Abstract
The aim was to evaluate the prediction of house dust mite allergy in children diagnosed with allergic disease based on their skin moisture and sebum levels. This is a case–control study including children with asthma, allergic rhinitis (AR), and atopic dermatitis (AD) and a healthy control group. The participants’ skin moisture and sebum levels were measured non-invasively using a digital device. A total of 421 patients and 143 healthy children were included. The median value of skin moisture percentage was statistically significantly lower in asthma, AR, and AD patients compared to the control group (p < 0.001 for each). The median value of skin sebum percentage was significantly lower in asthma and AD patients compared to the control group (p = 0.002 and p = 0.003, respectively). ROC analysis was performed to assess the predictive value of skin moisture percentage for house dust mite allergy in respiratory allergic diseases (asthma and AR) and AD separately. Using a cut-off point of 35.5% for skin moisture in asthma and AR patients, the sensitivity and specificity were 81.3% and 56.5%, respectively. Although the specificity is low, the high sensitivity value is promising. The non-invasive measurement of skin sebum and moisture could provide convenience to clinicians in the diagnosis and management of allergic diseases.
1. Introduction
In recent years, an increase in the frequency of allergic diseases has been observed [1]. Therefore, the prevention and management of allergic diseases are of great importance for child health. Allergic diseases such as asthma, allergic rhinitis (AR), and atopic dermatitis (AD) typically result from an over-reaction of the immune system and can also be associated with environmental factors [2,3]. These environmental factors include exposure to allergens, air pollution, and dietary habits [4,5].
Asthma is one of the most common allergic and chronic diseases in childhood [6]. The most common symptoms of asthma include recurrent attacks of wheezing, coughing, shortness of breath, and chest discomfort [7,8]. Allergic rhinitis is characterized by the inflammation of the mucous membrane inside the nose when exposed to allergens. It manifests with symptoms such as a runny nose, nasal itching, sneezing, and nasal congestion [9]. Atopic dermatitis is the most common non-infectious inflammatory skin disease and exhibits a chronic recurrent nature [10]. The pathophysiology of AD involves a disrupted skin barrier [11,12]. Due to the impaired skin barrier, irritants and allergens can penetrate the skin, initiating the inflammation process associated with AD [13].
The human skin plays important homeostatic roles such as reducing water loss and contributing to thermoregulation [14]. Additionally, our skin serves as the first line of defense against environmental factors [15]. The most important function of the skin is to act as a barrier between the body’s internal and external environments [12]. Maintaining the barrier function of the skin is crucial, as it prevents microorganisms, allergens, and mechanical and chemical irritants from entering the body [12,16].
The presence of sebum and moisture in the skin is necessary for healthy skin. The moisture and sebum balance in the skin affects its barrier function [17]. An impaired skin barrier is the primary abnormality leading to AD [18]. One study in the literature reported that the lipid composition of the skin is different in patients with atopic dermatitis, and these patients exhibit abnormal lipid metabolism in the sebaceous glands [19].
The pathophysiological mechanisms of allergic diseases show similarities. The allergic march refers to the sequential development of asthma, AR, and AD. Although patients with asthma and AR may not have skin findings, allergic diseases can exhibit similar clinical courses and pathophysiological mechanisms, leading to an impaired skin barrier in these patients. There are publications in the literature regarding the low levels of skin moisture and sebum in patients with AD. In a previous study conducted in our clinic with a total of 342 children, including patients with respiratory allergy (asthma and AR) (n = 232) and a control group (n = 110), we evaluated the skin sebum and moisture content of children without skin findings of dust mite allergy [20]. In this study, we aimed to increase our sample size to make the results more generalizable and representative. Additionally, this study included patients with AD in addition to those with allergic airway diseases. In this context, this study aimed to evaluate the skin moisture and sebum levels in children diagnosed with allergic diseases. Furthermore, this study aimed to determine a cut-off point for these values in predicting the diagnosis of allergen sensitivity. Establishing a cut-off point for non-invasively measured skin sebum and moisture levels in predicting the diagnosis of allergic diseases could facilitate clinicians in clinical practice and guide patients at high risk to allergy clinics.
2. Materials and Methods
2.1. Study Design, Type, and Sample
This study is a case–control study. Children aged 0–18 years who presented to our pediatric allergy and immunology outpatient clinic with a diagnosis of asthma, AR, and AD were included in the study. Patients with skin diseases other than AD (scabies, seborrheic dermatitis, contact dermatitis, etc.) were excluded from the study. Patients who had taken a bath/shower in the last 24 h, those who had used medications such as local steroids on the day of examination, and those who had used topical products such as moisturizers or additional products (soap, shampoo, detergent, etc.) on the day of examination were excluded from the study due to their potential impact on the skin barrier.
For the study, a control group was formed among children aged 0–18 years who presented to our hospital’s pediatric health and diseases outpatient clinic without a diagnosis of skin disease or any other chronic disease. During the data collection period (approximately 4 months), this study aimed to include all children diagnosed with AR/asthma who visited our pediatric allergy and immunology outpatient clinic and met the inclusion criteria as the patient group. This study aimed to include the maximum number of children in the control group during the data collection period.
2.2. Evaluations and Measurement of Skin Sebum and Moisture
The sociodemographic characteristics and skin sebum and moisture values of the patients and the control group were examined. Prior to measurement, children rested for nearly 10–20 min to reduce the effect of physical activity on skin moisture and sebum. At least 1 day before skin moisture and sebum measurement, patients were asked not to use moisturizing cream, sunscreen, or other creams, baby oils, or a coarse scrub in the bath/shower. The skin area to be measured was cleaned with plain water at home on the day of analysis. Measurements were performed in a room with a temperature of approximately 20 °C and air humidity between 40% and 60%. After each measurement, the device was cleaned with an alcohol-containing soft cloth.
Skin moisture and sebum levels were measured using a portable pen-shaped LCD Display Digital Skin Moist Oil Analyzer (Reyoung-Beauty, Shenzhen, China) from the cubital fossa. The device measures moisture and sebum levels using a non-invasive method called bioimpedance. Bioimpedance describes the ability of biological tissue to resist the flow of electric currents, which is a result of the passive electrical properties inherent in biological substances [21]. This device was commercially produced for measuring the moisture and sebum levels of the skin. Measurements were performed on the bare skin for a few seconds by placing the probe of the device on the antecubital fossa of the non-dominant upper limb. The device provides results for the sebum and moisture levels of the skin as percentages. The measurable moisture range is from 0% to 99.9%, and the measurable sebum range is from 16.0% to 63.0%. The device is lightweight, portable, and easy to use, with dimensions of 128 × 26 × 34 mm. While the device has not been validated for diagnostic tests on patients with allergic diseases, previous studies in the literature have utilized it to evaluate skin moisture and sebum content. Additionally, in our study, the eosinophil count, total IgE levels, and the presence of dust mite allergy were evaluated in the patient group. Laboratory values were retrieved from the hospital’s database. The presence of dust mite allergy was determined by having a positive skin prick test (SPT) and/or specific IgE test result.
2.3. Statistical Analysis
Data were analyzed with the SPSS (Statistical Package for Social Sciences for Windows 25.0, Armonk, NY, USA) program. Descriptive data were presented with the median, minimum, and maximum values, number (n), and percentages (%). The Chi-Square test was used for the comparison of categorized data. The conformity of continuous variables to normal distribution was examined with histograms, probability plots, and Kolmogorov–Smirnov/Shapiro–Wilk tests. The Mann–Whitney U test was used to compare continuous variables that did not conform to normal distribution. The capacity of the skin moisture (%) value in predicting test positivity for house dust mites (skin prick test and/or specific IgE positivity) was analyzed using ROC (Receiver Operating Characteristics) curve analysis. By examining the area under the ROC curve (AUC), we assessed the overall ability of the test to discriminate between positive and negative cases. When a significant cut-off value was observed, the sensitivity and specificity were presented. The ROC curve provides a visual representation of the trade-off between sensitivity and specificity, and the AUC value quantifies the test’s diagnostic accuracy, with a value closer to 1 indicating better performance. The statistical significance level was set at p < 0.05.
2.4. Ethics
Ethics committee approval was obtained from the Health Sciences University Ümraniye Training and Research Ethics Committee on 5 October 2023 with decision number 356. Before participating in the study, participants and parents were informed about the study, and their consent was obtained.
3. Results
In our study, 190 (33.7%) patients with AR, 131 (23.2%) patients with asthma, and 100 (17.7%) patients with AD were included. A total of 143 (25.4%) children were included in the control group. Of the patient group, 53.0% (n = 223) were male, and 47.0% (n = 198) were female. In the control group, 45.5% (n = 65) were male and 54.5% (n = 78) were female. There was no statistically significant difference between the patient and control groups in terms of gender and age (p > 0.05) (Table 1).

Table 1.
The age and gender of the patients and control group.
The laboratory parameters of the patients were evaluated. The median values of the absolute eosinophil and eosinophil counts (%) were 260.0 cells/µL (0–2260.0) and 3.2% (0–22.0), respectively, in asthma patients. The median value of total IgE was 99.5 IU/mL (3.0–3840.0). In AR patients, the median values of absolute eosinophils and eosinophils (%) were 300.0 cells/µL (30.0–4160.0) and 3.6% (0.4–21.0), respectively. The median value of total IgE was 177.0 IU/mL (1.0–1802.0). The median values of the absolute eosinophil and eosinophil counts (%) were 430.0 cells/µL (40.0–1840.0), 4.9% (0.5–23.7), respectively, in AD patients. The median value of total IgE was 85.0 IU/mL (2.0–1914.0) (Table 2).

Table 2.
The eosinophil counts, total IgE levels, and allergy test positivity of the patients.
Patients were evaluated for allergens they were sensitive to based on skin prick tests and/or specific IgE levels in the blood. House dust mite allergy was observed in asthma, AR, and AD patients at rates of 42.1% (n = 80), 61.1% (n = 80), and 54.0% (n = 54), respectively (Table 2).
The percentages of the skin moisture and skin sebum of the patients and control group were evaluated. The median value of skin moisture percentage was statistically significantly lower in asthma, AR, and AD patients compared to the control group (p < 0.001 for each). The median value of skin moisture percentage was 37.0% (19.0–69.0) in the control group, while it was 35.0% (12.0–56.0), 34.0% (11.0–56.0), and 31.0% (range: 10.0–45.0) in asthma, AR, and AD patients, respectively. The median value of skin sebum percentage was significantly lower in asthma and AD patients compared to the control group (p = 0.002 and p = 0.003, respectively). Although the median value of skin sebum percentage in AR patients was lower than in the control group, it did not reach statistical significance (p = 0.170) (Table 3).

Table 3.
Moisture and sebum of patient and control group.
The skin sebum and moisture ratios of patients with asthma, AR, and AD were evaluated according to the presence of house dust mite allergy. In all three patient groups, the skin moisture percentage was lower in those with house dust mite allergen sensitivity compared to those without. This difference was statistically significant in the asthma and AR patient groups (p < 0.001 for both). However, in AD patients, there was no significant relationship between the presence of house dust mite allergy and skin moisture percentage (p = 0.089). When the relationship between the presence of house dust mites and skin sebum percentages was evaluated, no statistically significant relationship was observed in any of the three patient groups (p > 0.005) (Table 4).

Table 4.
Moisture and sebum content of asthma, AR, and AD patients with and without house dust mite allergy.
ROC analysis was performed to evaluate the predictive value of skin moisture percentage for house dust mite allergy in respiratory allergic diseases (asthma and AR) and AD, separately. Taking 35.5 as the cut-off point for the percent skin moisture of asthma and AR patients, sensitivity and specificity were 81.3% and 56.5%, respectively. The AUC was 71.4% (95.0% CI: 65.8–77.0%) (p < 0.001). Taking 31.5 as the cut-off point for the percent skin moisture of AD patients, sensitivity and specificity were 64.8% and 52.2%, respectively. The AUC was 59.8% (95.0% CI: 48.6–71.0%) (p = 0.092) (Figure 1).
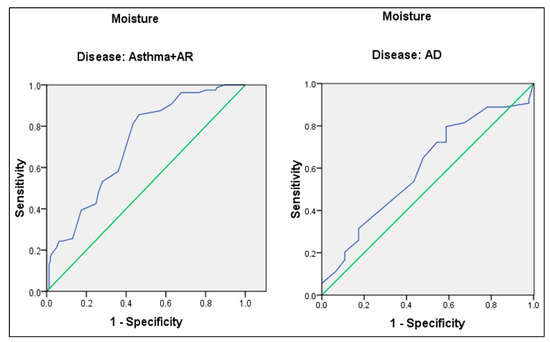
Figure 1.
ROC curve for skin moisture content of asthma, AR, and AD patients. Blue line: ROC curve, Green line: Diagonal reference line.
According to the ROC analysis, the area under curve was low to evaluate the predictive value of skin sebum percentage for house dust mite allergy in respiratory allergic diseases (asthma and AR) and AD.
4. Discussion
The prevalence of allergic diseases is increasing, and allergic diseases constitute a significant burden of disease in individuals and society. Therefore, the appropriate and practical management of allergic diseases is of utmost importance. More advanced diagnostic tests and facilities such as skin tests that should be applied for the diagnosis of allergic diseases are generally available in large centers such as tertiary hospitals in our country. In this case, it takes some time for children with allergic symptoms to access an allergy doctor and diagnostic center for diagnosis and evaluation. In some cases, there may even be a loss of follow-up. For this reason, in children whose clinical history and examination suggest allergic diseases and allergen sensitization, skin sebum and moisture measurements with non-invasive methods will make it possible to start the first-line treatment of high-risk children in terms of allergen sensitization until access is gained to a pediatric allergist.
In our study, the median values of skin moisture and skin sebum percentage, which we evaluated with a non-invasive method, were lower in asthma, AR, and AD patients compared to the control group. While this decrease in skin moisture was statistically significant in all three disease groups, the decrease in skin sebum was statistically significant only in asthma and AD patients compared to the control group. In our previous study conducted in our clinic, the skin sebum and moisture content of asthma and AR patients without skin manifestations were found to be significantly lower than the control group [20]. In a study conducted in our country in children with AD, the skin moisture and skin sebum content of children with AD were found to be lower than those of healthy children [22]. The results of this study and the results in the literature suggest that skin moisture and oil balance play an important role in the pathophysiology of AR and asthma as well as AD.
The skin sebum and moisture levels of asthma, AR, and AD patients were evaluated according to the presence of house dust allergen sensitization. In all three patient groups, the percentage of skin moisture was lower in patients with house dust mite allergen sensitization than in those without. This difference was statistically significant in asthma and AR patient groups. There was no statistically significant relationship between house dust mite allergen sensitization and skin sebum. Similar to our previous study, in asthma and AR patients with house dust mite allergen sensitization, the percentages of skin moisture and skin sebum were lower in patients with house dust mite allergen sensitization compared to those without house dust mite allergen sensitization. This decrease in skin moisture was statistically significant [20]. According to the results, it is likely that exposure to inhaled allergens may also affect the skin barrier, which may lead to the loss of skin moisture and a decrease in lipids. According to a study in the literature, it has been reported that even on non-eczematous skin, patients with AD experience impaired barrier function. This impairment was linked to increased sensitization to aeroallergens and could play a role in the development of allergic respiratory symptoms [23]. Further studies are required to elucidate the pathophysiological mechanism underlying the decrease in moisture and sebum content in the skin of asthma and AR patients without skin involvement.
In our study, ROC analysis was performed to evaluate the predictive value of skin moisture percentage for house dust mite allergy both in respiratory allergic diseases and AD. Using a cut-off point of 35.5% for skin moisture percentage, we found a sensitivity of 81.3% and a specificity of 56.5%. Even though the specificity is low, our results indicate that 81.3% of asthma and AR patients with a house dust mite allergy can be identified by measuring skin moisture, which corresponds to approximately four out of five patients—a notably high rate. Similarly, in our previous study, we found a cut-off point of 35.5% for skin moisture percentage in asthma and AR patients, with a sensitivity of 78.9% and a specificity of 57.9% [20]. In our study, in addition to our previous research, we calculated a cut-off point for skin moisture that could predict house dust mite sensitivity in AD patients. However, the sensitivity and specificity values were lower than those in asthma and AR patients (31.5 as the cut-off point, sensitivity and specificity were 64.8% and 52.2%, respectively). Multicenter studies with a higher sample size could be planned to evaluate a cut-off point for skin moisture with high sensitivity and specificity for predicting house dust mite sensitivity in AD patients. Establishing a cut-off point for skin moisture percentage to predict a house dust mite allergy could be a valuable alternative for patients who cannot undergo skin testing or specific IgE level assessment for house dust allergy.
Limitations and Strengths
There are no similar studies in the literature evaluating the capacity of skin moisture to predict house dust mite allergy in asthma, AR, and AD patients simultaneously. Thanks to our study, it was possible to evaluate skin sebum and moisture in both respiratory allergic diseases and AD with skin involvement and to compare them with the control group. The non-invasive assessment of skin moisture and sebum may shed light on other innovative approaches in the management of allergic diseases. Our study has limitations as well as strengths. Our data collection period was shorter than anticipated, which caused us to not reach the targeted high sample size. This situation creates a limitation in terms of the representation of the population. In addition, the fact that our study was conducted in a single center creates a limitation in terms of the generalizability of the results.
5. Conclusions
In our study with asthma, AR, and AD patients, the percentage of skin moisture was significantly lower in the patients than in the control group. Skin moisturization, which is one of the approaches to protect the function of the skin barrier while planning treatment in AD patients, is frequently applied in clinical practice. In patients with respiratory allergic diseases such as asthma and AR, skin involvement is not seen in all patients as it is in atopic dermatitis AD. Therefore, the use of moisturizers in these patients remains less emphasized.
In clinical practice, the measurement of skin moisture and sebum by a non-invasive method in children may be useful in evaluating skin moisture and sebum balance and protecting the barrier function of the skin. Since the presence of allergen sensitization such as house dust mites may be possible in values below 35.5%, which is the cut-off value we found for skin moisture in asthma and AR, our study results will be guiding in terms of referring these children to pediatric allergy clinics. There are studies in the literature regarding predictors of allergic situations [24,25]. Our study also contributes to this field in the literature. In addition, our study results may shed light on the adoption of holistic and innovative approaches in the treatment and management of allergic diseases.
Author Contributions
Conceptualization, S.Ç., U.A. and M.Y.Ö.; methodology, S.Ç., U.A. and M.Y.Ö.; validation, S.Ç., U.A. and M.Y.Ö.; formal analysis, S.Ç., U.A. and M.Y.Ö.; investigation, S.Ç., U.A. and M.Y.Ö.; resources, S.Ç., U.A. and M.Y.Ö.; data curation, U.A.; writing—original draft preparation, S.Ç., U.A. and M.Y.Ö.; writing—review and editing, S.Ç., U.A. and M.Y.Ö.; visualization, U.A.; supervision, M.Y.Ö. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of University of Health Sciences, Ümraniye Training and Research Hospital Istanbul, Türkiye (protocol code 356 date of 5 October 2023).
Informed Consent Statement
Written informed consent was obtained from mothers.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ha, J.; Lee, S.W.; Yon, D.K. Ten-year trends and prevalence of asthma, allergic rhinitis, and atopic dermatitis among the Korean population, 2008–2017. Clin. Exp. Pediatr. 2020, 63, 278. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.C.; Dahlin, A.; Wang, A.L. The role of environmental risk factors on the development of childhood allergic rhinitis. Children 2021, 8, 708. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, Y.; Zhang, H.; Hu, L.; Liu, J.; Wang, L.; Wang, Q. Pathogenesis of allergic diseases and implications for therapeutic interventions. Signal Transduct Target Ther. 2023, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Tiotiu, A.I.; Novakova, P.; Nedeva, D.; Chong-Neto, H.J.; Novakova, S.; Steiropoulos, P.; Kowal, K. Impact of air pollution on asthma outcomes. Int. J. Environ. Res. Public Health 2020, 17, 6212. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, E.; Małgorzewicz, S.; Gruchała-Niedoszytko, M.; Skotnicka, M.; Jassem, E. Dietary habits in children with respiratory allergies: A single-center polish pilot study. Nutrients 2020, 12, 1521. [Google Scholar] [CrossRef] [PubMed]
- Pijnenburg, M.W.; Fleming, L. Advances in understanding and reducing the burden of severe asthma in children. Lancet Respir. Med. 2020, 8, 1032–1044. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Feng, J.; Xia, J.; Wu, Q.; Yang, H.; Ma, Q. Frequency of signs and symptoms in persons with asthma. Respir. Care 2020, 65, 252–264. [Google Scholar] [CrossRef]
- Sarikloglou, E.; Fouzas, S.; Paraskakis, E. Prediction of Asthma Exacerbations in Children. J. Pers. Med. 2024, 14, 20. [Google Scholar] [CrossRef]
- Bernstein, J.A.; Bernstein, J.S.; Makol, R.; Ward, S. Allergic Rhinitis: A Review. JAMA 2024, 331, 866–877. [Google Scholar] [CrossRef]
- Grobe, W.; Bieber, T.; Novak, N. Pathophysiology of atopic dermatitis. J. Dtsch. Dermatol. Ges. 2019, 17, 433–440. [Google Scholar] [CrossRef]
- Peng, W.; Novak, N. Pathogenesis of atopic dermatitis. Clin. Exp. Allergy 2015, 45, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Seok, J.K.; Kang, H.C.; Cho, Y.-Y.; Lee, H.S.; Lee, J.Y. Skin Barrier Abnormalities and Immune Dysfunction in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 2867. [Google Scholar] [CrossRef] [PubMed]
- Kolb, L.; Ferrer-Bruker, S.J. Atopic Dermatitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK448071/ (accessed on 12 May 2024).
- Chambers, E.S.; Vukmanovic-Stejic, M. Skin barrier immunity and ageing. Immunology 2020, 160, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, M. Skin Barrier Function and the Microbiome. Int. J. Mol. Sci. 2022, 23, 13071. [Google Scholar] [CrossRef] [PubMed]
- Boer, M.; Duchnik, E.; Maleszka, R.; Marchlewicz, M. Structural and biophysical characteristics of human skin in maintaining proper epidermal barrier function. Postepy Dermatol. Alergol. 2016, 33, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ezerskaia, A.; Pereira, S.F.; Urbach, H.P.; Verhagen, R.; Varghese, B. Quantitative and simultaneous non-invasive measurement of skin hydration and sebum levels. Biomed. Opt. Express 2016, 7, 2311–2320. [Google Scholar] [CrossRef] [PubMed]
- van den Bogaard, E.H.; Elias, P.M.; Goleva, E.; Berdyshev, E.; Smits, J.P.H.; Danby, S.G.; Cork, M.J.; Leung, D.Y.M. Targeting Skin Barrier Function in Atopic Dermatitis. J. Allergy Clin. Immunol. Pract. 2023, 11, 1335–1346. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Qiu, Z.; Zhu, R.; Wang, S.; Gu, C.; Yao, X.; Li, W. Dysregulated lipidome of sebum in patients with atopic dermatitis. Allergy 2023, 78, 1524–1537. [Google Scholar] [CrossRef] [PubMed]
- Altaş, U.; Altaş, Z.M.; Ercan, N.; Özkars, M.Y. The Effect of House Dust Sensitization on Skin Sebum and Moisture in Children with Allergic Respiratory Diseases. Children 2023, 10, 1483. [Google Scholar] [CrossRef]
- Kekonen, A.; Bergelin, M.; Eriksson, J.-E.; Vaalasti, A.; Ylänen, H.; Kielosto, S.; Viik, J. Bioimpedance method for monitoring venous ulcers: Clinical proof-of-concept study. Biosens. Bioelectron. 2021, 178, 112974. [Google Scholar] [CrossRef]
- Güneş, H.; Nazik, H.; Özkars, M.Y.; Parlak, N.; Yildiz, A.; Duyuran, Ö.; Yalçin, B.A. The assessment of skin sebum and moisture content of infants with atopic dermatitis. Turk. J. Med. Sci. 2020, 50, 844–848. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, F.; Piacentini, G.L.; Piazza, M.; Sandri, M.; Boner, A.L.; Peroni, D.G. Correlation of skin barrier impairment in atopic dermatitis with aeroallergen sensitization. Allergy Asthma Proc. 2015, 36, e127–e133. [Google Scholar] [CrossRef] [PubMed]
- Aydin, E.; Callahan, D.L.; Chong, L.; Azizoglu, S.; Gokhale, M.; Suphioglu, C. The Plight of the Metabolite: Oxidative Stress and Tear Film Destabilisation Evident in Ocular Allergy Sufferers across Seasons in Victoria, Australia. Int. J. Mol. Sci. 2024, 25, 4019. [Google Scholar] [CrossRef] [PubMed]
- Muntean, I.A.; Bocsan, I.C.; Wiest, L.K.; Pintea, I.; Dobrican, C.T.; Duca, E.; Ureche, C.; Buzoianu, A.D.; Deleanu, D. Predictive Factors for Oral Immune Modulation in Cow Milk Allergy. Nutrients 2022, 14, 494. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).