The Degrees of Coronary Heart Disease and the Degrees of New-Onset Blepharitis: A Nationwide Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Participant Selection
2.3. Main Outcome
2.4. Confounding Factors
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarwar, N.; Thompson, A.J.; Di Angelantonio, E. Markers of inflammation and risk of coronary heart disease. Dis. Markers 2009, 26, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.O.; Budoff, M. Effect of statins on atherosclerotic plaque. Trends Cardiovasc. Med. 2019, 29, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Al-Lamee, R.K.; Nowbar, A.N.; Francis, D.P. Percutaneous coronary intervention for stable coronary artery disease. Heart 2019, 105, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Huang, J.; Zhu, K.; Chen, Q. Percutaneous coronary intervention versus coronary artery bypass grafting in patients with coronary heart disease and type 2 diabetes mellitus: Cumulative meta-analysis. Clin. Cardiol. 2021, 44, 899–906. [Google Scholar] [CrossRef] [PubMed]
- South, T. Coronary artery bypass surgery. Crit. Care Nurs. Clin. N. Am. 2011, 23, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, U.S.; Buggeskov, K.B.; Nielsen, E.E.; Sethi, N.J.; Carranza, C.L.; Gluud, C.; Jakobsen, J.C. Coronary artery bypass surgery plus medical therapy versus medical therapy alone for ischaemic heart disease: A protocol for a systematic review with meta-analysis and trial sequential analysis. Syst. Rev. 2019, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Zhong, P.; Li, Z.; Lin, Y.; Peng, Q.; Huang, M.; Jiang, L.; Li, C.; Kuang, Y.; Cui, S.; Yu, D.; et al. Retinal microvasculature impairments in patients with coronary artery disease: An optical coherence tomography angiography study. Acta Ophthalmol. 2022, 100, 225–233. [Google Scholar] [CrossRef]
- Wirtz, P.H.; von Känel, R. Psychological Stress, Inflammation, and Coronary Heart Disease. Curr. Cardiol. Rep. 2017, 19, 111. [Google Scholar] [CrossRef]
- Wu, X.F.; Huang, J.Y.; Chiou, J.Y.; Chen, H.H.; Wei, J.C.; Dong, L.L. Increased risk of coronary heart disease among patients with primary Sjögren’s syndrome: A nationwide population-based cohort study. Sci. Rep. 2018, 8, 2209. [Google Scholar] [CrossRef]
- Vural, U.; Kizilay, M.; Aglar, A.A. Coronary Involvement in Behçet’s Disease: What are its Risks and Prognosis? (Rare Cases and Literature Review). Braz. J. Cardiovasc. Surg. 2019, 34, 749–758. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.; Li, C.; Wu, W.; Liu, J.; Zhang, F.; Zheng, W. Coronary involvement in patients with Behçet’s disease. Clin. Rheumatol. 2019, 38, 2835–2841. [Google Scholar] [CrossRef] [PubMed]
- Priyamvara, A.; Dey, A.K.; Bandyopadhyay, D.; Katikineni, V.; Zaghlol, R.; Basyal, B.; Barssoum, K.; Amarin, R.; Bhatt, D.L.; Lavie, C.J. Periodontal Inflammation and the Risk of Cardiovascular Disease. Curr. Atheroscler. Rep. 2020, 22, 28. [Google Scholar] [CrossRef] [PubMed]
- Escobar, E. Hypertension and coronary heart disease. J. Hum. Hypertens. 2002, 16 (Suppl. S1), S61–S63. [Google Scholar] [CrossRef] [PubMed]
- Rana, J.S.; Nieuwdorp, M.; Jukema, J.W.; Kastelein, J.J. Cardiovascular metabolic syndrome—An interplay of, obesity, inflammation, diabetes and coronary heart disease. Diabetes Obes. Metab. 2007, 9, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Girkin, C.A.; Kannel, W.B.; Friedman, D.S.; Weinreb, R.N. Glaucoma risk factor assessment and prevention: Lessons from coronary heart disease. Am. J. Ophthalmol. 2004, 138, S11–S18. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, T.F.; Bonfioli, A.A. Blepharitis. Semin. Ophthalmol. 2010, 25, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Chen, H.C.; Lin, H.W.; Huang, J.Y.; Chao, S.C.; Yeh, C.B.; Lin, H.Y.; Yang, S.F. Blepharitis as an early sign of metabolic syndrome: A nationwide population-based study. Br. J. Ophthalmol. 2018, 102, 1283–1287. [Google Scholar] [CrossRef]
- Harrington, R.A. Targeting Inflammation in Coronary Artery Disease. N. Engl. J. Med. 2017, 377, 1197–1198. [Google Scholar] [CrossRef]
- Yang, J.; Feng, L.; Ren, J.; Wu, G.; Chen, S.; Zhou, Q.; Du, Z.; Zhang, S.; Hu, C.; Wu, X.; et al. Correlation between the severity of periodontitis and coronary artery stenosis in a Chinese population. Aust. Dent. J. 2013, 58, 333–338. [Google Scholar] [CrossRef]
- Roos, C.J.; Quax, P.H.; Jukema, J.W. Cardiovascular metabolic syndrome: Mediators involved in the pathophysiology from obesity to coronary heart disease. Biomark. Med. 2012, 6, 35–52. [Google Scholar] [CrossRef]
- Wang, N.; Mustafa, R.; Zuber, V.; Rodgers, A.; Dehghan, A. Association between systolic blood pressure and low-density lipoprotein cholesterol with coronary heart disease according to age. PLoS ONE 2023, 18, e0295004. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; Lucan, S.C.; O’Keefe, J.H. The Evidence for Saturated Fat and for Sugar Related to Coronary Heart Disease. Prog. Cardiovasc. Dis. 2016, 58, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sun, K.; Zhao, R.; Hu, J.; Hao, Z.; Wang, F.; Lu, Y.; Liu, F.; Zhang, Y. Inflammatory biomarkers of coronary heart disease. Front. Biosci. (Schol. Ed.) 2018, 10, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Garvin, P.; Jonasson, L.; Nilsson, L.; Falk, M.; Kristenson, M. Plasma Matrix Metalloproteinase-9 Levels Predict First-Time Coronary Heart Disease: An 8-Year Follow-Up of a Community-Based Middle Aged Population. PLoS ONE 2015, 10, e0138290. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ye, T.; Chen, L.; Jin, T.; Sheng, Y.; Wu, G.; Zong, G. Systemic immune-inflammation index predicts the severity of coronary stenosis in patients with coronary heart disease. Coron. Artery Dis. 2021, 32, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.M.; Chien, W.C.; Chen, Y.H.; Sun, C.A.; Chung, C.H.; Chen, J.T.; Chen, C.L. Increased Risk of Acute Coronary Syndrome in Ankylosing Spondylitis Patients with Uveitis: A Population-Based Cohort Study. Front. Immunol. 2022, 13, 890543. [Google Scholar] [CrossRef] [PubMed]
- Masood, F.; Ehrenpreis, E.D.; Rubin, G.; Russell, J.; Guru, S.; Luzzi, P. State of the art review: Coronary artery disease in patients with inflammatory bowel disease: Mechanisms, prevalence, and outcomes. Acta Cardiol. 2022, 77, 297–306. [Google Scholar] [CrossRef]
- Kopin, L.; Lowenstein, C. Dyslipidemia. Ann. Intern. Med. 2017, 167, ITC81–ITC96. [Google Scholar] [CrossRef]
- Shaya, G.E.; Leucker, T.M.; Jones, S.R.; Martin, S.S.; Toth, P.P. Coronary heart disease risk: Low-density lipoprotein and beyond. Trends Cardiovasc. Med. 2022, 32, 181–194. [Google Scholar] [CrossRef]
- Vassalle, C.; Bianchi, S.; Bianchi, F.; Landi, P.; Battaglia, D.; Carpeggiani, C. Oxidative stress as a predictor of cardiovascular events in coronary artery disease patients. Clin. Chem. Lab. Med. 2012, 50, 1463–1468. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; Karpecki, P.M.; Perez, V.L. Treatment of blepharitis: Recent clinical trials. Ocul. Surf. 2014, 12, 273–284. [Google Scholar] [CrossRef] [PubMed]
- McCulley, J.P.; Shine, W.E. Eyelid disorders: The meibomian gland, blepharitis, and contact lenses. Eye Contact Lens 2003, 29, S93–S95; discussion S115–S118, S192–S114. [Google Scholar] [CrossRef] [PubMed]
- Thygeson, P. Complications of staphylococcic blepharitis. Am. J. Ophthalmol. 1969, 68, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Nemet, A.Y.; Vinker, S.; Kaiserman, I. Associated morbidity of blepharitis. Ophthalmology 2011, 118, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.T.; Lee, S.H.; Chun, Y.S.; Kim, J.C. Tear cytokines and chemokines in patients with Demodex blepharitis. Cytokine 2011, 53, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Acera, A.; Rocha, G.; Vecino, E.; Lema, I.; Durán, J.A. Inflammatory markers in the tears of patients with ocular surface disease. Ophthalmic Res. 2008, 40, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Pinna, A.; Blasetti, F.; Zinellu, A.; Carru, C.; Solinas, G. Meibomian gland dysfunction and hypercholesterolemia. Ophthalmology 2013, 120, 2385–2389. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.M.; Chung, C.H.; Chen, Y.H.; Chien, W.C.; Chien, K.H. Statin Use Is Associated with a Lower Risk of Blepharitis: A Population-Based Study. Front. Med. 2022, 9, 820119. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.P.; Aggarwal, K.K.; Zhang, P.Y. Omega-3 fatty acids and cardiovascular disease. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 441–445. [Google Scholar]
- Macsai, M.S. The role of omega-3 dietary supplementation in blepharitis and meibomian gland dysfunction (an AOS thesis). Trans. Am. Ophthalmol. Soc. 2008, 106, 336–356. [Google Scholar]
- Hojman, L.; Karsulovic, C. Cardiovascular Disease-Associated Skin Conditions. Vasc. Health Risk Manag. 2022, 18, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Ascott, A.; Mulick, A.; Yu, A.M.; Prieto-Merino, D.; Schmidt, M.; Abuabara, K.; Smeeth, L.; Roberts, A.; Langan, S.M. Atopic eczema and major cardiovascular outcomes: A systematic review and meta-analysis of population-based studies. J. Allergy Clin. Immunol. 2019, 143, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, H.; Gharebaghi, R.; Heidary, F. Diabetes as a possible predisposer for blepharitis. Can. J. Ophthalmol. 2008, 43, 485. [Google Scholar] [CrossRef]
- Khamis, R.Y.; Ammari, T.; Mikhail, G.W. Gender differences in coronary heart disease. Heart 2016, 102, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Pakzad, R.; Heydarian, S.; Aghamirsalim, M.; Asadollahi, M.; Yekta, A.; Khabazkhoob, M. The prevalence of anterior blepharitis in an elderly population of Iran; The Tehran geriatric eye study. Cont. Lens Anterior Eye 2021, 44, 101429. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Fang, J.; Schieb, L.; Park, S.; Casper, M.; Gillespie, C. Prevalence and Trends of Coronary Heart Disease in the United States, 2011 to 2018. JAMA Cardiol. 2022, 7, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, P.; Wickramasinghe, K.; Wilkins, E.; Townsend, N. Trends in the epidemiology of cardiovascular disease in the UK. Heart 2016, 102, 1945–1952. [Google Scholar] [CrossRef]
- Zhu, K.F.; Wang, Y.M.; Zhu, J.Z.; Zhou, Q.Y.; Wang, N.F. National prevalence of coronary heart disease and its relationship with human development index: A systematic review. Eur. J. Prev. Cardiol. 2016, 23, 530–543. [Google Scholar] [CrossRef]
- Dalen, J.E.; Alpert, J.S.; Goldberg, R.J.; Weinstein, R.S. The epidemic of the 20(th) century: Coronary heart disease. Am. J. Med. 2014, 127, 807–812. [Google Scholar] [CrossRef]
- Voutilainen, A.; Brester, C.; Kolehmainen, M.; Tuomainen, T.P. Epidemiological analysis of coronary heart disease and its main risk factors: Are their associations multiplicative, additive, or interactive? Ann. Med. 2022, 54, 1500–1510. [Google Scholar] [CrossRef]
- Rouen, P.A.; White, M.L. Dry Eye Disease: Prevalence, Assessment, and Management. Home Healthc. Now 2018, 36, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Wróbel-Dudzińska, D.; Osial, N.; Stępień, P.W.; Gorecka, A.; Żarnowski, T. Prevalence of Dry Eye Symptoms and Associated Risk Factors among University Students in Poland. Int. J. Environ. Res. Public Health 2023, 20, 1313. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, K.; Matsumura, S.; Hatef, E.; Akpek, E.K. Interventions for chronic blepharitis. Cochrane Database Syst. Rev. 2012, 2012, CD005556. [Google Scholar] [CrossRef] [PubMed]

| Clinical Characteristics | Mild CHD Group (N = 593,048) | Severe CHD Group (N = 296,524) | ASD |
|---|---|---|---|
| Sex | 0.0000 | ||
| Male | 385,810 (65.05%) | 192,905 (65.05%) | |
| Female | 207,238 (34.95%) | 103,619 (34.95%) | |
| Age | 0.0009 | ||
| <40 | 15,667 (2.64%) | 7693 (2.60%) | |
| 40–49 | 47,793 (8.04%) | 24,844 (8.39%) | |
| 50–59 | 115,373 (19.45%) | 57,283 (19.32%) | |
| 60–69 | 178,836 (30.17%) | 88,296 (29.77%) | |
| >=70 | 235,379 (39.69%) | 118,408 (39.93%) | |
| Occupation | 0.0012 | ||
| Government employee | 28,914 (4.90%) | 12,882 (4.32%) | |
| Worker | 298,005 (50.23%) | 148,761 (50.20%) | |
| Farmer and fisherman | 121,002 (20.45%) | 62,890 (21.22%) | |
| Low-income | 7800 (1.28%) | 3557 (1.21%) | |
| Others | 137,397 (23.14%) | 68,434 (23.06%) | |
| Co-morbidity | |||
| Hypertension | 387,379 (65.32%) | 228,264 (76.38%) | 0.0197 |
| DM | 179,575 (30.28%) | 129,344 (43.62%) | 0.0362 |
| Hyperlipidemia | 252,164 (42.52%) | 172,251 (58.09%) | 0.0276 |
| Cerebrovascular disease | 55,035 (9.28%) | 38,044 (12.83%) | 0.0155 |
| Peripheral vascular disease | 16,012 (2.70%) | 10,912 (3.68%) | 0.0098 |
| Rheumatoid arthritis | 6998 (1.18%) | 3884 (1.31%) | 0.0004 |
| Sjogren syndrome | 8065 (1.36%) | 4389 (1.44%) | 0.0004 |
| Co-medication | |||
| Non-steroid anti-inflammatory drug | 353,872 (59.67%) | 178,033 (60.04%) | 0.0003 |
| Systemic corticosteroids | 106,867 (18.02%) | 64,494 (21.75%) | 0.0034 |
| Biguanides | 112,679 (19.00%) | 72,293 (24.38%) | 0.0062 |
| Dipeptidyl peptidase-4 inhibitor | 64,939 (10.95%) | 50,202 (16.93%) | 0.0267 |
| Glucagon-like peptide-1 agonists | 949 (0.16%) | 1127 (0.38%) | 0.0010 |
| Sodium–glucose cotransporter-2 inhibitors | 8718 (1.47%) | 6909 (2.33%) | 0.0009 |
| Statin | 225,002 (37.94%) | 140,226 (47.29%) | 0.0388 |
| Event | Mild CHD Group | Severe CHD Group | p Value |
|---|---|---|---|
| Blepharitis | |||
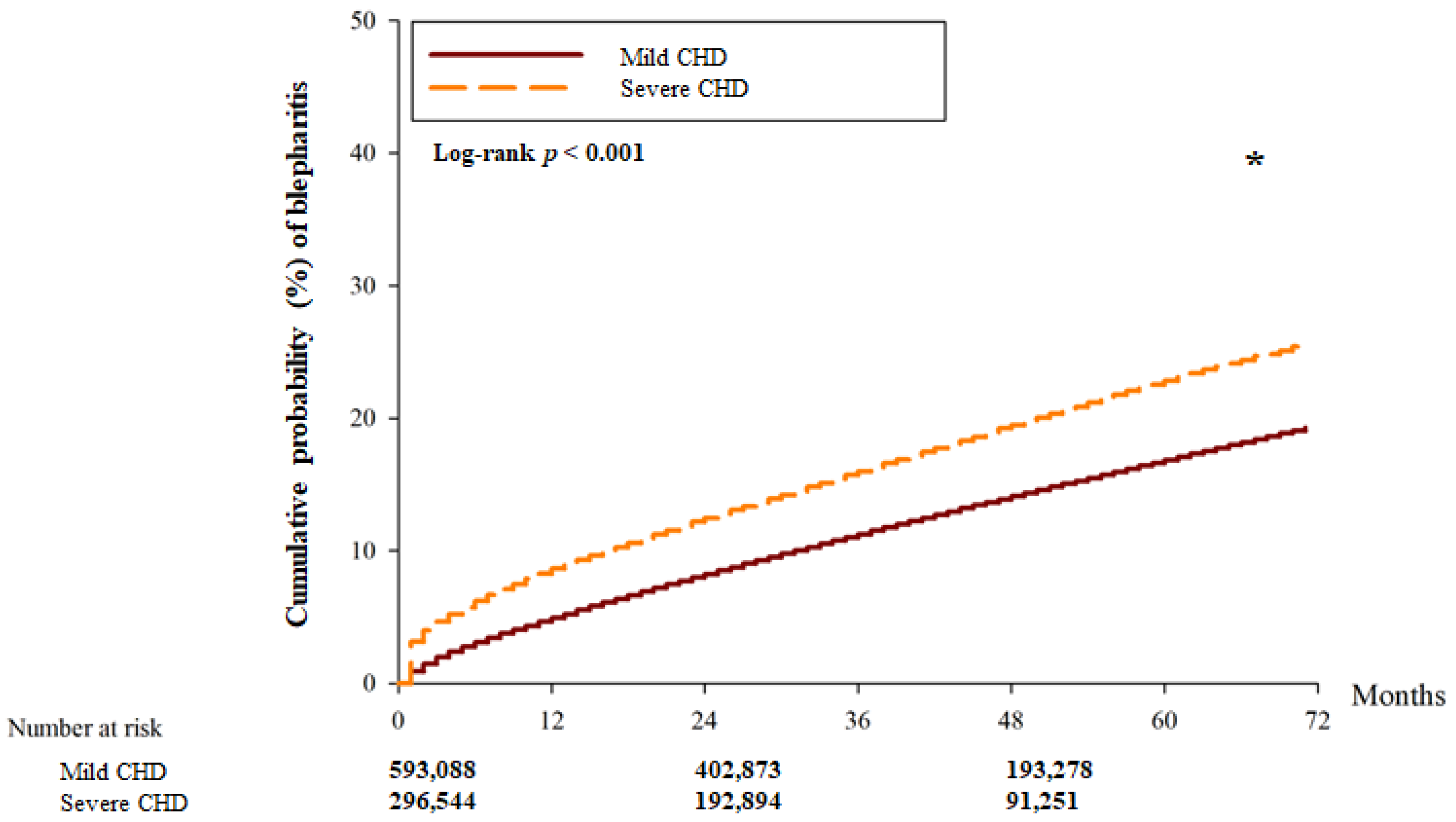
| Person–months | 20,253,262 | 9,716,179 | |
| Event | 22,161 | 15,369 | |
| Crude HR (95% CI) | Reference | 1.274 (1.051–1.885) | |
| aHR (95% CI) | Reference | 1.275 (1.051–1.912) * | 0.0285 * |
| Severe blepharitis | |||
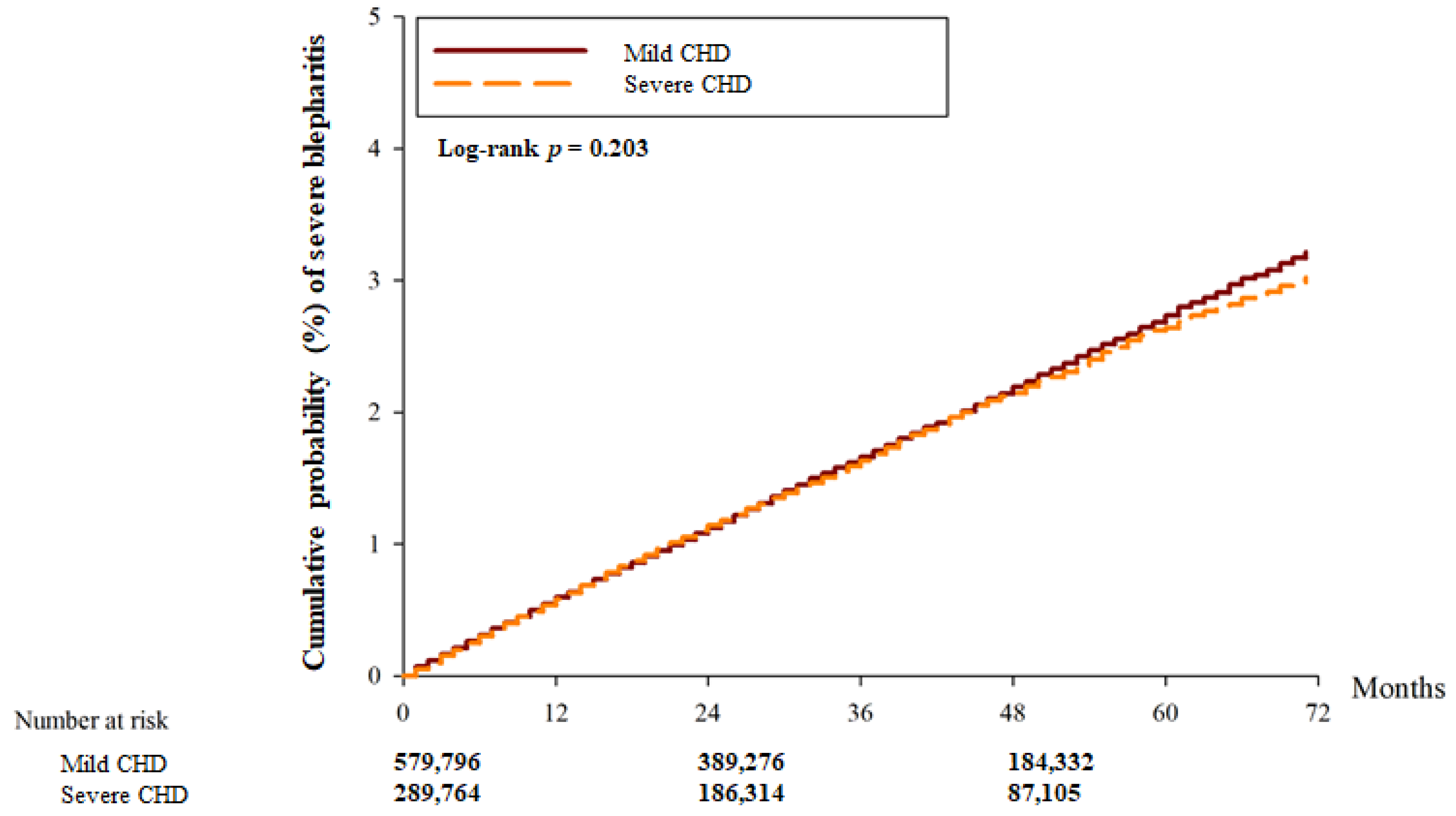
| Person–months | 20,570,555 | 9,861,096 | |
| Event | 9597 | 4500 | |
| Crude HR (95% CI) | Reference | 1.077 (0.943–1.213) | |
| aHR (95% CI) | Reference | 0.981 (0.945–1.020) | 0.3453 |
| Event | aHR | 95% CI | p for Interaction |
|---|---|---|---|
| Blepharitis | 0.4571 | ||
| Male | 1.160 | 0.960–1.592 | |
| Female | 1.303 | 1.064–2.043 | |
| Severe blepharitis | 0.2671 | ||
| Male | 0.999 | 0.952–1.048 | |
| Female | 0.958 | 0.901–1.019 |
| Event | aHR | 95% CI | p for Interaction |
|---|---|---|---|
| Blepharitis | 0.0115 * | ||
| <60 | 0.917 | 0.872–1.265 | |
| 60–69 | 1.046 | 0.905–1.788 | |
| >70 | 1.432 | 1.201–2.171 | |
| Severe blepharitis | 0.5498 | ||
| <60 | 0.956 | 0.886–1.030 | |
| 60–69 | 0.958 | 0.896–1.025 | |
| >70 | 1.016 | 0.959–1.076 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-Y.; Yang, S.-F.; Chang, Y.-L.; Huang, J.-Y.; Chang, C.-K. The Degrees of Coronary Heart Disease and the Degrees of New-Onset Blepharitis: A Nationwide Cohort Study. Diagnostics 2024, 14, 1349. https://doi.org/10.3390/diagnostics14131349
Lee C-Y, Yang S-F, Chang Y-L, Huang J-Y, Chang C-K. The Degrees of Coronary Heart Disease and the Degrees of New-Onset Blepharitis: A Nationwide Cohort Study. Diagnostics. 2024; 14(13):1349. https://doi.org/10.3390/diagnostics14131349
Chicago/Turabian StyleLee, Chia-Yi, Shun-Fa Yang, Yu-Ling Chang, Jing-Yang Huang, and Chao-Kai Chang. 2024. "The Degrees of Coronary Heart Disease and the Degrees of New-Onset Blepharitis: A Nationwide Cohort Study" Diagnostics 14, no. 13: 1349. https://doi.org/10.3390/diagnostics14131349
APA StyleLee, C.-Y., Yang, S.-F., Chang, Y.-L., Huang, J.-Y., & Chang, C.-K. (2024). The Degrees of Coronary Heart Disease and the Degrees of New-Onset Blepharitis: A Nationwide Cohort Study. Diagnostics, 14(13), 1349. https://doi.org/10.3390/diagnostics14131349






