Diagnostic Yield and Outcomes of Small Bowel Capsule Endoscopy in Patients with Small Bowel Bleeding Receiving Antithrombotics
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Definitions
2.3. Capsule Procedure
2.4. Endpoints
- To assess the diagnostic yield of SBCE among patients presenting with SBB.
- To assess the therapeutic intervention rate in patients with positive findings at SBCE.
- To assess the rebleeding rate in patients with SBB undergoing SBCE.
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pennazio, M.; Rondonotti, E.; Despott, E.; Dray, X.; Keuchel, M.; Moreels, T.; Sanders, D.S.; Spada, C.; Carretero, C.; Valdivia, P.C.; et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2022. Endoscopy 2023, 55, 58–95. [Google Scholar] [CrossRef] [PubMed]
- Robertson, K.D.; Singh, R. Capsule Endoscopy. [Updated 8 August 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482306/ (accessed on 1 January 2024).
- Gerson, L.B.; Fidler, J.L.; Cave, D.R.; Leighton, J.A. ACG Clinical Guideline: Diagnosis and management of small bowel bleeding. Am. J. Gastroenterol. 2015, 110, 1265–1287. [Google Scholar] [CrossRef] [PubMed]
- Viazis, N.; Anastasiou, G.; Karamanolis, D. Small bowel capsule endoscopy for the investigation of obscure gastrointestinal bleeding: When we should do and what should we expect. Acta Gastroenterol. Belg. 2016, 79, 355–362. [Google Scholar] [PubMed]
- Cortegoso Valdivia, P.; Skonieczna-Żydecka, K.; Elosua, A.; Sciberras, M.; Piccirelli, S.; Rullan, M.; Tabone, T.; Gawel, K.; Stachowski, A.; Lemiński, A.; et al. Indications, detection, completion and retention rates of capsule endoscopy in two decades of use: A systematic review and meta-analysis. Diagnostics 2022, 12, 1105. [Google Scholar] [CrossRef] [PubMed]
- Levine, I.; Hong, S.; Bhakta, D.; McNeill, M.B.; Gross, S.A.; Latorre, M. Diagnostic yield of inpatient capsule endoscopy. BMC Gastroenterol. 2022, 22, 236. [Google Scholar] [CrossRef] [PubMed]
- Pennazio, M.; Santucci, R.; Rondonotti, E.; Abbiati, C.; Beccari, G.; Rossini, F.P.; De Franchis, R. Outcome of patients with obscure gastrointestinal bleeding after capsule endoscopy: Report of 100 consecutive cases. Gastroenterology 2004, 126, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, M.W.; Nessel, C.C.; Hellkamp, A.S.; Mahaffey, K.W.; Piccini, J.P.; Suh, E.Y.; Becker, R.C.; Singer, D.E.; Halperin, J.L.; Hankey, G.J.; et al. Gastrointestinal bleeding in patients with atrial fibrillation treated with rivaroxaban or warfarin: ROCKET AF trial. J. Am. Coll. Cardiol. 2015, 66, 2271–2281. [Google Scholar] [CrossRef] [PubMed]
- Abraham, N.S.; Hlatky, M.A.; Antman, E.M.; Bhatt, D.L.; Bjorkman, D.J.; Clark, C.B.; Furberg, C.D.; Johnson, D.A.; Kahi, C.J.; Laine, L. ACCF/ACG/AHA 2010 expert consensus document on the concomitant use of proton pump inhibitors and thienopyridines: A focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: A report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Am. J. Gastroenterol. 2010, 105, 2533–2549. [Google Scholar]
- Maiden, L.; Thjodleifsson, B.; Seigal, A.; Bjarnason, I.I.; Scott, D.; Birgisson, S.; Bjarnason, I. Long-term effects of nonsteroidal anti-infammatory drugs and cyclooxygenase-2 selective agents on the small bowel: A cross-sectional capsule enteroscopy study. Clin. Gastroenterol. Hepatol. 2007, 5, 1040–1045. [Google Scholar] [CrossRef]
- Lanas, A.; Dumonceau, J.M.; Hunt, R.H.; Fujishiro, M.; Scheiman, J.M.; Gralnek, I.M.; Campbell, H.E.; Rostom, A.; Villanueva, C.; Sung, J.J.Y. Non-variceal upper gastrointestinal bleeding. Nat Rev Dis Primers. 2018, 4, 18020. [Google Scholar] [CrossRef] [PubMed]
- Lepileur, L.; Dray, X.; Antonietti, M.; Iwanicki-Caron, I.; Grigioni, S.; Chaput, U.; Di–Fiore, A.; Alhameedi, R.; Marteau, P.; Ducrotté, P.; et al. Factors associated with diagnosis of obscure gastrointestinal bleeding by video capsule enteroscopy. Clin. Gastroenterol. Hepatol. 2012, 10, 1376–1380. [Google Scholar] [CrossRef] [PubMed]
- Shiotani, A.; Honda, K.; Murao, T.; Ishii, M.; Fujita, M.; Matsumoto, H.; Tarumi, K.-I.; Kamada, T.; Sakakibara, T.; Haruma, K. Combination of low-dose aspirin and thienopyridine exacerbates small bowel injury. Scand. J. Gastroenterol. 2011, 46, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Curdia Goncalves, T.; Arieira, C.; Monteiro, S.; Rosa, B.; Moreira, M.J.; Cotter, J. ORBIT score: An useful predictor of small bowel rebleeding in patients under chronic anticoagulation. Scand. J. Gastroenterol. 2018, 53, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Saurin, J.C.; Delvaux, M.; Vahedi, K.; Gaudin, J.L.; Villarejo, J.; Florent, C.; Gay, G.; Ponchon, T. Clinical impact of capsule endoscopy compared to push enteroscopy: 1-year follow-up study. Endoscopy 2005, 37, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Viazis, N.; Sgouros, S.; Papaxoinis, K.; Vlachogiannakos, J.; Bergele, C.; Sklavos, P.; Panani, A.; Avgerinos, A. Bowel preparation increases the diagnostic yield of capsule endoscopy: A prospective, randomized, controlled study. Gastrointest Endosc. 2004, 60, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Smecuol, E.; Pinto Sanchez, M.; Suarez, A.; Argonz, J.E.; Sugai, E.; Vazquez, H.; Litwin, N.; Piazuelo, E.; Meddings, J.B.; Bai, J.C.; et al. Low dose aspirin affects the small bowel mucosa: Results of a pilot study with a multidimensional assessment. Clin. Gastroenterol. Hepatol. 2009, 7, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Ehrhard, F.; Nazeyrollas, P.; Brixi, H.; Heurgue-Berlot, A.; Thiefin, G. Proximal predominance of small bowel injury associated with uncoated low dose aspirin therapy: A video capsule study in chronic users. Eur. J. Gastroenterol. Hepatol. 2013, 25, 1265–1272. [Google Scholar] [CrossRef]
- Boal Carvalho, P.; Rosa, B.; Moreira, M.; Cotter, J. New evidence on the impact of antithrombotics in patients submitted to small bowel capsule endoscopy for the evaluation of obscure gastrointestinal bleeding. Gastroenterol. Res. Pract. 2013, 2014, 709217. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Kawano, S.; Okamoto, Y.; Obayashi, Y.; Baba, Y.; Sakae, H.; Abe, M.; Gotoda, T.; Inokuchi, T.; Kanzaki, H.; et al. Clinical outcome of patients with obscure gastrointestinal bleeding during antithrombotic drug therapy. Ther. Adv. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Nagata, N.; Niikura, R.; Yamada, A.; Sakurai, T.; Shimbo, T.; Kobayashi, Y.; Okamoto, M.; Mitsuno, Y.; Ogura, K.; Hirata, Y.; et al. Acute middle gastrointestinal bleeding risk associated with NSAIDs, antithrombotic drugs and PPIs: A multicenter case-control study. PLoS ONE 2016, 11, e0151332. [Google Scholar] [CrossRef]
- Domper Arnal, M.J.; Hijos-Mallada, G.; Lanas, A. Gastrointestinal and cardiovascular adverse events associated with NSAIDs. Expert Opin Drug Saf. 2022, 21, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Tziatzios, G.; Gkolfakis, P.; Papanikolaou, I.; Triantafyllou, K. Antithrombotic treatment is associated with small bowel video capsule endoscopy positive findings in obscure gastrointestinal bleeding: A systematic review and meta-analysis. Dig. Dis. Sci. 2019, 64, 15–24. [Google Scholar] [CrossRef]
- Pennazio, M.; Cortegoso Valdivia, P.; Triantafyllou, K.; Gralnek, I.M. Diagnosis and management of small bowel bleeding. Best. Pract. Res. Clin. Gastroenterol. 2023, 64–65, 101844. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Scaramella, L.; Tontini, G.E.; Topa, M.; Conte, D.; Sidhu, R.; Rondonotti, E.; Penagini, R.; Vecchi, M. Clinical impact of videocapsule and double balloon enteroscopy on small bowel bleeding: Results from a large monocentric cohort in the last 19 years. Dig. Liver Dis. 2022, 54, 251–257. [Google Scholar] [CrossRef]
- Valdivia, P.; Skonieczna-Zydecka, K.; Pennazio, M.; Rondonotti, E.; Marlicz, W.; Toth, E.; Koulaouzidis, A. Capsule endoscopy transit-related indicators in choosing the insertion route for double-balloon enteroscopy: A systematic review. Endosc. Int. Open 2021, 9, E163–E170. [Google Scholar]
- Singeap, A.M.; Sfarti, C.; Minea, H.; Chiriac, S.; Cuciureanu, T.; Nastasa, R.; Stanciu, C.; Trifan, A. Small bowel capsule endoscopy and enteroscopy: A shoulder to shoulder race. J. Clin. Med. 2023, 12, 7328. [Google Scholar] [CrossRef]
- Kim, J.H.; Nam, S.J. Prediction models for recurrence in patients with small bowel bleeding. World J. Clin. Cases 2023, 11, 3949–3957. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Watanabe, T.; Shimada, S.; Nadatani, Y.; Otani, K.; Tanigawa, T.; Miyazaki, T.; Iimuro, M.; Fujiwara, Y. Does discontinuation of antithrombotics affect the diagnostic yield of small bowel capsule endoscopy in patients demonstrating obscure gastrointestinal bleeding? J. Clin. Biochem. Nutr. 2018, 63, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Kim, J.; Jeon, S.; Jeen, Y.T.; Im, J.P.; Cheung, D.Y.; Choi, M.-G.; Lee, K.J.; Ye, B.D.; Shim, K.-N.; et al. Long term outcome of capsule endoscopy in obscure gastrointestinal bleeding: A nationwide analysis. Endoscopy 2014, 46, 59–65. [Google Scholar] [PubMed]
- Tai, F.W.D.; Chetcuti-Zammit, S.; Sidhu, R. Sall bowel angioectasia—The clinical and cost impact of different management strategies. Clin. Res. Hepatol. Gastroenterol. 2023, 47, 102193. [Google Scholar] [CrossRef]
- Kim, S.E.; Kim, H.J.; Koh, M.; Kim, M.C.; Kim, J.S.; Nam, J.H.; Cho, Y.K.; Choe, A.R.; Research Group for Capsule Endoscopy and Enteroscopy of the Korean Society of Gastrointestinal Endoscopy. A practical approach for small bowel bleeding. Clin. Endosc. 2023, 56, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Compean, D.; Cueto-Aguilera, A.; Jimenez Rodriquez, A.; Gonzales, J.; Garza, H. Diagnostic and therapeutic challenges of gastrointestinal angiodysplasias: A critical review and view points. World J. Gastroenterol. 2019, 21, 2549–2564. [Google Scholar] [CrossRef] [PubMed]
- Bollinger, E.; Raines, D.; Saitta, P. Distribution of bleeding gastrointestinal angioectasias in a Western population. World J. Gastroenterol. 2012, 18, 6235–6239. [Google Scholar] [CrossRef] [PubMed]
- Holleran, G.; Hall, B.; Hussey, M.; McNamara, D. Small bowel angiodysplasia and novel disease associations: A cohort study. Scand. J. Gastroenterol. 2013, 48, 433–438. [Google Scholar] [CrossRef]
- Xu, Y.; Siegal, D. Anticoagulant associated gastrointestinal bleeding: Framework for decisions about whether, when and how to resume anticoagulants. J. Thromb. Haemost. 2021, 10, 2303–2393. [Google Scholar] [CrossRef]
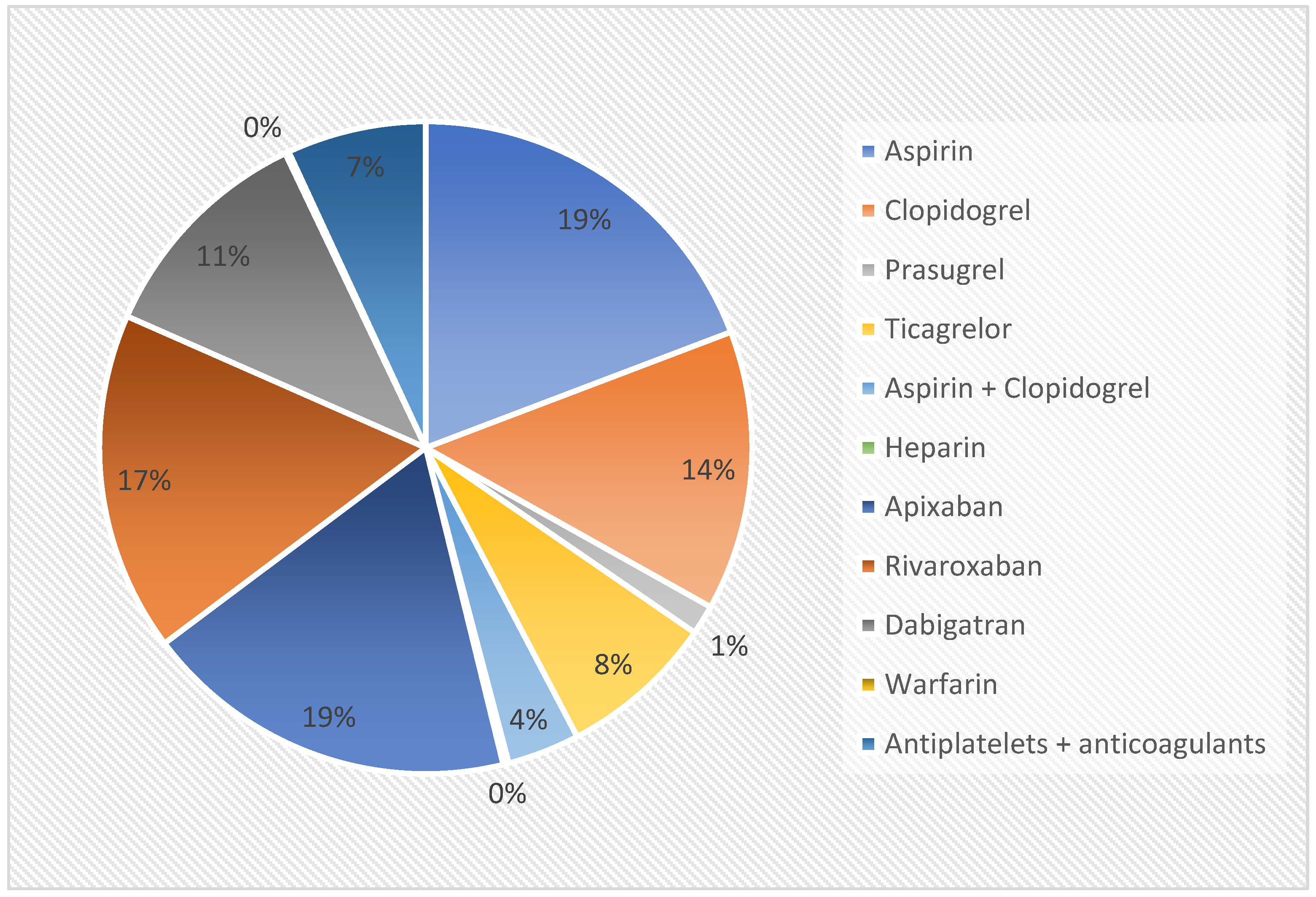
| Whole Population | Patients Receiving Antithrombotics | Patients Not Receiving Antithrombotics | p Value | |
|---|---|---|---|---|
| Age, mean (SD), years | 52.6 ± 27.3 | 56.1 ± 29.1 | 49.9 ± 25.4 | <0.0001 |
| Sex, males/females, number of patients | 4360/4041 | 1302/1233 | 3058/2828 | 0.61 |
| Comorbidities, n (%) | ||||
| Hypertension | 2854 (33.9%) | 804 | 2050 | 0.006 |
| Diabetes mellitus | 1982 (23.5%) | (31.7%) | (34.9%) | 0.05 |
| Chronic kidney disease | 1077 (12.8%) | 631 | 1351 | 0.0009 |
| Liver cirrhosis | 233 (2.8%) | (24.9%) | (23.0%) | <0.0001 |
| Ischemic heart disease | 2106 (25.1%) | 278 | 801 | <0.0001 |
| Hematologic disease | 349 (4.2%) | (10.9%) | (13.6%) | <0.0001 |
| Whole Population (n = 8401) | Patients on Antithrombotics (n = 2535) | Patients without Antithrombotics (n = 5866) | p Value | |
|---|---|---|---|---|
| Overt Bleeding, n (%) | 2389 (28.4) | 963 (38) | 1426 (24.2) | <0.0001 |
| Melena, n (%) | 1733 (20.6) | 647 (25.5) | 1086 (18.5) | <0.0001 |
| Hematochezia, n (%) | 656 (7.8) | 316 (12.5) | 340 (5.8) | <0.0001 |
| Occult bleeding, n (%) | 6012 (71.5) | 1572 (62) | 4440 (75.7) | <0.0001 |
| Iron deficiency anemia, n (%) | 4396 (52.3) | 1127 (44.5) | 3269 (55.7) | <0.0001 |
| Positive fecal occult blood testing, n (%) | 1616 (19.2) | 445 (17.6) | 1171 (20) | 0.01 |
| Whole Population (n = 8401) | Patients on Antithrombotics (n = 2535) | Patients without Antithrombotics (n = 5866) | p Value | |
|---|---|---|---|---|
| P0 lesions, n (%) | 3976 (47.3%) | 723 (28.5) | 3253 (55.4) | <0.0001 |
| P1 lesions, n (%) | 2209 (26.3%) | 709 (27.9) | 1500 (25.6) | 0.02 |
| Isolated erosions or red spots | 1208 (14.3%) | 356 (14.1) | 852 (14.5) | 0.60 |
| Venous ectasias | 864 (10.3) | 302 (11.9) | 562 (9.5) | 0.001 |
| Small polyps or submucosal tumors | 137 (1.6%) | 51 (2.0) | 86 (1.4) | 0.07 |
| P2 lesions, n (%) | 2216 (26.3%) | 1103 (43.5) | 1113 (18.9) | <0.0001 |
| Mucosal breaks | 582 (6.9%) | 241 (9.5) | 341 (5.8) | <0.0001 |
| Angiodysplasias | 1459 (17.4%) | 781 (30.8) | 678 (11.5) | <0.0001 |
| Small bowel tumors | 175 (2.1%) | 81 (3.2) | 94 (1.6) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viazis, N.; Christodoulou, D.; Papastergiou, V.; Mousourakis, K.; Kozompoli, D.; Stasinos, G.; Dimopoulou, K.; Apostolopoulos, P.; Fousekis, F.; Liatsos, C.; et al. Diagnostic Yield and Outcomes of Small Bowel Capsule Endoscopy in Patients with Small Bowel Bleeding Receiving Antithrombotics. Diagnostics 2024, 14, 1361. https://doi.org/10.3390/diagnostics14131361
Viazis N, Christodoulou D, Papastergiou V, Mousourakis K, Kozompoli D, Stasinos G, Dimopoulou K, Apostolopoulos P, Fousekis F, Liatsos C, et al. Diagnostic Yield and Outcomes of Small Bowel Capsule Endoscopy in Patients with Small Bowel Bleeding Receiving Antithrombotics. Diagnostics. 2024; 14(13):1361. https://doi.org/10.3390/diagnostics14131361
Chicago/Turabian StyleViazis, Nikos, Dimitris Christodoulou, Vasilis Papastergiou, Konstantinos Mousourakis, Dimitra Kozompoli, Giannis Stasinos, Konstantina Dimopoulou, Periklis Apostolopoulos, Fotios Fousekis, Christos Liatsos, and et al. 2024. "Diagnostic Yield and Outcomes of Small Bowel Capsule Endoscopy in Patients with Small Bowel Bleeding Receiving Antithrombotics" Diagnostics 14, no. 13: 1361. https://doi.org/10.3390/diagnostics14131361
APA StyleViazis, N., Christodoulou, D., Papastergiou, V., Mousourakis, K., Kozompoli, D., Stasinos, G., Dimopoulou, K., Apostolopoulos, P., Fousekis, F., Liatsos, C., Kyriakos, N., Argyropoulos, T., & Tribonias, G. (2024). Diagnostic Yield and Outcomes of Small Bowel Capsule Endoscopy in Patients with Small Bowel Bleeding Receiving Antithrombotics. Diagnostics, 14(13), 1361. https://doi.org/10.3390/diagnostics14131361






