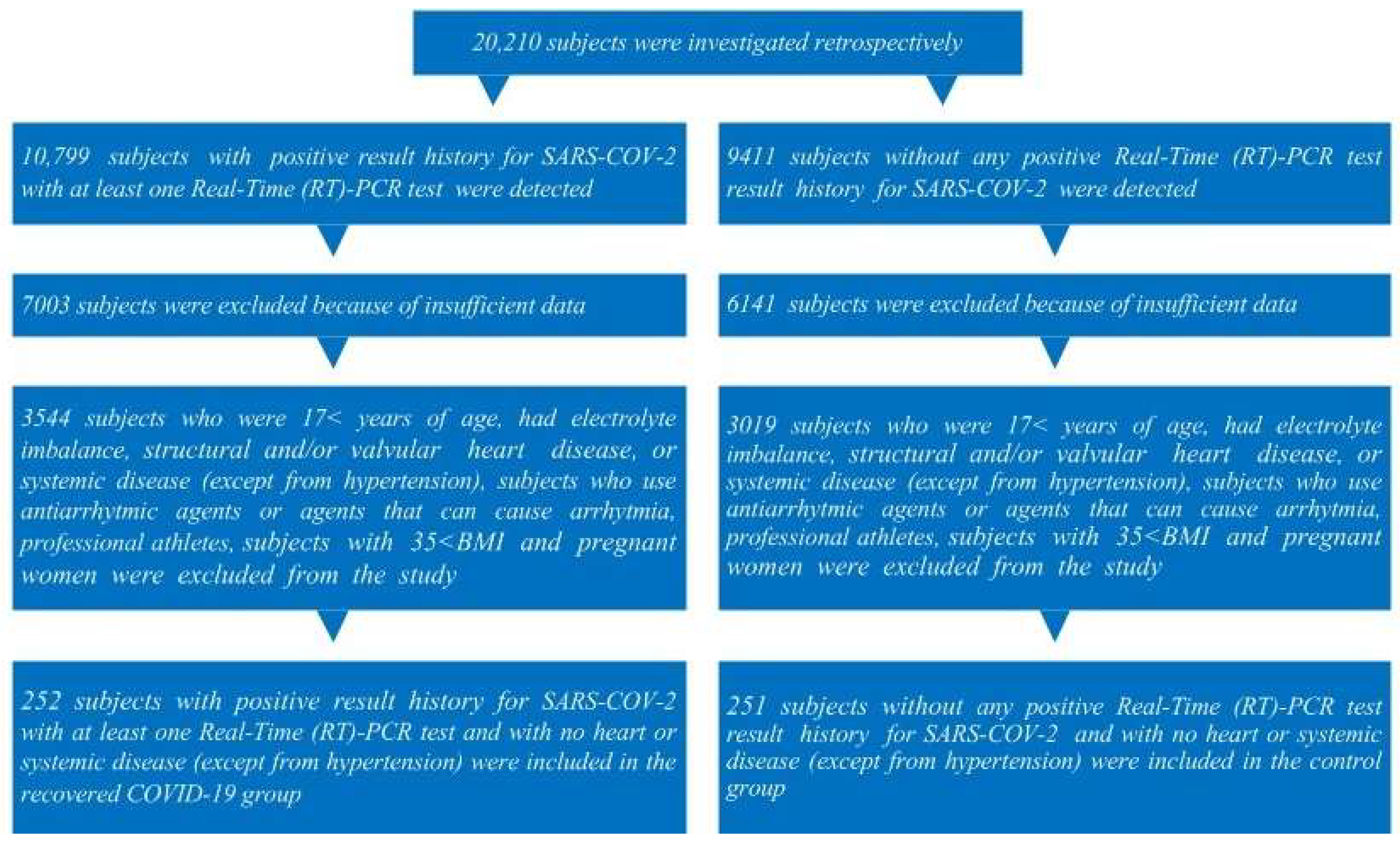
2. Materials and Methods
2.1. Study Population
2.2. Laboratory Measurements
2.3. PCR Analysis
2.4. Chest CT
2.5. Echocardiographic Measurements
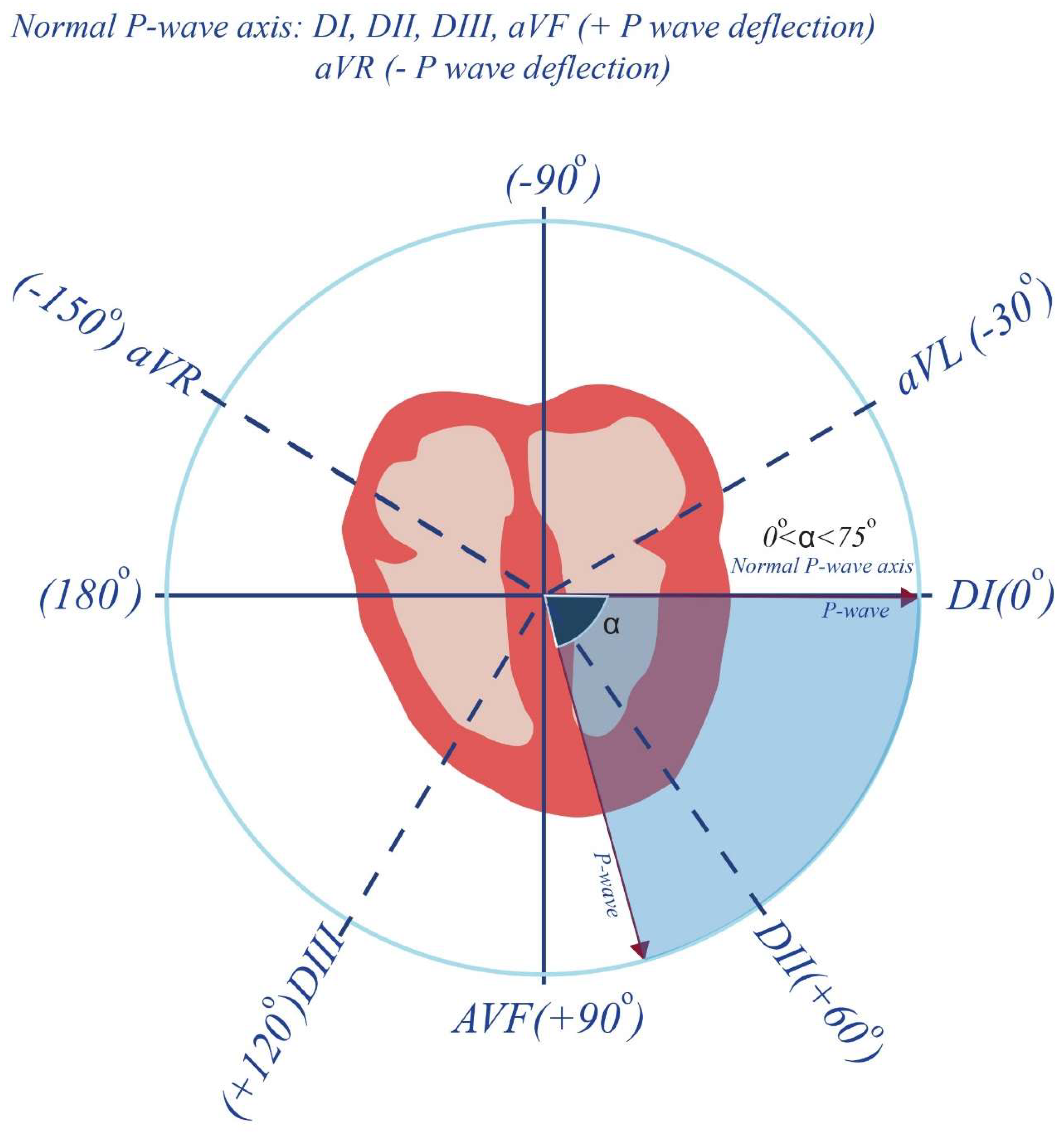
2.6. Electrocardiographic Measurements
2.7. Statistical Evaluation
3. Results
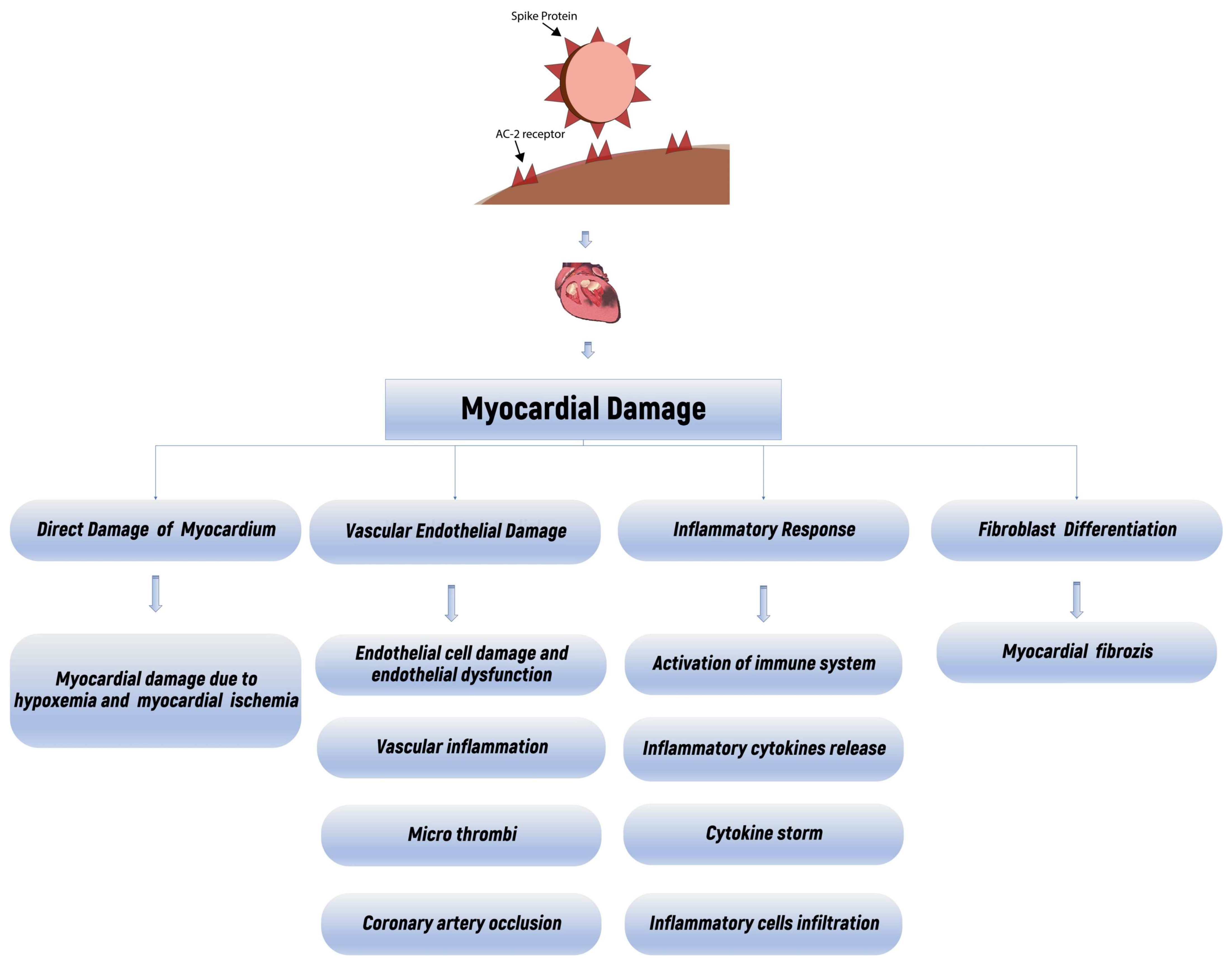
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Gawad, M.; Zaghloul, M.S.; Abd-Elsalam, S.; Hashem, M.; Lashen, S.A.; Mahros, A.M.; Mohammed, A.Q.; Hassan, A.M.; Bekhit, A.N.; Mohammed, W.; et al. Post-COVID-19 Syndrome Clinical Manifestations: A Systematic Review. Antiinflamm. Antiallergy Agents. Med. Chem. 2022, 21, 115–120. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent symptoms in patients after acute COVID-19. JAMA 2020, 324, 603–635. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, M.W.; Kim, S.S.; Lindsell, C.J.; Rose, E.B.; Shapiro, N.I.; Files, D.C.; Gibbs, K.W.; Erickson, H.L.; Steingrub, J.S.; Smithline, H.A.; et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network—United States. 2020. MMWR Morb. Mortal. Wkly. 2020, 69, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Mallick, D.; Goyal, L.; Chourasia, P.; Zapata, M.R.; Yashi, K.; Surani, S. COVID-19 Induced Postural Orthostatic Tachycardia Syndrome (POTS): A Review. Cureus 2023, 15, e36955. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized patients With 2019 Novel Coronavirus-Infected pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef]
- Li, Y.; Shah, A.J.; Soliman, E.Z. Effect of electrocardiographic P-wave axis on mortality. Am. J. Cardiol. 2014, 113, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Lazzeroni, D.; Bini, M.; Camaiora, U.; Castiglioni, P.; Moderato, L.; Ugolotti, P.T.; Brambilla, L.; Brambilla, V.; Coruzzi, P. Predictive role of P-wave axis abnormalities in secondary cardiovascular prevention. Eur. J. Prev. Cardiol. 2017, 24, 1994–1999. [Google Scholar] [CrossRef]
- Sharma, S.; Aggarwal, A.; Sharma, R.K.; Patras, E.; Singhal, A. Correlation of chest CT severity score with clinical parameters in COVID-19 pulmonary disease in a tertiary care hospital in Delhi during the pandemic period. Egypt J. Radiol. Nucl. Med. 2022, 53, 166. [Google Scholar] [CrossRef]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.; et al. Recommendations for chamber quantification. Eur. J. Echocardiogr. 2006, 7, 79–108. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Yilmaz, S. Major depressive disorder is an independent predictor of the electrocardiographic frontal QRS-T angle. Bratisl. Lek. Listy 2023, 124, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Oudit, G.Y.; Kassiri, Z.; Jiang, C.; Liu, P.P.; Poutanen, S.M.; Penninger, J.; Butany, J. SARS coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur. J. Clin. Investig. 2009, 39, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; He, L.; Zhang, Q.; Huang, Z.; Che, X.; Hou, J.; Wang, H.; Shen, H.; Qiu, L.; Li, Z.; et al. Organ distribution of severe acute respiratory syndrome (SARS) associated corona-virus (SARS-CoV) in SARS patients: Implications for pathogenesis and virus transmission pathways. J. Pathol. 2004, 203, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.Y.; Redwood, S.; Prendergast, B.; Chen, M. Coronaviruses and the cardiovascular system: Acute and long-term implications. Eur. Heart J. 2020, 41, 1798–1800. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, J.; Zhao, F. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, X.; Yang, P.; Zhang, S. COVID-19 Complicated by Acute Pulmonary Embolism. Radiol. Cardiothorc. Imaging 2020, 2, e200067. [Google Scholar] [CrossRef]
- Turner, A.J.; Hiscox, J.A.; Hooper, N.M. ACE2: From vasopeptidase to SARS virus receptor. Trends. Pharmacol. Sci. 2004, 25, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Stasi, C.; Fallani, S.; Voller, F.; Silvestri, C. Treatment for COVID-19: An overview. Eur. J. Pharmacol. 2020, 889, 173644. [Google Scholar] [CrossRef] [PubMed]
- Roux, C.; Kachenoura, N.; Raissuni, Z.; Mousseaux, E.; Young, J.; Graves, M.J.; Jublanc, C.; Cluzel, P.; Chanson, P.; Kamenický, P.; et al. Effects of Cortisol on the Heart: Characterization of Myocardial Involvement in Cushing’s Disease by Longitudinal Cardiac MRI T1 Mapping. J. Magn. Reson. Imaging 2017, 45, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, G.; Dilley, R.; Fullerton, M.J.; Funder, J.W. Experimental cardiac fibrosis: Differential time course of responses to mineralocorticoid-salt administration. Endocrinology 2001, 142, 3625–3631. [Google Scholar] [CrossRef] [PubMed]
- Rangel, M.O.; O’Neal, W.T.; Soliman, E.Z. Usefulness of the electrocardiographic P-wave axis as a predictor of atrial fibrillation. Am. J. Cardiol. 2016, 117, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Kleiven, Ø.; Ørn, S. P-wave axis as a predictor of mortality. Eur. J. Prev. Cardiol. 2017, 24, 1991–1993. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2023, 401, e21–e33. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Raman, B.; Bluemke, D.A.; Luscher, T.F.; Neubauer, S. Long COVID: Post-acute sequelae of COVID-19 with acardiovascular focus. Eur. Heart J. 2022, 43, 1157–1172. [Google Scholar] [CrossRef]
- Visco, V.; Vitale, C.; Rispoli, A.; Izzo, C.; Virtuoso, N.; Ferruzzi, G.J.; Santopietro, M.; Melfi, A.; Rusciano, M.R.; Maglio, A.; et al. Post-COVID-19 Syndrome: Involvement and Interactions between Respiratory, Cardiovascular and Nervous Systems. J. Clin. Med. 2022, 11, 524. [Google Scholar] [CrossRef]
- Saha, S.A.; Russo, A.M.; Chung, M.K.; Deering, T.F.; Lakkireddy, D.; Gopinathannair, R. COVID-19 and Cardiac Arrhythmias: A Contemporary Review. Curr. Treat. Options Cardiovasc. Med. 2022, 24, 87–107. [Google Scholar] [CrossRef] [PubMed]
- Babapoor-Farrokhran, S.; Gill, D.; Walker, J.; Rasekhi, R.T.; Bozorgnia, B.; Amanullah, A. Myocardial injury and COVID-19: Possible mechanisms. Life Sci. 2020, 253, 117723. [Google Scholar] [CrossRef] [PubMed]
- Lazzerini, P.E.; Laghi-Pasini, F.; Boutjdir, M.; Capecchi, P.L. Inflammatory cytokines and cardiac arrhythmias: Thelesson from COVID-19. Nat. Rev. Immunol. 2022, 22, 270–272. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Yue, H.; Liang, W.; Wu, Z. Effects of COVID-19 on Arrhythmia. J. Cardiovasc. Dev. Dis. 2022, 9, 292. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; Assaf, G.S.; Mc Corkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. eClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef] [PubMed]
- Huseynov, A.; Akin, I.; Duerschmied, D.; Scharf, R.E. Cardiac Arrhythmias in Post-COVID Syndrome: Prevalence, Pathology, Diagnosis, and Treatment. Viruses 2023, 15, 389. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Xie, Y.; Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021, 594, 259–264. [Google Scholar] [CrossRef]
- Maheshwari, A.; Norby, F.L.; Soliman, E.Z.; Koene, R.J.; Rooney, M.R.; O’Neal, W.T.; Alonso, A.; Chen, L.Y. Abnormal P-Wave Axis and Ischemic Stroke: The ARIC Study (Atherosclerosis Risk in Communities). Stroke 2017, 48, 2060–2065. [Google Scholar] [CrossRef]
- Haloot, J.; Bhavaraju-Sanka, R.; Pillarisetti, J.; Verduzco-Gutierrez, M. Autonomic Dysfunction Related to Postacute SARS-CoV-2 Syndrome. Phys. Med. Rehabil. Clin. N. Am. 2023, 34, 563–572. [Google Scholar] [CrossRef]
- Huang, B.; Yan, H.; Hu, L.; Cao, G.; Wang, G.; Meng, J.; Li, W.; Liu, G.; Wang, J.; Le, W.; et al. The Contribution of Psychological Distress to Resting Palpitations in Patients Who Recovered from Severe COVID-19. Int. J. Gen. Med. 2021, 14, 9371–9378. [Google Scholar] [CrossRef]
- Keshtkar, A.; Bahrami, B. Relationship between COVID-19 Anxiety and Hypochondriasis in Retirees Aged 60 to 70 Years in Shiraz. J. Environ. Treat. Tech. 2021, 9, 737–740. [Google Scholar]
- Kasi, L.S.; Moorthy, B.A. Case Report on Care-Seeking Type Illness Anxiety Disorder After COVID-19 Infection. Case Rep. Psychiatry 2023, 2023, 3003499. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Cohen, C. Post-COVID-19 panic disorder in older adults: Two case reports. Am. J. Geriatr. Psychiatry 2021, 29, 58–59. [Google Scholar] [CrossRef]
- Javelot, H.; Weiner, L. Panic and pandemic: Narrative review of the literatüre on the links and risks of panic disorder as a consequence of the SARS-CoV-2 pandemic. Encephale 2021, 47, 38–42. [Google Scholar] [CrossRef] [PubMed]
| Individual Lobar Scores Based on Percentage of Involvement | COVID-19 Chest CT Severity Index Based on the Involvements of the Five Lobes | ||
|---|---|---|---|
| Lobar Involvement | Score | Total Score | Severity (Category) |
| 0–5% | 1 | <8 | Mild |
| 5–25% | 2 | 8–15 | Moderate |
| 26–49% | 3 | 16–25 | Severe |
| 50–75% | 4 | ||
| >75% | 5 | ||
| The Controls (Noun: 251) | Subgroup I (Recovered Mild COVID-19 Group, Noun: 142) | Subgroup II (Recovered Moderate COVID-19 Group, Noun: 57) | Subgroup III (Recovered Severe COVID-19 Group, Noun: 53) | p | |
|---|---|---|---|---|---|
| Age (Years) | 45.0 (26.0–55.0) | 44.0 (32.75–51.0) | 46.0 (39.50–55.50) | 50.0 (41.0–55.0) | a 1.0,b 0.29, c <0.001, d 0.02, e <0.001, f 0.68 |
| Gender (Female) (%) | 52.6 | 50.0 | 47.4 | 41.5 | a 0.62, b 0.57, c 0.19, d 0.86, e 0.29, f 0.67 |
| HT (%) | 23.1 | 23.2 | 31.6 | 35.8 | a 0.97, b 0.24, c 0.78, d 0.30, e 0.11, f 0.78 |
| Smoking (%) | 30.3 | 34.5 | 38.6 | 37.7 | a 0.39, b 0.29, c 0.37, d 0.70, e 0.80, f 1.00 |
| aPwa (%) | (4.0%) | (5.6%) | (7.0%) | (22.6%) | a 0.61, b 0.30, c <0.001, d 0.74, e 0.001, f 0.04 |
| Abnormal P-Wave Axis | ||
|---|---|---|
| R | p | |
| Recovered COVID-19 subgroups | −0.194 | 0.002 |
| Post COVID-19 recovery duration (week) | 0.047 | 0.461 |
| Number of positive PCR tests for COVID-19 | −0.110 | 0.081 |
| Recovered COVID-19 subject’s chest CT severity score | −0.245 | <0.001 |
| β | S.E. | Hazard Ratio | 95% Confidence Interval | p | ||
|---|---|---|---|---|---|---|
| HT | 0.997 | 0.470 | 2.710 | 1.079 | 6.804 | 0.03 |
| Smoking | 0.561 | 0.469 | 1.753 | 0.699 | 4.400 | 0.23 |
| Age | −0.028 | 0.023 | 0.973 | 0.929 | 1.018 | 0.23 |
| (Recovered Severe COVID-19 Subgroup) | 1.265 | 0.474 | 3.542 | 1.398 | 8.969 | 0.01 |
| Gender | 0.455 | 0.482 | 1.576 | 0.613 | 4.054 | 0.34 |
| Post COVID-19 recovery duration (week) | 0.007 | 0.008 | 1.007 | 0.992 | 1.022 | 0.38 |
| Number of positive PCR tests for COVID-19 | −0.418 | 0.384 | 0.658 | 0.310 | 1.398 | 0.27 |
| Constant | −1.422 | 2.144 | 0.241 | 0.50 | ||
| β | S.E. | Hazard Ratio | 95% Confidence Interval | p | ||
|---|---|---|---|---|---|---|
| HT | 1.003 | 0.479 | 2.725 | 1.065 | 6.972 | 0.03 |
| Smoking | 0.600 | 0.483 | 1.823 | 0.707 | 4.701 | 0.21 |
| Age | −0.023 | 0.024 | 0.977 | 0.932 | 1.023 | 0.32 |
| Recovered COVID-19 subject’s chest CT severity score | −0.110 | 0.033 | 0.896 | 0.840 | 0.955 | <0.001 |
| Gender | 0.378 | 0.491 | 1.460 | 0.558 | 3.819 | 0.44 |
| Post COVID-19 recovery duration (week) | 0.005 | 0.008 | 1.005 | 0.991 | 1.020 | 0.48 |
| Number of positive PCR tests for COVID-19 | −0.505 | 0.389 | 0.603 | 0.282 | 1.293 | 0.20 |
| Constant | 1.977 | 1.919 | 7.218 | 0.30 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yılmaz, M.; Mirzaoğlu, Ç. Recovery from Severe COVID-19 Is an Independent Predictor of Electrocardiographic Abnormal P-Wave Axis. Diagnostics 2024, 14, 1326. https://doi.org/10.3390/diagnostics14131326
Yılmaz M, Mirzaoğlu Ç. Recovery from Severe COVID-19 Is an Independent Predictor of Electrocardiographic Abnormal P-Wave Axis. Diagnostics. 2024; 14(13):1326. https://doi.org/10.3390/diagnostics14131326
Chicago/Turabian StyleYılmaz, Mücahid, and Çetin Mirzaoğlu. 2024. "Recovery from Severe COVID-19 Is an Independent Predictor of Electrocardiographic Abnormal P-Wave Axis" Diagnostics 14, no. 13: 1326. https://doi.org/10.3390/diagnostics14131326
APA StyleYılmaz, M., & Mirzaoğlu, Ç. (2024). Recovery from Severe COVID-19 Is an Independent Predictor of Electrocardiographic Abnormal P-Wave Axis. Diagnostics, 14(13), 1326. https://doi.org/10.3390/diagnostics14131326







