Tissue Inhibitor of Metalloproteinases-2 (TIMP-2) as a Prognostic Biomarker in Acute Kidney Injury: A Narrative Review
Abstract
:1. Introduction
2. Tissue Inhibitor of Metalloproteinases-2
2.1. General Characteristics
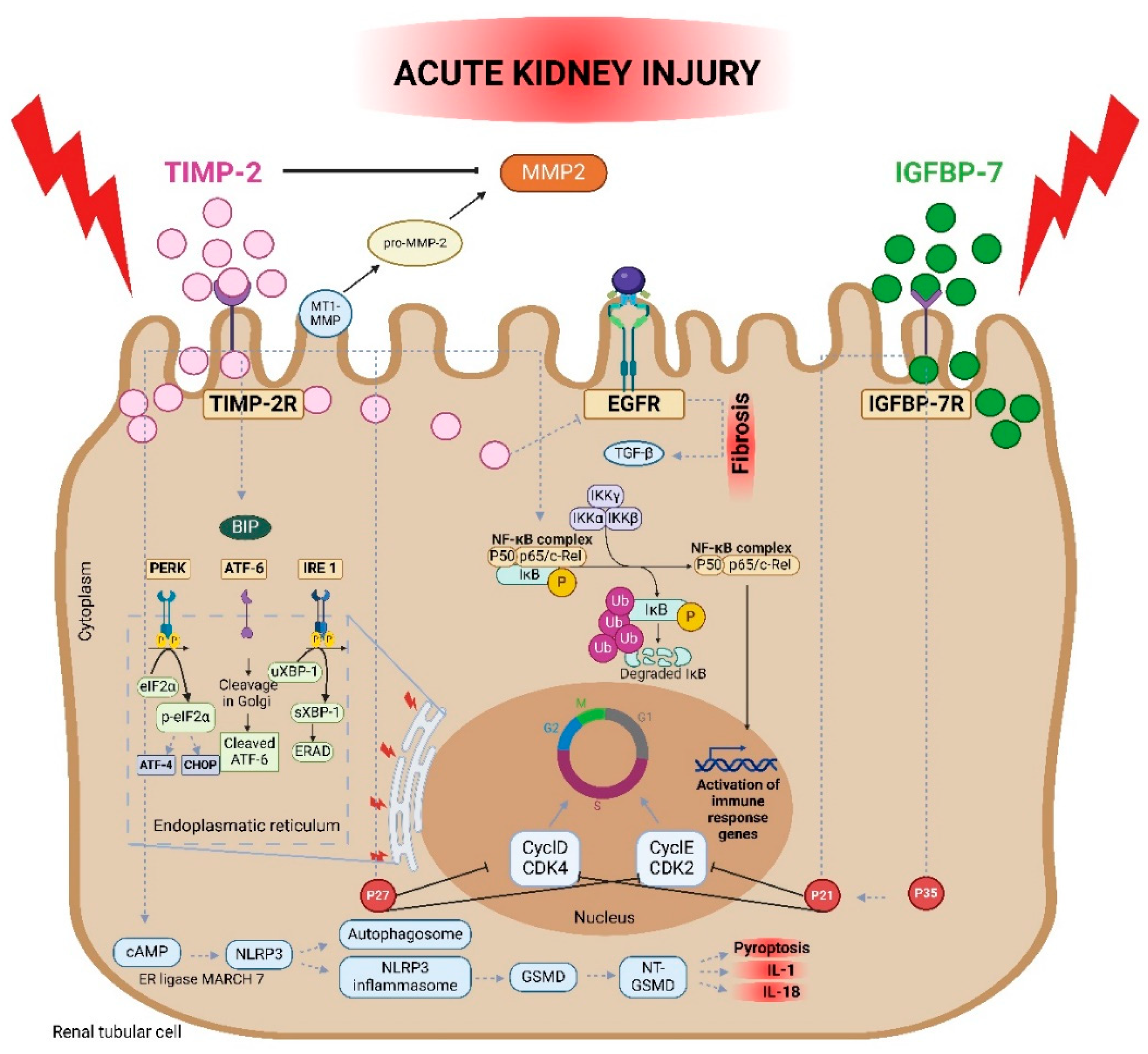
2.2. Mechanism of Action
3. Analytical Aspects
4. Clinical Evidence on TIMP-2
4.1. Preclinical Studies
4.2. Clinical Studies
4.2.1. All-Cause Acute Kidney Injury
4.2.2. Acute Kidney Injury after Surgery
4.2.3. Septic-Associated Acute Kidney Injury
4.2.4. Acute Kidney Injury after Cardiac Arrest and after Cardiac Surgery
4.2.5. Acute Kidney Injury in Critically Ill Patients
4.2.6. Acute Kidney Injury in Children
4.2.7. Acute Kidney Injury in Geriatrics
4.2.8. Acute Kidney Injury and COVID-19
4.2.9. Drug- and Contrast-Induced Acute Kidney Injury
5. Clinical Application
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mehta, R.L.; Cerdá, J.; Burdmann, E.A.; Tonelli, M.; García-García, G.; Jha, V.; Susantitaphong, P.; Rocco, M.; Vanholder, R.; Sever, M.S.; et al. International Society of Nephrology’s 0by25 Initiative for Acute Kidney Injury (Zero Preventable Deaths by 2025): A Human Rights Case for Nephrology. Lancet 2015, 385, 2616–2643. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.A.J.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of Acute Kidney Injury in Critically Ill Patients: The Multinational AKI-EPI Study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Ronco, C.; Mehta, R.L.; Asfar, P.; Boisramé-Helms, J.; Darmon, M.; Diehl, J.-L.; Duranteau, J.; Hoste, E.A.J.; Olivier, J.-B.; et al. Acute Kidney Injury in the ICU: From Injury to Recovery: Reports from the 5th Paris International Conference. Ann. Intensive Care 2017, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- James, M.T.; Pannu, N.; Hemmelgarn, B.R.; Austin, P.C.; Tan, Z.; McArthur, E.; Manns, B.J.; Tonelli, M.; Wald, R.; Quinn, R.R.; et al. Derivation and External Validation of Prediction Models for Advanced Chronic Kidney Disease Following Acute Kidney Injury. JAMA 2017, 318, 1787. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Sawhney, S.; Marks, A.; Fluck, N.; Levin, A.; McLernon, D.; Prescott, G.; Black, C. Post-Discharge Kidney Function Is Associated with Subsequent Ten-Year Renal Progression Risk among Survivors of Acute Kidney Injury. Kidney Int. 2017, 92, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.; Devarajan, P. Biomarkers for the Early Detection of Acute Kidney Injury. Pediatr. Nephrol. 2008, 23, 2151–2157. [Google Scholar] [CrossRef] [PubMed]
- Parikh, C.R.; Devarajan, P. New Biomarkers of Acute Kidney Injury. Crit. Care Med. 2008, 36, S159–S165. [Google Scholar] [CrossRef] [PubMed]
- Alge, J.L.; Arthur, J.M. Biomarkers of AKI: A Review of Mechanistic Relevance and Potential Therapeutic Implications. Clin. J. Am. Soc. Nephrol. 2015, 10, 147–155. [Google Scholar] [CrossRef]
- Malhotra, R.; Siew, E.D. Biomarkers for the Early Detection and Prognosis of Acute Kidney Injury. CJASN 2017, 12, 149–173. [Google Scholar] [CrossRef]
- Srisawat, N.; Murugan, R.; Kellum, J.A. Repair or Progression after AKI: A Role for Biomarkers? Nephron Clin. Pract. 2014, 127, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Kashani, K.; Al-Khafaji, A.; Ardiles, T.; Artigas, A.; Bagshaw, S.M.; Bell, M.; Bihorac, A.; Birkhahn, R.; Cely, C.M.; Chawla, L.S.; et al. Discovery and Validation of Cell Cycle Arrest Biomarkers in Human Acute Kidney Injury. Crit. Care 2013, 17, R25. [Google Scholar] [CrossRef] [PubMed]
- Arpino, V.; Brock, M.; Gill, S.E. The Role of TIMPs in Regulation of Extracellular Matrix Proteolysis. Matrix Biol. 2015, 44–46, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Rysz, J.; Banach, M.; Stolarek, R.A.; Pasnik, J.; Cialkowska-Rysz, A.; Koktysz, R.; Piechota, M.; Baj, Z. Serum Matrix Metalloproteinases MMP-2 and MMP-9 and Metalloproteinase Tissue Inhibitors TIMP-1 and TIMP-2 in Diabetic Nephropathy. J. Nephrol. 2007, 20, 444–452. [Google Scholar] [PubMed]
- Ahmed, A.K.; Haylor, J.L.; El Nahas, A.M.; Johnson, T.S. Localization of Matrix Metalloproteinases and Their Inhibitors in Experimental Progressive Kidney Scarring. Kidney Int. 2007, 71, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.M.; Fridman, R. TIMP-2 (Tissue Inhibitor of Metalloproteinase-2) Regulates MMP-2 (Matrix Metalloproteinase-2) Activity in the Extracellular Environment after pro-MMP-2 Activation by MT1 (Membrane Type 1)-MMP. Biochem. J. 2003, 374, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.P.; Martin, D.R.; Hammerman, M.R. Extracellular Matrix-Related Genes in Kidney after Ischemic Injury: Potential Role for TGF-Beta in Repair. Am. J. Physiol. 1998, 275, F894–F903. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chi, K.; Wu, D.; Hong, Q. Insulin-Like Growth Factor Binding Proteins in Kidney Disease. Front. Pharmacol. 2021, 12, 807119. [Google Scholar] [CrossRef]
- Bode, W.; Maskos, K. Structural Basis of the Matrix Metalloproteinases and Their Physiological Inhibitors, the Tissue Inhibitors of Metalloproteinases. Biol. Chem. 2003, 384, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Worley, J.R.; Thompkins, P.B.; Lee, M.H.; Hutton, M.; Soloway, P.; Edwards, D.R.; Murphy, G.; Knäuper, V. Sequence Motifs of Tissue Inhibitor of Metalloproteinases 2 (TIMP-2) Determining Progelatinase A (proMMP-2) Binding and Activation by Membrane-Type Metalloproteinase 1 (MT1-MMP). Biochem. J. 2003, 372, 799–809. [Google Scholar] [CrossRef]
- Grossman, M.; Tworowski, D.; Dym, O.; Lee, M.-H.; Levy, Y.; Murphy, G.; Sagi, I. The Intrinsic Protein Flexibility of Endogenous Protease Inhibitor TIMP-1 Controls Its Binding Interface and Affects Its Function. Biochemistry 2010, 49, 6184–6192. [Google Scholar] [CrossRef]
- Maskos, K.; Lang, R.; Tschesche, H.; Bode, W. Flexibility and Variability of TIMP Binding: X-Ray Structure of the Complex Between Collagenase-3/MMP-13 and TIMP-2. J. Mol. Biol. 2007, 366, 1222–1231. [Google Scholar] [CrossRef]
- Jiang, A.; Pei, D. Distinct Roles of Catalytic and Pexin-like Domains in Membrane-Type Matrix Metalloproteinase (MMP)-Mediated Pro-MMP-2 Activation and Collagenolysis. J. Biol. Chem. 2003, 278, 38765–38771. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C.J.; Overall, C.M. TIMP Independence of Matrix Metalloproteinase (MMP)-2 Activation by Membrane Type 2 (MT2)-MMP Is Determined by Contributions of Both the MT2-MMP Catalytic and Hemopexin C Domains. J. Biol. Chem. 2006, 281, 26528–26539. [Google Scholar] [CrossRef]
- Tam, E.M.; Wu, Y.I.; Butler, G.S.; Stack, M.S.; Overall, C.M. Collagen Binding Properties of the Membrane Type-1 Matrix Metalloproteinase (MT1-MMP) Hemopexin C Domain. J. Biol. Chem. 2002, 277, 39005–39014. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Barrantes, S.; Toth, M.; Bernardo, M.M.; Yurkova, M.; Gervasi, D.C.; Raz, Y.; Sang, Q.A.; Fridman, R. Binding of Active (57 kDa) Membrane Type 1-Matrix Metalloproteinase (MT1-MMP) to Tissue Inhibitor of Metalloproteinase (TIMP)-2 Regulates MT1-MMP Processing and Pro-MMP-2 Activation. J. Biol. Chem. 2000, 275, 12080–12089. [Google Scholar] [CrossRef] [PubMed]
- Meléndez, J.; Maldonado, V.; Bingle, C.D.; Selman, M.; Pardo, A. Cloning and Expression of Guinea Pig TIMP-2. Expression in Normal and Hyperoxic Lung Injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 278, L737–L743. [Google Scholar] [CrossRef]
- Qi, W.; Chen, X.; Holian, J.; Mreich, E.; Twigg, S.; Gilbert, R.E.; Pollock, C.A. Transforming Growth Factor-Beta1 Differentially Mediates Fibronectin and Inflammatory Cytokine Expression in Kidney Tubular Cells. Am. J. Physiol. Ren. Physiol. 2006, 291, F1070–F1077. [Google Scholar] [CrossRef]
- Gifford, C.C.; Tang, J.; Costello, A.; Khakoo, N.S.; Nguyen, T.Q.; Goldschmeding, R.; Higgins, P.J.; Samarakoon, R. Negative Regulators of TGF-Β1 Signaling in Renal Fibrosis; Pathological Mechanisms and Novel Therapeutic Opportunities. Clin. Sci. 2021, 135, 275–303. [Google Scholar] [CrossRef]
- Leivonen, S.-K.; Lazaridis, K.; Decock, J.; Chantry, A.; Edwards, D.R.; Kähäri, V.-M. TGF-β-Elicited Induction of Tissue Inhibitor of Metalloproteinases (TIMP)-3 Expression in Fibroblasts Involves Complex Interplay between Smad3, P38α, and ERK1/2. PLoS ONE 2013, 8, e57474. [Google Scholar] [CrossRef]
- Chung, H.; Ramachandran, R.; Hollenberg, M.D.; Muruve, D.A. Proteinase-Activated Receptor-2 Transactivation of Epidermal Growth Factor Receptor and Transforming Growth Factor-β Receptor Signaling Pathways Contributes to Renal Fibrosis. J. Biol. Chem. 2013, 288, 37319–37331. [Google Scholar] [CrossRef]
- Stetler-Stevenson, W.G.; Aznavoorian, S.; Liotta, L.A. Tumor Cell Interactions with the Extracellular Matrix During Invasion and Metastasis. Annu. Rev. Cell Biol. 1993, 9, 541–573. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Famulski, K.; Lee, J.; Das, S.K.; Wang, X.; Halloran, P.; Oudit, G.Y.; Kassiri, Z. TIMP2 and TIMP3 Have Divergent Roles in Early Renal Tubulointerstitial Injury. Kidney Int. 2014, 85, 82–93. [Google Scholar] [CrossRef]
- Elnokeety, M.M.; Hussein, W.M.; Ahmed Abdelrazek, S.; Momtaz, M. Cell Cycle Arrest Biomarkers for the Early Detection of Acute Allograft Dysfunction and Acute Rejection in Living Donor Kidney Transplantation: A Cross-Sectional Study from Egypt. Korean J. Transplant. 2023, 37, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.; Lu, Z.; Wu, J.; Qin, X.; Wang, Z.; Zhang, Z.; Zheng, L.; Zhao, J. BTN3A2 Serves as a Prognostic Marker and Favors Immune Infiltration in Triple-negative Breast Cancer. J. Cell. Biochem. 2020, 121, 2643–2654. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.-W.; Li, H.; Guedez, L.; Wingfield, P.T.; Diaz, T.; Salloum, R.; Wei, B.; Stetler-Stevenson, W.G. TIMP-2 Mediated Inhibition of Angiogenesis. Cell 2003, 114, 171–180. [Google Scholar] [CrossRef]
- Seo, D.-W.; Kim, S.H.; Eom, S.-H.; Yoon, H.J.; Cho, Y.-R.; Kim, P.-H.; Kim, Y.K.; Han, J.-W.; Diaz, T.; Wei, B.; et al. TIMP-2 Disrupts FGF-2-Induced Downstream Signaling Pathways. Microvasc. Res. 2008, 76, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Liu, N.; Tolbert, E.; Ponnusamy, M.; Ma, L.; Gong, R.; Bayliss, G.; Yan, H.; Zhuang, S. Sustained Activation of EGFR Triggers Renal Fibrogenesis after Acute Kidney Injury. Am. J. Pathol. 2013, 183, 160–172. [Google Scholar] [CrossRef]
- Hoegy, S.E.; Oh, H.-R.; Corcoran, M.L.; Stetler-Stevenson, W.G. Tissue Inhibitor of Metalloproteinases-2 (TIMP-2) Suppresses TKR-Growth Factor Signaling Independent of Metalloproteinase Inhibition*. J. Biol. Chem. 2001, 276, 3203–3214. [Google Scholar] [CrossRef]
- Li, Y.-M.; Zhang, J.; Su, L.-J.; Kellum, J.A.; Peng, Z.-Y. Downregulation of TIMP2 Attenuates Sepsis-Induced AKI through the NF-Κb Pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 558–569. [Google Scholar] [CrossRef]
- Sun, J. Matrix Metalloproteinases and Tissue Inhibitor of Metalloproteinases Are Essential for the Inflammatory Response in Cancer Cells. J. Signal Transduct. 2010, 2010, 985132. [Google Scholar] [CrossRef]
- Jiang, N.; Huang, R.; Zhang, J.; Xu, D.; Li, T.; Sun, Z.; Su, L.; Peng, Z. TIMP2 Mediates Endoplasmic Reticulum Stress Contributing to Sepsis-Induced Acute Kidney Injury. FASEB J. 2022, 36, e22228. [Google Scholar] [CrossRef]
- Mei, Y.; Thompson, M.D.; Cohen, R.A.; Tong, X. Endoplasmic Reticulum Stress and Related Pathological Processes. J. Pharmacol. Biomed. Anal. 2013, 1, 1000107. [Google Scholar]
- Xu, D.; Jiang, J.; Liu, Y.; Pang, J.; Suo, J.; Li, Y.; Peng, Z. TIMP2 Protects against Sepsis-Associated Acute Kidney Injury by cAMP/NLRP3 Axis-Mediated Pyroptosis. Am. J. Physiol.-Cell Physiol. 2024, 326, C1353–C1366. [Google Scholar] [CrossRef]
- Fujimoto, N.; Zhang, J.; Iwata, K.; Shinya, T.; Okada, Y.; Hayakawa, T. A One-Step Sandwich Enzyme Immunoassay for Tissue Inhibitor of Metalloproteinases-2 Using Monoclonal Antibodies. Clin. Chim. Acta 1993, 220, 31–45. [Google Scholar] [CrossRef]
- Kunz, P.; Sähr, H.; Lehner, B.; Fischer, C.; Seebach, E.; Fellenberg, J. Elevated Ratio of MMP2/MMP9 Activity Is Associated with Poor Response to Chemotherapy in Osteosarcoma. BMC Cancer 2016, 16, 223. [Google Scholar] [CrossRef] [PubMed]
- Henriquez, J.; Zhou, J.; Li, J.; Crawford, R.; Kaminski, N. Application of Gene Specific mRNA Level Determinations in Individual Cells Using Flow Cytometry-Based PrimeFlowTM in Immunotoxicology. Toxicol. Appl. Pharmacol. 2017, 337, 39–44. [Google Scholar] [CrossRef]
- Pieper-Fürst, U.; Stöcklein, W.F.M.; Warsinke, A. Gold Nanoparticle-Enhanced Surface Plasmon Resonance Measurement with a Highly Sensitive Quantification for Human Tissue Inhibitor of Metalloproteinases-2. Anal. Chim. Acta 2005, 550, 69–76. [Google Scholar] [CrossRef]
- Fan, W.; Ankawi, G.; Zhang, J.; Digvijay, K.; Giavarina, D.; Yin, Y.; Ronco, C. Current Understanding and Future Directions in the Application of TIMP-2 and IGFBP7 in AKI Clinical Practice. Clin. Chem. Lab. Med. 2019, 57, 567–576. [Google Scholar] [CrossRef]
- Gocze, I.; Koch, M.; Renner, P.; Zeman, F.; Graf, B.M.; Dahlke, M.H.; Nerlich, M.; Schlitt, H.J.; Kellum, J.A.; Bein, T. Urinary Biomarkers TIMP-2 and IGFBP7 Early Predict Acute Kidney Injury after Major Surgery. PLoS ONE 2015, 10, e0120863. [Google Scholar] [CrossRef]
- Chindarkar, N.S.; Chawla, L.S.; Straseski, J.A.; Jortani, S.A.; Uettwiller-Geiger, D.; Orr, R.R.; Kellum, J.A.; Fitzgerald, R.L. Reference Intervals of Urinary Acute Kidney Injury (AKI) Markers [IGFBP7]∙[TIMP2] in Apparently Healthy Subjects and Chronic Comorbid Subjects without AKI. Clin. Chim. Acta 2016, 452, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.-Y.; Zhou, F.; Kellum, J.A. Cross-Species Validation of Cell Cycle Arrest Markers for Acute Kidney Injury in the Rat during Sepsis. ICMx 2016, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ren, S.; Long, L.; Zhao, H.; Shen, L. Evaluation of the Efficiency of TIMP-2 as a Biomarker for Acute Kidney Injury in Sepsis. Bull. Exp. Biol. Med. 2023, 174, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zeng, Y.; Lv, L.; Chen, Y.; Yan, Y.; Luo, L.; Pan, R.; Jiang, J.; Wei, X. Predictive Value of Urinary Cell Cycle Arrest Biomarkers for All Cause-Acute Kidney Injury: A Meta-Analysis. Sci. Rep. 2023, 13, 6037. [Google Scholar] [CrossRef] [PubMed]
- Titeca-Beauport, D.; Daubin, D.; Chelly, J.; Zerbib, Y.; Brault, C.; Diouf, M.; Slama, M.; Vinsonneau, C.; Klouche, K.; Maizel, J. The Urine Biomarkers TIMP2 and IGFBP7 Can Identify Patients Who Will Experience Severe Acute Kidney Injury Following a Cardiac Arrest: A Prospective Multicentre Study. Resuscitation 2019, 141, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shen, Q.; Zhou, X. The Predictive Value of [TIMP-2]*[IGFBP7] in Adverse Outcomes for Acute Kidney Injury: A Systematic Review and Meta-Analysis. Ren. Fail. 2023, 45, 2253933. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Yu, J.; Wang, H.; Zheng, R. The Value of Urine Cell Cycle Arrest Biomarkers to Predict Persistent Acute Kidney Injury: A Systematic Review and Meta-Analysis. Clin. Nephrol. 2021, 96, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Guo, Q.; Yang, B.; Xie, P.; Zhang, J.; Lu, W.; Ronco, C.; Jiang, G. Tissue Inhibitor Metalloproteinase-2·IGF-Binding Protein 7 for the Prediction of Acute Kidney Injury Following Cardiac Surgery. Cardiorenal Med. 2024, 14, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Lacquaniti, A.; Ceresa, F.; Campo, S.; Barbera, G.; Caruso, D.; Palazzo, E.; Patanè, F.; Monardo, P. Acute Kidney Injury and Sepsis after Cardiac Surgery: The Roles of Tissue Inhibitor Metalloproteinase-2, Insulin-like Growth Factor Binding Protein-7, and Mid-Regional Pro-Adrenomedullin. J. Clin. Med. 2023, 12, 5193. [Google Scholar] [CrossRef]
- Yu, P.-J.; Rodriguez, G.; Cassiere, H.; Bocchieri, K.; Hirsch, J.S.; Chang, T.Y.; Jhaveri, K.D.; Hentz, R.; Fishbane, S.; Sharma, P.D.; et al. Use of TIMP-2 and IGFBP-7 for Prediction of Postoperative Acute Kidney Injury after Cardiac Surgery. Clin. Nephrol. 2022, 98, 288–295. [Google Scholar] [CrossRef]
- Bihorac, A.; Chawla, L.S.; Shaw, A.D.; Al-Khafaji, A.; Davison, D.L.; DeMuth, G.E.; Fitzgerald, R.; Gong, M.N.; Graham, D.D.; Gunnerson, K.; et al. Validation of Cell-Cycle Arrest Biomarkers for Acute Kidney Injury Using Clinical Adjudication. Am. J. Respir. Crit. Care Med. 2014, 189, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Chui, H.; McMahon, K.R.; Rassekh, S.R.; Schultz, K.R.; Blydt-Hansen, T.D.; Mammen, C.; Pinsk, M.; Cuvelier, G.D.E.; Carleton, B.C.; Tsuyuki, R.T.; et al. Urinary TIMP-2*IGFBP-7 to Diagnose Acute Kidney Injury in Children Receiving Cisplatin. Pediatr. Nephrol. 2024, 39, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Greco, M.; De Rosa, S.; Boehm, F.; Spano, S.; Aceto, R.; Voza, A.; Reggiani, F.; Calatroni, M.; Castellani, G.; Costantini, E.; et al. Kinetics of the Cell Cycle Arrest Biomarkers (TIMP2 and IGFBP7) for the Diagnosis of Acute Kidney Injury in Critically Ill COVID-19 Patients. Diagnostics 2023, 13, 317. [Google Scholar] [CrossRef] [PubMed]
- Westhoff, J.H.; Tönshoff, B.; Waldherr, S.; Pöschl, J.; Teufel, U.; Westhoff, T.H.; Fichtner, A. Urinary Tissue Inhibitor of Metalloproteinase-2 (TIMP-2) • Insulin-Like Growth Factor-Binding Protein 7 (IGFBP7) Predicts Adverse Outcome in Pediatric Acute Kidney Injury. PLoS ONE 2015, 10, e0143628. [Google Scholar] [CrossRef] [PubMed]
- Waskowski, J.; Pfortmueller, C.A.; Schenk, N.; Buehlmann, R.; Schmidli, J.; Erdoes, G.; Schefold, J.C. (TIMP2) x (IGFBP7) as Early Renal Biomarker for the Prediction of Acute Kidney Injury in Aortic Surgery (TIGER). A Single Center Observational Study. PLoS ONE 2021, 16, e0244658. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Jia, H.-M.; Zheng, X.; Jiang, Y.-J.; Zhang, T.-E.; Li, W.-X. Urinary Cell Cycle Biomarkers for the Prediction of Renal Non-Recovery in Patients with Septic Acute Kidney Injury: A Prospective Study. Clin. Exp. Nephrol. 2023, 27, 1051–1059. [Google Scholar] [CrossRef]
- Titeca-Beauport, D.; Diouf, M.; Daubin, D.; Vong, L.V.; Belliard, G.; Bruel, C.; Zerbib, Y.; Vinsonneau, C.; Klouche, K.; Maizel, J. The Combination of Kidney Function Variables with Cell Cycle Arrest Biomarkers Identifies Distinct Subphenotypes of Sepsis-Associated Acute Kidney Injury: A Post-Hoc Analysis (the PHENAKI Study). Ren. Fail. 2024, 46, 2325640. [Google Scholar] [CrossRef] [PubMed]
- Su, L.-J.; Li, Y.-M.; Kellum, J.A.; Peng, Z.-Y. Predictive Value of Cell Cycle Arrest Biomarkers for Cardiac Surgery-Associated Acute Kidney Injury: A Meta-Analysis. Br. J. Anaesth. 2018, 121, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Naorungroj, T.; Yanase, F.; Bittar, I.; Eastwood, G.; Bellomo, R. The Relationship between Nephrocheck® Test Values, Outcomes, and Urinary Output in Critically Ill Patients at Risk of Acute Kidney Injury. Acta Anaesthesiol. Scand. 2022, 66, 1219–1227. [Google Scholar] [CrossRef]
- Abouhadid, M.A.; Gawad, T.A.A.; Gebaly, H.H.E.; Abdallah, A.A.; Refay, A.S.E.; Helmy, N.M.; Allam, A.M. Urinary Tissue Inhibitor of Metalloproteinase-2 as an Early Predictor for Acute Kidney Injury in Critically Ill Children. Int. J. Health Sci. 2023, 17, 22–28. [Google Scholar]
- Vandenberghe, W.; De Loor, J.; Francois, K.; Vandekerckhove, K.; Herck, I.; Vande Walle, J.; Peperstraete, H.; Bové, T.; De Wolf, D.; Nuytinck, L.; et al. Potential of Urine Biomarkers CHI3L1, NGAL, TIMP-2, IGFBP7, and Combinations as Complementary Diagnostic Tools for Acute Kidney Injury after Pediatric Cardiac Surgery: A Prospective Cohort Study. Diagnostics 2023, 13, 1047. [Google Scholar] [CrossRef]
- Ramírez, M.; Chakravarti, S.; Busovsky-McNeal, M.; McKinstry, J.; Al-qaqaa, Y.; Sahulee, R.; Kumar, T.K.S.; Li, X.; Goldberg, J.D.; Gefen, A.M.; et al. Elevated Levels of Urinary Biomarkers TIMP-2 and IGFBP-7 Predict Acute Kidney Injury in Neonates after Congenital Heart Surgery. J. Pediatr. Intensive Care 2022, 11, 153–158. [Google Scholar] [CrossRef]
- Cheng, L.; Jia, H.-M.; Zheng, X.; Jiang, Y.-J.; Xin, X.; Li, W.-X. Association between the Levels of Urinary Cell Cycle Biomarkers and Non-Recovery of Renal Function among Critically Ill Geriatric Patients with Acute Kidney Injury. Ren. Fail. 2024, 46, 2304099. [Google Scholar] [CrossRef]
- Weiss, R.; von Groote, T.; Ostermann, M.; Lumlertgul, N.; Weerapolchai, K.; Garcia, M.I.M.; Cano, J.M.M.; Del Corral, B.D.; Broch-Porcar, M.J.; Perez Carrasco, M.; et al. The Role of Cell Cycle Arrest Biomarkers for Predicting Acute Kidney Injury in Critically Ill COVID-19 Patients: A Multicenter, Observational Study. Crit. Care Med. 2023, 51, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Casas-Aparicio, G.; Alvarado-de la Barrera, C.; Escamilla-Illescas, D.; León-Rodríguez, I.; Del Río-Estrada, P.M.; Calderón-Dávila, N.; González-Navarro, M.; Olmedo-Ocampo, R.; Castillejos-López, M.; Figueroa-Hernández, L.; et al. Role of Urinary Kidney Stress Biomarkers for Early Recognition of Subclinical Acute Kidney Injury in Critically Ill COVID-19 Patients. Biomolecules 2022, 12, 275. [Google Scholar] [CrossRef]
- Akalya, K.; Murali, T.M.; Vathsala, A.; Teo, B.-W.; Low, S.; Dharmasegaran, D.; Koh, L.-P.; Bonney, G.K.; Hong, W.-Z.; Da, Y.; et al. Elevated Urinary Tissue Inhibitor of Metalloproteinase-2 and Insulin-Like Growth Factor Binding Protein-7 Predict Drug-Induced Acute Kidney Injury. Curr. Drug Metab. 2022, 23, 223–232. [Google Scholar] [CrossRef]
- Murugan, R.; Boudreaux-Kelly, M.Y.; Kellum, J.A.; Palevsky, P.M.; Weisbord, S. Contrast-Associated Acute Kidney Injury and Cardiovascular Events: A Secondary Analysis of the PRESERVE Cohort. Clin. Kidney J. 2023, 16, 2626–2638. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Kang, Z.; Li, Z.; Xun, M. Urinary NGAL, IGFBP-7, and TIMP-2: Novel Biomarkers to Predict Contrast Medium-Induced Acute Kidney Injury in Children. Ren. Fail. 2022, 44, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Ankawi, G.; Yang, B.; Garzotto, F.; Passannante, A.; Breglia, A.; Digvijay, K.; Ferrari, F.; Brendolan, A.; Raffaele, B.; et al. Tissue Inhibitor Metalloproteinase-2 (TIMP-2) • IGF-Binding Protein-7 (IGFBP7) Levels Are Associated with Adverse Outcomes in Patients in the Intensive Care Unit with Acute Kidney Injury. Kidney Int. 2019, 95, 1486–1493. [Google Scholar] [CrossRef]
- Guzzi, L.M.; Bergler, T.; Binnall, B.; Engelman, D.T.; Forni, L.; Germain, M.J.; Gluck, E.; Göcze, I.; Joannidis, M.; Koyner, J.L.; et al. Clinical Use of [TIMP-2]•[IGFBP7] Biomarker Testing to Assess Risk of Acute Kidney Injury in Critical Care: Guidance from an Expert Panel. Crit. Care 2019, 23, 225. [Google Scholar] [CrossRef]
- Bank, J.R.; Ruhaak, R.; Soonawala, D.; Mayboroda, O.; Romijn, F.P.; Van Kooten, C.; Cobbaert, C.M.; De Fijter, J.W. Urinary TIMP-2 Predicts the Presence and Duration of Delayed Graft Function in Donation After Circulatory Death Kidney Transplant Recipients. Transplantation 2019, 103, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Daubin, D.; Cristol, J.P.; Dupuy, A.M.; Kuster, N.; Besnard, N.; Platon, L.; Buzançais, A.; Brunot, V.; Garnier, F.; Jonquet, O.; et al. Urinary Biomarkers IGFBP7 and TIMP-2 for the Diagnostic Assessment of Transient and Persistent Acute Kidney Injury in Critically Ill Patients. PLoS ONE 2017, 12, e0169674. [Google Scholar] [CrossRef] [PubMed]
- Zdziechowska, M.; Gluba-Brzózka, A.; Poliwczak, A.R.; Franczyk, B.; Kidawa, M.; Zielinska, M.; Rysz, J. Serum NGAL, KIM-1, IL-18, L-FABP: New Biomarkers in the Diagnostics of Acute Kidney Injury (AKI) Following Invasive Cardiology Procedures. Int. Urol. Nephrol. 2020, 52, 2135–2143. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Zhao, L.; Peng, W.; Wu, F.-H.; Zhang, G.-B.; Yang, B.; Huo, W.-Q. Value of Urine IL-8, NGAL and KIM-1 for the Early Diagnosis of Acute Kidney Injury in Patients with Ureteroscopic Lithotripsy Related Urosepsis. Chin. J. Traumatol. 2022, 25, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.S.; Lee, Y.; Jang, H.M.; Cho, J.; Hyun, M.C.; Kim, Y.H.; Hwang, S.-K.; Cho, M.H. Variation in Clinical Usefulness of Biomarkers of Acute Kidney Injury in Young Children Undergoing Cardiac Surgery. Clin. Exp. Pediatr. 2020, 63, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Musiał, K.; Stojanowski, J.; Miśkiewicz-Bujna, J.; Kałwak, K.; Ussowicz, M. KIM-1, IL-18, and NGAL, in the Machine Learning Prediction of Kidney Injury among Children Undergoing Hematopoietic Stem Cell Transplantation—A Pilot Study. Int. J. Mol. Sci. 2023, 24, 15791. [Google Scholar] [CrossRef] [PubMed]
- Rumpel, J.; Spray, B.J.; Chock, V.Y.; Kirkley, M.J.; Slagle, C.L.; Frymoyer, A.; Cho, S.-H.; Gist, K.M.; Blaszak, R.; Poindexter, B.; et al. Urine Biomarkers for the Assessment of Acute Kidney Injury in Neonates with Hypoxic Ischemic Encephalopathy Receiving Therapeutic Hypothermia. J. Pediatr. 2022, 241, 133–140.e3. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, M.; Zarbock, A.; Goldstein, S.; Kashani, K.; Macedo, E.; Murugan, R.; Bell, M.; Forni, L.; Guzzi, L.; Joannidis, M.; et al. Recommendations on Acute Kidney Injury Biomarkers from the Acute Disease Quality Initiative Consensus Conference: A Consensus Statement. JAMA Netw. Open 2020, 3, e2019209. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.C.M.; Zager, R.A. Mechanisms Underlying Increased TIMP2 and IGFBP7 Urinary Excretion in Experimental AKI. JASN 2018, 29, 2157–2167. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Zhao, Z.; Meng, Y.; Bian, J.; Bao, R.; Zhu, K.; Yang, T. IGFBP7 Regulates Sepsis-Induced Epithelial-Mesenchymal Transition through ERK1/2 Signaling. ABBS 2019, 51, 799–806. [Google Scholar] [CrossRef]
| AKI | Study | Key Findings | Ref |
|---|---|---|---|
| AKI | Meta-analysis of 20 trials involving 3625 patients | Pooled sensitivity: 0.79; specificity: 0.70; AUC: 0.81. | [54] |
| Surgical Patients | Study with 107 patients post-surgery | [TIMP-2] × [IGFBP-7] showed a good predictive value for AKI with an AUC of 0.85. | [33] |
| Cardiac Arrest | Prospective multicenter study with 115 patients post-cardiac arrest | [TIMP-2] × [IGFBP-7] had strong discriminative capacity with an AUC of 0.91 for severe AKI. | [54] |
| Cardiac Surgery | Cohort study of 557 patients | Combined TIMP-2 and IGFBP-7 showed high predictive accuracy for AKI with an AUC of 0.70. | [58] |
| SA-AKI | Prospective observational study with 198 patients | Combined TIMP-2 and IGFBP-7 had an AUC of 0.782 for non-recovery of kidney function, improved to 0.822 with a clinical model. | [59] |
| Critically Ill Patients | Multicenter trial with 420 patients in ICUs | High sensitivity (92%) and improved predictive power with [TIMP-2] × [IGFBP-7] compared to clinical variables alone. | [60] |
| Pediatric AKI | Prospective cohort of 42 critically ill neonates | Elevated urine TIMP-2 levels linked to AKI, preceding traditional biomarkers. | [61] |
| Patients with COVID-19 | Multicenter observational study with 300 patients with COVID-19-related ARDS | High predictive value for AKI at enrollment with an AUC-ROC of 0.89. | [62] |
| Drug-induced AKI | Study with 21 patients with AKI and 28 controls receiving nephrotoxic drugs | Significantly higher TIMP-2 and IGFBP-7 levels 2–3 days before AKI onset. | [63] |
| CA-AKI | Prospective trial with 172 children post-contrast medium injection | Elevated [TIMP-2] × [IGFBP-7] levels significant for predicting AKI with an AUC-ROC of 0.811. | [64] |
| Biomarker | Mechanism | Advantages | Disadvantages | Ref |
|---|---|---|---|---|
| TIMP-2 | Inhibits MMPs, involved in ECM turnover and cell cycle arrest | Early detection of AKI, reflects cellular stress before significant damage | Biological variability, affected by underlying conditions | [55,59,63,64,70] |
| NGAL | Expressed in neutrophils, upregulated in response to kidney injury | Rapid increase post-injury | Reflects injury post-damage, not early predictive | [54,61] |
| KIM-1 | Expressed in damaged proximal tubule cells | Specific for epithelial cell injury | Late indicator, reflects post-injury | [57,62] |
| IL-18 | Pro-inflammatory cytokine involved in inflammatory response | Useful in inflammatory AKI | Less specific for kidney injury | [56,69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delrue, C.; Speeckaert, M.M. Tissue Inhibitor of Metalloproteinases-2 (TIMP-2) as a Prognostic Biomarker in Acute Kidney Injury: A Narrative Review. Diagnostics 2024, 14, 1350. https://doi.org/10.3390/diagnostics14131350
Delrue C, Speeckaert MM. Tissue Inhibitor of Metalloproteinases-2 (TIMP-2) as a Prognostic Biomarker in Acute Kidney Injury: A Narrative Review. Diagnostics. 2024; 14(13):1350. https://doi.org/10.3390/diagnostics14131350
Chicago/Turabian StyleDelrue, Charlotte, and Marijn M. Speeckaert. 2024. "Tissue Inhibitor of Metalloproteinases-2 (TIMP-2) as a Prognostic Biomarker in Acute Kidney Injury: A Narrative Review" Diagnostics 14, no. 13: 1350. https://doi.org/10.3390/diagnostics14131350





