Central Vein Sign and Paramagnetic Rim Lesions: Susceptibility Changes in Brain Tissues and Their Implications for the Study of Multiple Sclerosis Pathology
Abstract
1. Introduction
2. Physical Principles
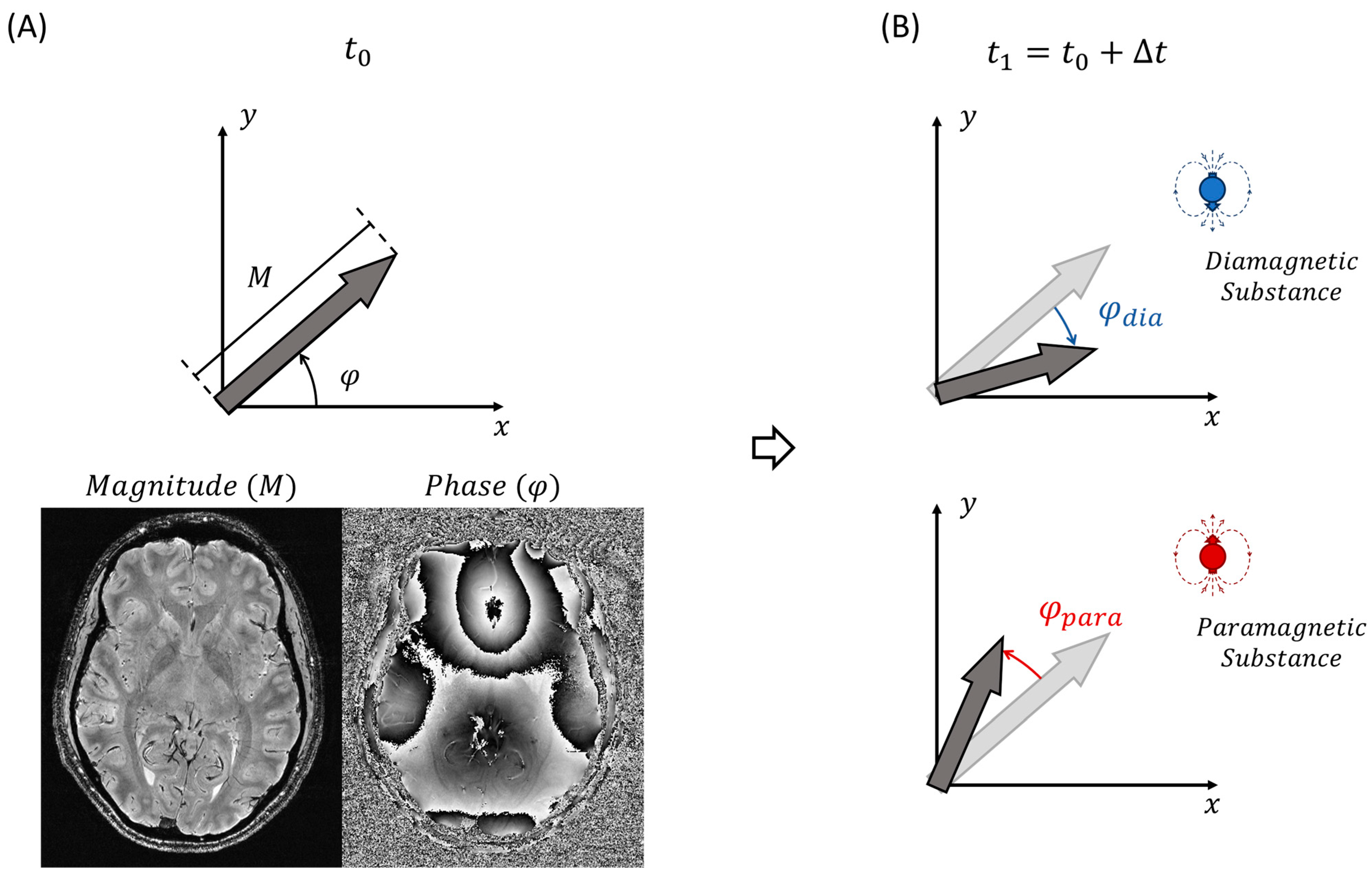
2.1. Magnetic Susceptibility
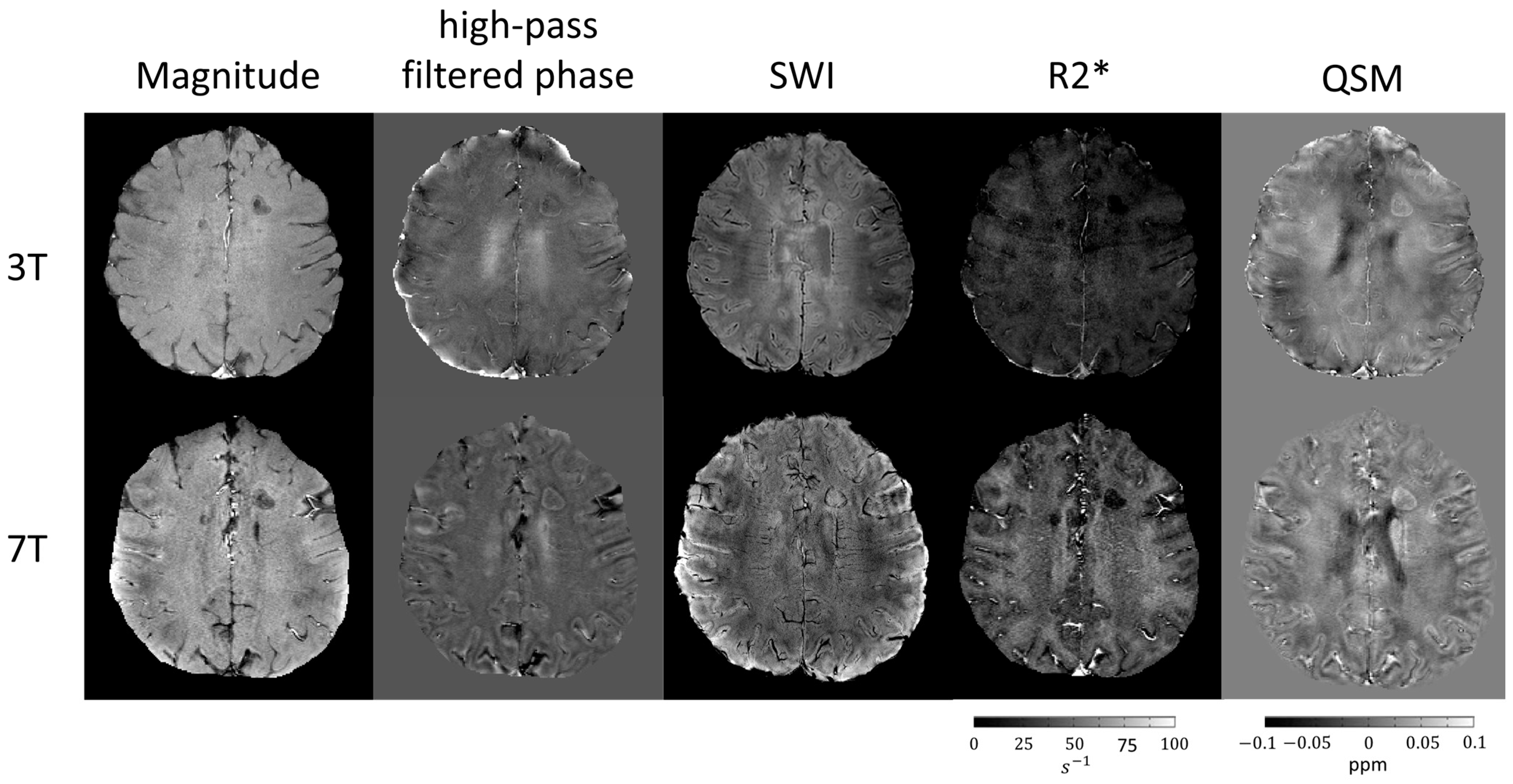
2.2. Susceptibility-Weighted Techniques
2.3. Iron and Myelin on Susceptibility-Weighted Images
2.4. Deoxyhemoglobin in Susceptibility-Weighted Images
3. Application of Susceptibility-Weighted Techniques in Multiple Sclerosis
3.1. Central Vein Sign
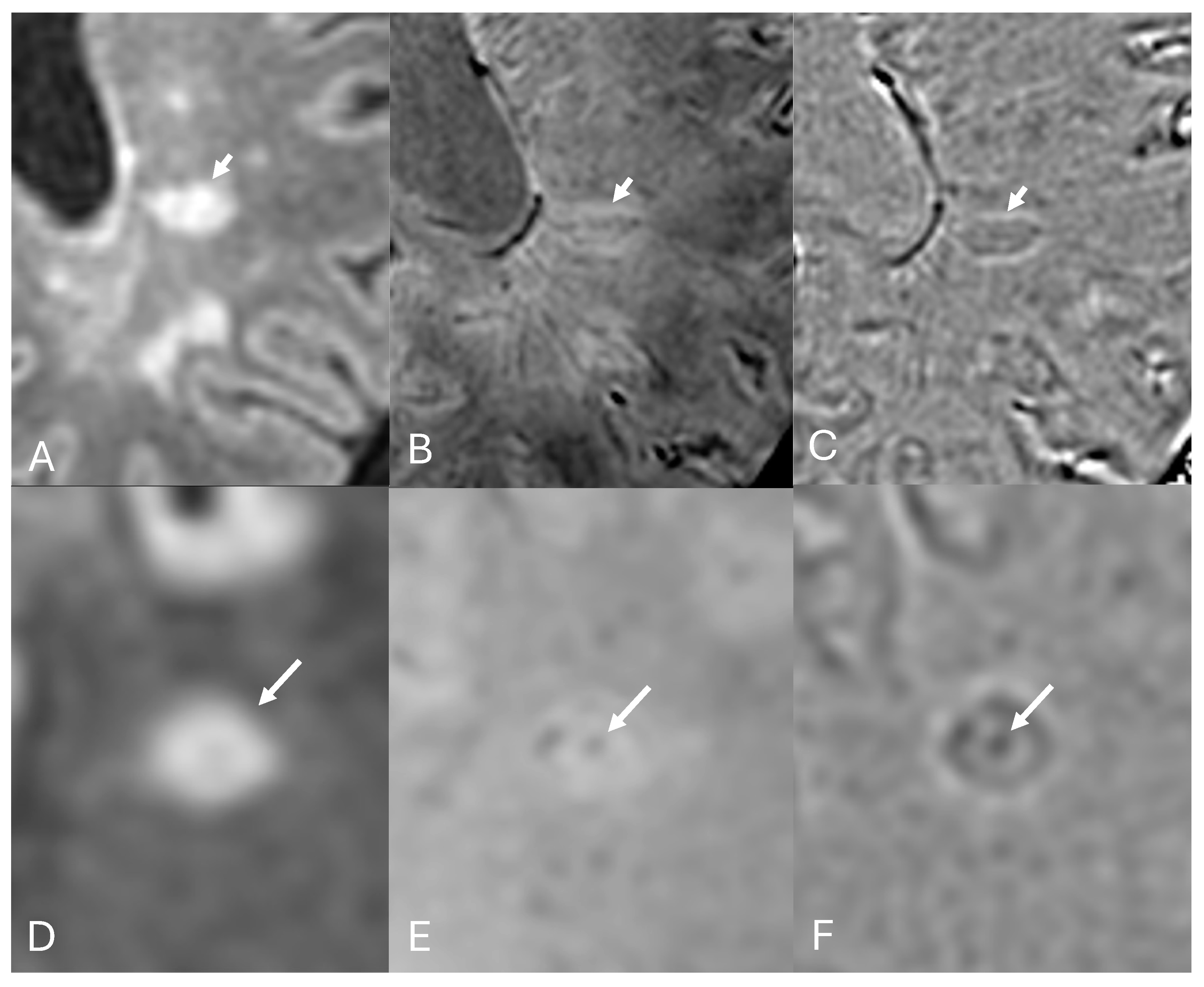
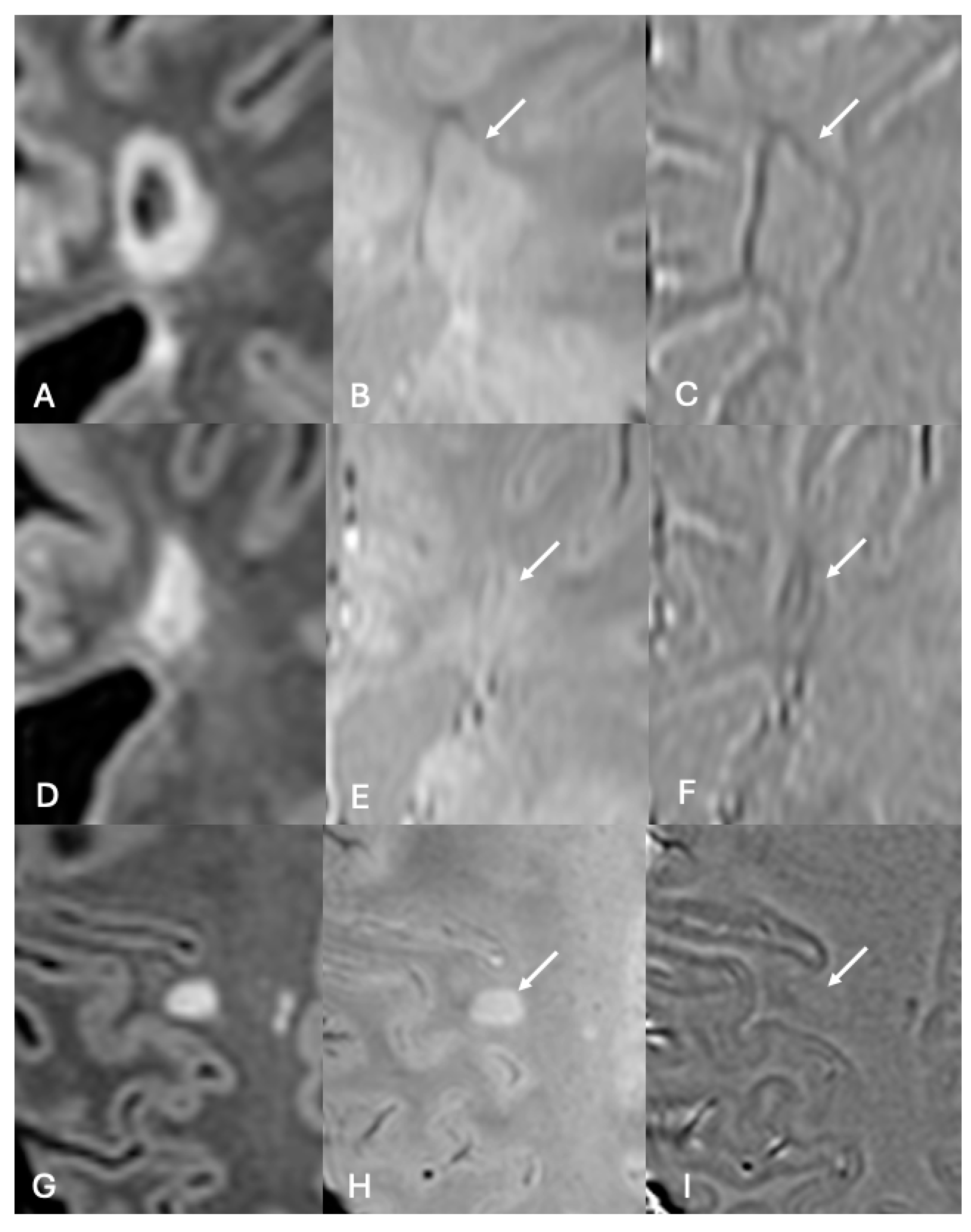
3.2. Paramagnetic Rim Lesions
3.3. Iron Homeostasis, Demyelination, Remyelination, and Chronic Active Lesions
3.4. Persistent Paramagnetic Rim and Slowly Expanding Lesions
4. Conclusions and Future Directions
Funding
Conflicts of Interest
References
- Kau, T.; Taschwer, M.; Deutschmann, H.; Schönfelder, M.; Weber, J.R.; Hausegger, K.A. The “central vein sign”: Is there a place for susceptibility weighted imaging in possible multiple sclerosis? Eur. Radiol. 2013, 23, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Sinnecker, T.; Clarke, M.A.; Meier, D.; Enzinger, C.; Calabrese, M.; De Stefano, N.; Pitiot, A.; Giorgio, A.; Schoonheim, M.M.; Paul, F.; et al. Evaluation of the Central Vein Sign as a Diagnostic Imaging Biomarker in Multiple Sclerosis. JAMA Neurol. 2019, 76, 1446–1456. [Google Scholar] [CrossRef] [PubMed]
- Maggi, P.; Absinta, M.; Sati, P.; Perrotta, G.; Massacesi, L.; Dachy, B.; Pot, C.; Meuli, R.; Reich, D.S.; Filippi, M.; et al. The “central vein sign” in patients with diagnostic "red flags" for multiple sclerosis: A prospective multicenter 3T study. Mult. Scler. J. 2020, 26, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Landes-Chateau, C.; Levraut, M.; Okuda, D.T.; Themelin, A.; Cohen, M.; Kantarci, O.H.; Siva, A.; Pelletier, D.; Mondot, L.; Lebrun-Frenay, C. The diagnostic value of the central vein sign in radiologically isolated syndrome. Ann. Clin. Transl. Neurol. 2024, 11, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H. Targets of therapy in progressive MS. Mult. Scler. J. 2017, 23, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Metz, I.; Gavrilova, R.H.; Weigand, S.D.; Frischer, J.M.; Popescu, B.F.; Guo, Y.; Gloth, M.; Tobin, W.O.; Zalewski, N.L.; Lassmann, H.; et al. Magnetic Resonance Imaging Correlates of Multiple Sclerosis Immunopathological Patterns. Ann. Neurol. 2021, 90, 440–454. [Google Scholar] [CrossRef]
- Cortese, R.; Collorone, S.; Ciccarelli, O.; Toosy, A.T. Advances in brain imaging in multiple sclerosis. Ther. Adv. Neurol. Disord. 2019, 12, 1756286419859722. [Google Scholar] [CrossRef]
- Haller, S.; Haacke, E.M.; Thurnher, M.M.; Barkhof, F. Susceptibility-weighted Imaging: Technical Essentials and Clinical Neurologic Applications. Radiology 2021, 299, 3–26. [Google Scholar] [CrossRef]
- Mehta, V.; Pei, W.; Yang, G.; Li, S.; Swamy, E.; Boster, A.; Schmalbrock, P.; Pitt, D. Iron is a sensitive biomarker for inflammation in multiple sclerosis lesions. PLoS ONE 2013, 8, e57573. [Google Scholar] [CrossRef]
- Absinta, M.; Sati, P.; Schindler, M.; Leibovitch, E.C.; Ohayon, J.; Wu, T.; Meani, A.; Filippi, M.; Jacobson, S.; Cortese, I.C.; et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J. Clin. Investig. 2016, 126, 2597–2609. [Google Scholar] [CrossRef]
- Altokhis, A.I.; Hibbert, A.M.; Allen, C.M.; Mougin, O.; Alotaibi, A.; Lim, S.Y.; Constantinescu, C.S.; Abdel-Fahim, R.; Evangelou, N. Longitudinal clinical study of patients with iron rim lesions in multiple sclerosis. Mult. Scler. J. 2022, 28, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Popescu, B.F.; Frischer, J.M.; Webb, S.M.; Tham, M.; Adiele, R.C.; Robinson, C.A.; Fitz-Gibbon, P.D.; Weigand, S.D.; Metz, I.; Nehzati, S.; et al. Pathogenic implications of distinct patterns of iron and zinc in chronic MS lesions. Acta Neuropathol. 2017, 134, 45–64. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Teunissen, C.; Langkammer, C. Iron and Neurodegeneration in Multiple Sclerosis. Mult. Scler. Int. 2011, 2011, 606807. [Google Scholar] [CrossRef]
- Cheli, V.T.; Correale, J.; Paez, P.M.; Pasquini, J.M. Iron Metabolism in Oligodendrocytes and Astrocytes, Implications for Myelination and Remyelination. ASN Neuro 2020, 12, 1759091420962681. [Google Scholar] [CrossRef]
- Haacke, E.M.; Makki, M.; Ge, Y.; Maheshwari, M.; Sehgal, V.; Hu, J.; Selvan, M.; Wu, Z.; Latif, Z.; Xuan, Y.; et al. Characterizing iron deposition in multiple sclerosis lesions using susceptibility weighted imaging. J. Magn. Reson. Imaging 2009, 29, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Arrighini, G.P.; Maestro, M.; Moccia, R. Magnetic Properties of Polyatomic Molecules. I. Magnetic Susceptibility of H2O, NH3, CH4, H2O2. J. Chem. Phys. 1968, 49, 882–889. [Google Scholar] [CrossRef]
- Kawamura, Y.; Sakurai, I.; Ikegami, A.; Iwayanagi, S. Magneto-Orientation of Phospholipids. Mol. Cryst. Liq. Cryst. 1981, 67, 77–87. [Google Scholar] [CrossRef]
- Schenck, J.F. Health and Physiological Effects of Human Exposure to Whole-Body Four-Tesla Magnetic Fields during MRI. Ann. N. Y. Acad. Sci. 1992, 649, 285–301. [Google Scholar] [CrossRef]
- Oshima, S.; Fushimi, Y.; Okada, T.; Takakura, K.; Liu, C.; Yokota, Y.; Arakawa, Y.; Sawamoto, N.; Miyamoto, S.; Togashi, K. Brain MRI with Quantitative Susceptibility Mapping: Relationship to CT Attenuation Values. Radiology 2020, 294, 600–609. [Google Scholar] [CrossRef]
- Duyn, J.H.; Schenck, J. Contributions to magnetic susceptibility of brain tissue. NMR Biomed. 2017, 30, e3546. [Google Scholar] [CrossRef] [PubMed]
- Deistung, A.; Schäfer, A.; Schweser, F.; Biedermann, U.; Turner, R.; Reichenbach, J.R. Toward in vivo histology: A comparison of quantitative susceptibility mapping (QSM) with magnitude-, phase-, and R2⁎-imaging at ultra-high magnetic field strength. Neuroimage 2013, 65, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Yablonskiy, D.A.; Haacke, E.M. Theory of NMR signal behavior in magnetically inhomogeneous tissues: The static dephasing regime. Magn. Reson. Med. 1994, 32, 749–763. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Nguyen, T.D.; Pandya, S.; Zhang, Y.; Hurtado Rúa, S.; Kovanlikaya, I.; Kuceyeski, A.; Liu, Z.; Wang, Y.; Gauthier, S.A. Combining Quantitative Susceptibility Mapping with Automatic Zero Reference (QSM0) and Myelin Water Fraction Imaging to Quantify Iron-Related Myelin Damage in Chronic Active MS Lesions. AJNR Am. J. Neuroradiol. 2018, 39, 303–310. [Google Scholar]
- Kaunzner, U.W.; Kang, Y.; Zhang, S.; Morris, E.; Yao, Y.; Pandya, S.; Hurtado Rua, S.M.; Park, C.; Gillen, K.M.; Nguyen, T.D.; et al. Quantitative susceptibility mapping identifies inflammation in a subset of chronic multiple sclerosis lesions. Brain 2019, 142, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Sedlacik, J.; Rauscher, A.; Reichenbach, J.R. Quantification of modulated blood oxygenation levels in single cerebral veins by investigating their MR signal decay. Z. Med. Phys. 2009, 19, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Mehemed, T.M.; Yamamoto, A. High-pass-filtered phase image: Left- versus right-handed MR imaging systems. AJNR Am. J. Neuroradiol. 2013, 34, E72. [Google Scholar] [CrossRef] [PubMed]
- Halefoglu, A.M.; Yousem, D.M. Susceptibility weighted imaging: Clinical applications and future directions. World J. Radiol. 2018, 10, 30–45. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wei, H.; Gong, N.J.; Cronin, M.; Dibb, R.; Decker, K. Quantitative Susceptibility Mapping: Contrast Mechanisms and Clinical Applications. Tomography 2015, 1, 3–17. [Google Scholar] [CrossRef] [PubMed]
- QSM Challenge 2.0 Organization Committee; Bilgic, B.; Langkammer, C.; Marques, J.P.; Meineke, J.; Milovic, C.; Schweser, F. QSM reconstruction challenge 2.0: Design and report of results. Magn. Reson. Med. 2021, 86, 1241–1255. [Google Scholar]
- Haacke, E.M.; Liu, S.; Buch, S.; Zheng, W.; Wu, D.; Ye, Y. Quantitative susceptibility mapping: Current status and future directions. Magn. Reson. Imaging 2015, 33, 1–25. [Google Scholar] [CrossRef]
- Liu, Z.; Spincemaille, P.; Yao, Y.; Zhang, Y.; Wang, Y. MEDI+0: Morphology enabled dipole inversion with automatic uniform cerebrospinal fluid zero reference for quantitative susceptibility mapping. Magn. Reson. Med. 2018, 79, 2795–2803. [Google Scholar] [CrossRef] [PubMed]
- Cronin, M.J.; Wang, N.; Decker, K.S.; Wei, H.; Zhu, W.-Z.; Liu, C. Exploring the origins of echo-time-dependent quantitative susceptibility mapping (QSM) measurements in healthy tissue and cerebral microbleeds. Neuroimage 2017, 149, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Kudo, K.; Fujima, N.; Yoshikawa, M.; Ikebe, Y.; Sato, R.; Shirai, T.; Bito, Y.; Uwano, I.; Miyata, M. Quantitative Susceptibility Mapping: Basic Methods and Clinical Applications. RadioGraphics 2022, 42, 1161–1176. [Google Scholar] [CrossRef] [PubMed]
- Eskreis-Winkler, S.; Deh, K.; Gupta, A.; Liu, T.; Wisnieff, C.; Jin, M.; Gauthier, S.A.; Wang, Y.; Spincemaille, P. Multiple sclerosis lesion geometry in quantitative susceptibility mapping (QSM) and phase imaging. J. Magn. Reson. Imaging 2015, 42, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Karsa, A.; Punwani, S.; Shmueli, K. The effect of low resolution and coverage on the accuracy of susceptibility mapping. Magn. Reson. Med. 2019, 81, 1833–1848. [Google Scholar] [CrossRef] [PubMed]
- Absinta, M.; Sati, P.; Fechner, A.; Schindler, M.K.; Nair, G.; Reich, D.S. Identification of Chronic Active Multiple Sclerosis Lesions on 3T MRI. AJNR Am. J. Neuroradiol. 2018, 39, 1233–1238. [Google Scholar] [CrossRef]
- Gillen, K.M.; Mubarak, M.; Park, C.; Ponath, G.; Zhang, S.; Dimov, A.; Levine-Ritterman, M.; Toro, S.; Huang, W.; Amici, S.; et al. QSM is an imaging biomarker for chronic glial activation in multiple sclerosis lesions. Ann. Clin. Transl. Neurol. 2021, 8, 877–886. [Google Scholar] [CrossRef]
- Harrison, D.M.; Li, X.; Liu, H.; Jones, C.K.; Caffo, B.; Calabresi, P.A.; van Zijl, P. Lesion Heterogeneity on High-Field Susceptibility MRI Is Associated with Multiple Sclerosis Severity. AJNR Am. J. Neuroradiol. 2016, 37, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.; Jensen, J.H.; Johnson, G. Intravascular contrast agent T2* relaxivity in brain tissue. NMR Biomed. 2013, 26, 392–399. [Google Scholar] [CrossRef]
- Hsu, C.C.; Haacke, E.M.; Heyn, C.C.; Watkins, T.W.; Krings, T. The T1 shine through effect on susceptibility weighted imaging: An under recognized phenomenon. Neuroradiology 2018, 60, 235–237. [Google Scholar] [CrossRef]
- El-Koussy, M.; Schenk, P.; Kiefer, C.; Osman, O.M.; Mordasini, P.; Ozdoba, C.; Schroth, G.; Gönner, F. Susceptibility-weighted imaging of the brain: Does gadolinium administration matter? Eur. J. Radiol. 2012, 81, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Stankiewicz, J.M.; Neema, M.; Ceccarelli, A. Iron and multiple sclerosis. Neurobiol. Aging 2014, 35 (Suppl. S2), S51–S58. [Google Scholar] [CrossRef] [PubMed]
- Birkl, C.; Birkl-Toeglhofer, A.M.; Kames, C.; Goessler, W.; Haybaeck, J.; Fazekas, F.; Ropele, S.; Rauscher, A. The influence of iron oxidation state on quantitative MRI parameters in post mortem human brain. Neuroimage 2020, 220, 117080. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, C.; Duong, T.Q.; van Zijl, P.C.M.; Li, X. Susceptibility tensor imaging (STI) of the brain. NMR Biomed. 2017, 30, e3540. [Google Scholar] [CrossRef] [PubMed]
- Puwal, S.; Roth, B.J.; Basser, P.J. Heterogeneous anisotropic magnetic susceptibility of the myelin-water layers causes local magnetic field perturbations in axons. NMR Biomed. 2017, 30, e3628. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.G.; Lee, J.; Yun, Y.H.; Yoo, S.H.; Jang, J.; Oh, S.H.; Nam, Y.; Jung, S.; Kim, S.; Fukunaga, M.; et al. χ-separation: Magnetic susceptibility source separation toward iron and myelin mapping in the brain. Neuroimage 2021, 240, 118371. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gong, N.J.; Chaim, K.T.; Otaduy, M.C.G.; Liu, C. Decompose quantitative susceptibility mapping (QSM) to sub-voxel diamagnetic and paramagnetic components based on gradient-echo MRI data. Neuroimage 2021, 242, 118477. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Zohrabian, V.M.; Osa, E.O.; Xu, J.; Jaggi, H.; Herbert, J.; Haacke, E.M.; Grossman, R.I. Diminished visibility of cerebral venous vasculature in multiple sclerosis by susceptibility-weighted imaging at 3.0 Tesla. J. Magn. Reson. Imaging 2009, 29, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, R.; Miletich, R.S.; Kinkel, P.R.; Emmet, M.L.; Kinkel, W.R. High-resolution fluorodeoxyglucose positron emission tomography shows both global and regional cerebral hypometabolism in multiple sclerosis. J. Neuroimaging Off. J. Am. Soc. Neuroimaging 1998, 8, 228–234. [Google Scholar] [CrossRef]
- Sparacia, G.; Agnello, F.; Iaia, A.; Banco, A.; Galia, M.; Midiri, M. Multiple sclerosis: Prevalence of the ‘central vein’ sign in white matter lesions on gadolinium-enhanced susceptibility-weighted images. Neuroradiol. J. 2021, 34, 470–475. [Google Scholar] [CrossRef]
- Maggi, P.; Mazzoni, L.N.; Moretti, M.; Grammatico, M.; Chiti, S.; Massacesi, L. SWI enhances vein detection using gadolinium in multiple sclerosis. Acta Radiol. Open 2015, 4, 2047981614560938. [Google Scholar] [CrossRef] [PubMed]
- Sati, P.; Thomasson, D.M.; Li, N.; Pham, D.L.; Biassou, N.M.; Reich, D.S.; Butman, J.A. Rapid, high-resolution, whole-brain, susceptibility-based MRI of multiple sclerosis. Mult. Scler. J. 2014, 20, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Sati, P.; George, I.C.; Shea, C.D.; Gaitan, M.I.; Reich, D.S. FLAIR*: A combined MR contrast technique for visualizing white matter lesions and parenchymal veins. Radiology 2012, 265, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Zohrabian, V.M.; Grossman, R.I. Seven-Tesla magnetic resonance imaging: New vision of microvascular abnormalities in multiple sclerosis. Arch. Neurol. 2008, 65, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Campion, T.; Smith, R.J.P.; Altmann, D.R.; Brito, G.C.; Turner, B.P.; Evanson, J.; George, I.C.; Sati, P.; Reich, D.S.; Miquel, M.E.; et al. FLAIR* to visualize veins in white matter lesions: A new tool for the diagnosis of multiple sclerosis? Eur. Radiol. 2017, 27, 4257–4263. [Google Scholar] [CrossRef] [PubMed]
- Maggi, P.; Absinta, M.; Grammatico, M.; Vuolo, L.; Emmi, G.; Carlucci, G.; Spagni, G.; Barilaro, A.; Repice, A.M.; Emmi, L.; et al. Central vein sign differentiates Multiple Sclerosis from central nervous system inflammatory vasculopathies. Ann. Neurol. 2018, 83, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Lamot, U.; Avsenik, J.; Šega, S.; Šurlan Popovič, K. Presence of central veins and susceptibility weighted imaging for evaluating lesions in multiple sclerosis and leukoaraiosis. Mult. Scler. Relat. Disord. 2017, 13, 67–72. [Google Scholar] [CrossRef]
- Cagol, A.; Cortese, R.; Barakovic, M.; Schaedelin, S.; Ruberte, E.; Absinta, M.; Barkhof, F.; Calabrese, M.; Castellaro, M.; Ciccarelli, O.; et al. Diagnostic Performance of Cortical Lesions and the Central Vein Sign in Multiple Sclerosis. JAMA Neurol. 2024, 81, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.A.; Samaraweera, A.P.; Falah, Y.; Pitiot, A.; Allen, C.M.; Dineen, R.A.; Tench, C.R.; Morgan, P.S.; Evangelou, N. Single Test to ARrive at Multiple Sclerosis (STAR-MS) diagnosis: A prospective pilot study assessing the accuracy of the central vein sign in predicting multiple sclerosis in cases of diagnostic uncertainty. Mult. Scler. J. 2020, 26, 433–441. [Google Scholar] [CrossRef]
- Cortese, R.; Magnollay, L.; Tur, C.; Abdel-Aziz, K.; Jacob, A.; De Angelis, F.; Yiannakas, M.C.; Prados, F.; Ourselin, S.; Yousry, T.A.; et al. Value of the central vein sign at 3T to differentiate MS from seropositive NMOSD. Neurology 2018, 90, e1183–e1190. [Google Scholar] [CrossRef]
- Gao, C.; Su, L.; Li, H.; Song, T.; Liu, Y.; Duan, Y.; Shi, F.D. Susceptibility-weighted image features in AQP4-negative-NMOSD versus MS. Mult. Scler. Relat. Disord. 2024, 82, 105406. [Google Scholar] [CrossRef] [PubMed]
- Sati, P.; Oh, J.; Constable, R.T.; Evangelou, N.; Guttmann, C.R.G.; Henry, R.G.; Klawiter, E.C.; Mainero, C.; Massacesi, L.; McFarland, H.; et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: A consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat. Rev. Neurol. 2016, 12, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Mistry, N.; Abdel-Fahim, R.; Samaraweera, A.; Mougin, O.; Tallantyre, E.; Tench, C.; Jaspan, T.; Morris, P.; Morgan, P.S.; Evangelou, N. Imaging central veins in brain lesions with 3-T T2*-weighted magnetic resonance imaging differentiates multiple sclerosis from microangiopathic brain lesions. Mult. Scler. J. 2016, 22, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.A.; Pareto, D.; Pessini-Ferreira, L.; Arrambide, G.; Alberich, M.; Crescenzo, F.; Cappelle, S.; Tintoré, M.; Sastre-Garriga, J.; Auger, C.; et al. Value of 3T Susceptibility-Weighted Imaging in the Diagnosis of Multiple Sclerosis. AJNR Am. J. Neuroradiol. 2020, 41, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Kaisey, M.; Solomon, A.J.; Guerrero, B.L.; Renner, B.; Fan, Z.; Ayala, N.; Luu, M.; Diniz, M.A.; Sati, P.; Sicotte, N.L. Preventing multiple sclerosis misdiagnosis using the “central vein sign”: A real-world study. Mult. Scler. Relat. Disord. 2021, 48, 102671. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, J.D.; Sati, P.; Solomon, A.; Pham, D.L.; Watts, R.; Martin, M.L.; Ontaneda, D.; Schindler, M.K.; Reich, D.S.; Shinohara, R.T. Automated Integration of Multimodal MRI for the Probabilistic Detection of the Central Vein Sign in White Matter Lesions. AJNR Am. J. Neuroradiol. 2018, 39, 1806–1813. [Google Scholar] [CrossRef]
- Maggi, P.; Fartaria, M.J.; Jorge, J.; La Rosa, F.; Absinta, M.; Sati, P.; Meuli, R.; Du Pasquier, R.; Reich, D.S.; Cuadra, M.B.; et al. CVSnet: A machine learning approach for automated central vein sign assessment in multiple sclerosis. NMR Biomed. 2020, 33, e4283. [Google Scholar] [CrossRef]
- Daboul, L.; O’Donnell, C.M.; Amin, M.; Rodrigues, P.; Derbyshire, J.; Azevedo, C.; Bar-Or, A.; Caverzasi, E.; Calabresi, P.A.; Cree, B.A.; et al. A multicenter pilot study evaluating simplified central vein assessment for the diagnosis of multiple sclerosis. Mult. Scler. J. 2024, 30, 25–34. [Google Scholar] [CrossRef]
- Solomon, A.J.; Watts, R.; Ontaneda, D.; Absinta, M.; Sati, P.; Reich, D.S. Diagnostic performance of central vein sign for multiple sclerosis with a simplified three-lesion algorithm. Mult. Scler. J. 2018, 24, 750–757. [Google Scholar] [CrossRef]
- Okromelidze, L.; Patel, V.; Singh, R.B.; Lopez Chiriboga, A.S.; Tao, S.; Zhou, X.; Straub, S.; Westerhold, E.M.; Gupta, V.; Agarwal, A.K.; et al. Central Vein Sign in Multiple Sclerosis: A Comparison Study of the Diagnostic Performance of 3T versus 7T MRI. AJNR Am. J. Neuroradiol. 2023, 45, 76–81. [Google Scholar] [CrossRef]
- Tallantyre, E.C.; Morgan, P.S.; Dixon, J.E.; Al-Radaideh, A.; Brookes, M.J.; Evangelou, N.; Morris, P.G. A Comparison of 3T and 7T in the Detection of Small Parenchymal Veins Within MS Lesions. Investig. Radiol. 2009, 44, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Castellaro, M.; Tamanti, A.; Pisani, A.I.; Pizzini, F.B.; Crescenzo, F.; Calabrese, M. The Use of the Central Vein Sign in the Diagnosis of Multiple Sclerosis: A Systematic Review and Meta-analysis. Diagnostics 2020, 10, 1025. [Google Scholar] [CrossRef] [PubMed]
- Sparacia, G.; Agnello, F.; Gambino, A.; Sciortino, M.; Midiri, M. Multiple sclerosis: High prevalence of the ‘central vein’ sign in white matter lesions on susceptibility-weighted images. Neuroradiol. J. 2018, 31, 356–361. [Google Scholar] [CrossRef]
- Samaraweera, A.P.; Clarke, M.A.; Whitehead, A.; Falah, Y.; Driver, I.D.; Dineen, R.A.; Morgan, P.S.; Evangelou, N. The Central Vein Sign in Multiple Sclerosis Lesions Is Present Irrespective of the T2* Sequence at 3 T. J. Neuroimaging Off. J. Am. Soc. Neuroimaging 2017, 27, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, J.J.; Versluis, M.J.; Luijten, P.R.; Petridou, N. Fast high resolution whole brain T2* weighted imaging using echo planar imaging at 7T. Neuroimage 2011, 56, 1902–1907. [Google Scholar] [CrossRef] [PubMed]
- Absinta, M.; Nair, G.; Monaco, M.C.G.; Maric, D.; Lee, N.J.; Ha, S.K.; Luciano, N.J.; Sati, P.; Jacobson, S.; Reich, D.S. The "central vein sign" in inflammatory demyelination: The role of fibrillar collagen type I. Ann. Neurol. 2019, 85, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Grabner, G.; Dal-Bianco, A.; Schernthaner, M.; Vass, K.; Lassmann, H.; Trattnig, S. Analysis of multiple sclerosis lesions using a fusion of 3.0 T FLAIR and 7.0 T SWI phase: FLAIR SWI. J. Magn. Reson. Imaging 2011, 33, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Lapucci, C.; Tazza, F.; Rebella, S.; Boffa, G.; Sbragia, E.; Bruschi, N.; Mancuso, E.; Mavilio, N.; Signori, A.; Roccatagliata, L.; et al. Central vein sign and diffusion MRI differentiate microstructural features within white matter lesions of multiple sclerosis patients with comorbidities. Front. Neurol. 2023, 14, 1084661. [Google Scholar] [CrossRef]
- Buch, S.; Subramanian, K.; Jella, P.K.; Chen, Y.; Wu, Z.; Shah, K.; Bernitsas, E.; Ge, Y.; Haacke, E.M. Revealing vascular abnormalities and measuring small vessel density in multiple sclerosis lesions using USPIO. Neuroimage Clin. 2021, 29, 102525. [Google Scholar] [CrossRef]
- Li, J.; Huang, W.; Luo, X.; Wen, Y.; Cho, J.; Kovanlikaya, I.; Gauthier, S.A.; Nguyen, T.D.; Spincemaille, P.; Wang, Y. The central vein sign in multiple sclerosis lesions: Susceptibility relaxation optimization from a routine MRI multiecho gradient echo sequence. J. Neuroimaging Off. J. Am. Soc. Neuroimaging 2022, 32, 48–56. [Google Scholar] [CrossRef]
- Suthiphosuwan, S.; Sati, P.; Guenette, M.; Montalban, X.; Reich, D.S.; Bharatha, A.; Oh, J. The Central Vein Sign in Radiologically Isolated Syndrome. AJNR Am. J. Neuroradiol. 2019, 40, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Suthiphosuwan, S.; Sati, P.; Absinta, M.; Dewey, B.; Guenette, M.; Selchen, D.; Bharatha, A.; Donaldson, E.; Reich, D.S.; et al. Cognitive impairment, the central vein sign, and paramagnetic rim lesions in RIS. Mult. Scler. J. 2021, 27, 2199–2208. [Google Scholar] [CrossRef] [PubMed]
- Levasseur, V.A.; Xiang, B.; Salter, A.; Yablonskiy, D.A.; Cross, A.H. Stronger Microstructural Damage Revealed in Multiple Sclerosis Lesions With Central Vein Sign by Quantitative Gradient Echo MRI. J. Cent. Nerv. Syst. Dis. 2022, 14, 11795735221084842. [Google Scholar] [CrossRef]
- Reichl, M.; Wittayer, M.; Weber, C.E.; Platten, M.; Gass, A.; Eisele, P. Consistency of the "central vein sign" in chronic multiple sclerosis lesions. Mult. Scler. Relat. Disord. 2022, 58, 103530. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.E.; Kraemer, M.; Dabringhaus, A.; Ebert, A.; Platten, M.; Gass, A.; Eisele, P. Venous Diameter Changes in Chronic Active Multiple Sclerosis Lesions. J. Neuroimaging Off. J. Am. Soc. Neuroimaging 2021, 31, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Correale, J.; Gaitán, M.I.; Ysrraelit, M.C.; Fiol, M.P. Progressive multiple sclerosis: From pathogenic mechanisms to treatment. Brain 2017, 140, 527–546. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, T.; Moccia, M.; Coetzee, T.; Cohen, J.A.; Correale, J.; Graves, J.; Marrie, R.A.; Montalban, X.; Yong, V.W.; Thompson, A.J.; et al. Multiple sclerosis progression: Time for a new mechanism-driven framework. Lancet Neurol. 2023, 22, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Gillen, K.M.; Mubarak, M.; Nguyen, T.D.; Pitt, D. Significance and In Vivo Detection of Iron-Laden Microglia in White Matter Multiple Sclerosis Lesions. Front. Immunol. 2018, 9, 255. [Google Scholar] [CrossRef]
- Maggi, P.; Sati, P.; Nair, G.; Cortese, I.C.M.; Jacobson, S.; Smith, B.R.; Nath, A.; Ohayon, J.; van Pesch, V.; Perrotta, G.; et al. Paramagnetic Rim Lesions are Specific to Multiple Sclerosis: An International Multicenter 3T MRI Study. Ann. Neurol. 2020, 88, 1034–1042. [Google Scholar] [CrossRef]
- Absinta, M.; Sati, P.; Masuzzo, F.; Nair, G.; Sethi, V.; Kolb, H.; Ohayon, J.; Wu, T.; Cortese, I.C.M.; Reich, D.S. Association of Chronic Active Multiple Sclerosis Lesions With Disability In Vivo. JAMA Neurol. 2019, 76, 1474–1483. [Google Scholar] [CrossRef]
- Jang, J.; Nam, Y.; Choi, Y.; Shin, N.Y.; An, J.Y.; Ahn, K.J.; Kim, B.S.; Lee, K.S.; Kim, W. Paramagnetic Rims in Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorder: A Quantitative Susceptibility Mapping Study with 3-T MRI. J. Clin. Neurol. 2020, 16, 562–572. [Google Scholar] [CrossRef]
- Dal-Bianco, A.; Grabner, G.; Kronnerwetter, C.; Weber, M.; Kornek, B.; Kasprian, G.; Berger, T.; Leutmezer, F.; Rommer, P.S.; Trattnig, S.; et al. Long-term evolution of multiple sclerosis iron rim lesions in 7 T MRI. Brain 2021, 144, 833–847. [Google Scholar] [CrossRef] [PubMed]
- Dal-Bianco, A.; Schranzer, R.; Grabner, G.; Lanzinger, M.; Kolbrink, S.; Pusswald, G.; Altmann, P.; Ponleitner, M.; Weber, M.; Kornek, B.; et al. Iron Rims in Patients With Multiple Sclerosis as Neurodegenerative Marker? A 7-Tesla Magnetic Resonance Study. Front. Neurol. 2021, 12, 632749. [Google Scholar] [CrossRef] [PubMed]
- Preziosa, P.; Rocca, M.A.; Filippi, M. Central vein sign and iron rim in multiple sclerosis: Ready for clinical use? Curr. Opin. Neurol. 2021, 34, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Ikonomidou, V.N.; Cantor, F.K.; Ohayon, J.M.; Duyn, J.; Bagnato, F. Heterogeneity of Multiple Sclerosis White Matter Lesions Detected With T2*-Weighted Imaging at 7.0 Tesla. J. Neuroimaging Off. J. Am. Soc. Neuroimaging 2015, 25, 799–806. [Google Scholar] [CrossRef]
- Dal-Bianco, A.; Grabner, G.; Kronnerwetter, C.; Weber, M.; Höftberger, R.; Berger, T.; Auff, E.; Leutmezer, F.; Trattnig, S.; Lassmann, H.; et al. Slow expansion of multiple sclerosis iron rim lesions: Pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 2017, 133, 25–42. [Google Scholar] [CrossRef]
- Micheletti, L.; Maldonado, F.R.; Watal, P.; Toronchik, M.S.; Erripa, J.I.; Princich, J.P.; Rugilo, C. Utility of paramagnetic rim lesions on 1.5-T susceptibility phase imaging for the diagnosis of pediatric multiple sclerosis. Pediatr. Radiol. 2022, 52, 97–103. [Google Scholar] [CrossRef]
- Hemond, C.C.; Reich, D.S.; Dundamadappa, S.K. Paramagnetic Rim Lesions in Multiple Sclerosis: Comparison of Visualization at 1.5-T and 3-T MRI. AJR Am. J. Roentgenol. 2022, 219, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Calvi, A.; Clarke, M.A.; Prados, F.; Chard, D.; Ciccarelli, O.; Alberich, M.; Pareto, D.; Rodríguez Barranco, M.; Sastre-Garriga, J.; Tur, C.; et al. Relationship between paramagnetic rim lesions and slowly expanding lesions in multiple sclerosis. Mult. Scler. J. 2023, 29, 352–362. [Google Scholar] [CrossRef]
- Zhang, H.; Nguyen, T.D.; Zhang, J.; Marcille, M.; Spincemaille, P.; Wang, Y.; Gauthier, S.A.; Sweeney, E.M. QSMRim-Net: Imbalance-aware learning for identification of chronic active multiple sclerosis lesions on quantitative susceptibility maps. Neuroimage Clin. 2022, 34, 102979. [Google Scholar] [CrossRef]
- Zhang, S.; Nguyen, T.D.; Hurtado Rúa, S.M.; Kaunzner, U.W.; Pandya, S.; Kovanlikaya, I.; Spincemaille, P.; Wang, Y.; Gauthier, S.A. Quantitative Susceptibility Mapping of Time-Dependent Susceptibility Changes in Multiple Sclerosis Lesions. AJNR Am. J. Neuroradiol. 2019, 40, 987–993. [Google Scholar] [PubMed]
- Barquero, G.; La Rosa, F.; Kebiri, H.; Lu, P.J.; Rahmanzadeh, R.; Weigel, M.; Fartaria, M.J.; Kober, T.; Théaudin, M.; Du Pasquier, R.; et al. RimNet: A deep 3D multimodal MRI architecture for paramagnetic rim lesion assessment in multiple sclerosis. Neuroimage Clin. 2020, 28, 102412. [Google Scholar] [CrossRef] [PubMed]
- Maggi, P.; Kuhle, J.; Schädelin, S.; van der Meer, F.; Weigel, M.; Galbusera, R.; Mathias, A.; Lu, P.J.; Rahmanzadeh, R.; Benkert, P.; et al. Chronic White Matter Inflammation and Serum Neurofilament Levels in Multiple Sclerosis. Neurology 2021, 97, e543–e553. [Google Scholar] [CrossRef] [PubMed]
- Meaton, I.; Altokhis, A.; Allen, C.M.; Clarke, M.A.; Sinnecker, T.; Meier, D.; Enzinger, C.; Calabrese, M.; De Stefano, N.; Pitiot, A.; et al. Paramagnetic rims are a promising diagnostic imaging biomarker in multiple sclerosis. Mult. Scler. J. 2022, 28, 2212–2220. [Google Scholar] [CrossRef]
- Sinnecker, T.; Schumacher, S.; Mueller, K.; Pache, F.; Dusek, P.; Harms, L.; Ruprecht, K.; Nytrova, P.; Chawla, S.; Niendorf, T.; et al. MRI phase changes in multiple sclerosis vs neuromyelitis optica lesions at 7T. Neurol. Neuroimmunol. Neuroinflammation 2016, 3, e259. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.J.; Lebel, R.M.; Eissa, A.; Blevins, G.; Catz, I.; Lu, J.Q.; Resch, L.; Johnson, E.S.; Emery, D.J.; Warren, K.G.; et al. Multiple sclerosis: Validation of MR imaging for quantification and detection of iron. Radiology 2013, 267, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, N.; Wittayer, M.; Weber, C.E.; Schirmer, L.; Platten, M.; Gass, A.; Eisele, P. MRI predictors for the conversion from contrast-enhancing to iron rim multiple sclerosis lesions. J. Neurol. 2022, 269, 4414–4420. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.E.; Wittayer, M.; Kraemer, M.; Dabringhaus, A.; Bail, K.; Platten, M.; Schirmer, L.; Gass, A.; Eisele, P. Long-term dynamics of multiple sclerosis iron rim lesions. Mult. Scler. Relat. Disord. 2022, 57, 103340. [Google Scholar] [CrossRef]
- Chen, W.; Gauthier, S.A.; Gupta, A.; Comunale, J.; Liu, T.; Wang, S.; Pei, M.; Pitt, D.; Wang, Y. Quantitative susceptibility mapping of multiple sclerosis lesions at various ages. Radiology 2014, 271, 183–192. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Q.; Yan, Z.; Shi, Z.; Xu, Y.; Liu, Y.; Li, Y. Enlarged choroid plexus related to iron rim lesions and deep gray matter atrophy in relapsing-remitting multiple sclerosis. Mult. Scler. Relat. Disord. 2023, 75, 104740. [Google Scholar] [CrossRef]
- Frischer, J.M.; Weigand, S.D.; Guo, Y.; Kale, N.; Parisi, J.E.; Pirko, I.; Mandrekar, J.; Bramow, S.; Metz, I.; Brück, W.; et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann. Neurol. 2015, 78, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Luchetti, S.; Fransen, N.L.; van Eden, C.G.; Ramaglia, V.; Mason, M.; Huitinga, I. Progressive multiple sclerosis patients show substantial lesion activity that correlates with clinical disease severity and sex: A retrospective autopsy cohort analysis. Acta Neuropathol. 2018, 135, 511–528. [Google Scholar] [CrossRef]
- Hemond, C.C.; Baek, J.; Ionete, C.; Reich, D.S. Paramagnetic rim lesions are associated with pathogenic CSF profiles and worse clinical status in multiple sclerosis: A retrospective cross-sectional study. Mult. Scler. J. 2022, 28, 2046–2056. [Google Scholar] [CrossRef] [PubMed]
- Krajnc, N.; Schmidbauer, V.; Leinkauf, J.; Haider, L.; Bsteh, G.; Kasprian, G.; Leutmezer, F.; Kornek, B.; Rommer, P.S.; Berger, T.; et al. Paramagnetic rim lesions lead to pronounced diffuse periplaque white matter damage in multiple sclerosis. Mult. Scler. J. 2023, 29, 1406–1417. [Google Scholar] [CrossRef] [PubMed]
- Reeves, J.A.; Mohebbi, M.; Zivadinov, R.; Bergsland, N.; Dwyer, M.G.; Salman, F.; Schweser, F.; Jakimovski, D. Reliability of paramagnetic rim lesion classification on quantitative susceptibility mapping (QSM) in people with multiple sclerosis: Single-site experience and systematic review. Mult. Scler. Relat. Disord. 2023, 79, 104968. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.A.; Manning, A.R.; Chen, L.; Liu, F.; Cao, Q.; Bar-Or, A.; Shinohara, R.T.; Sweeney, E.; Schindler, M.K. Early Magnetic Resonance Imaging Features of New Paramagnetic Rim Lesions in Multiple Sclerosis. Ann. Neurol. 2023, 94, 736–744. [Google Scholar] [CrossRef]
- Shi, Z.; Pan, Y.; Yan, Z.; Ding, S.; Hu, H.; Wei, Y.; Luo, D.; Xu, Y.; Zhu, Q.; Li, Y. Microstructural alterations in different types of lesions and their perilesional white matter in relapsing-remitting multiple sclerosis based on diffusion kurtosis imaging. Mult. Scler. Relat. Disord. 2023, 71, 104572. [Google Scholar] [CrossRef]


| Compound/Structure | Magnetic Susceptibility (ppm) | Reference |
|---|---|---|
| Water (at 37 °C) | −9.04 | Arrighini et al., 1968 [17] |
| Phopholipids * | −9.68 | Kawamura et al., 1981 [18] |
| Lipids | −10.0 | Schenck, 1992 [19] |
| Cortical bone | −8.7 | Schenck, 1992 [19] |
| Choroid plexus | −0.14 | Oshima et al., 2020 [20] |
| Calcified lesions | −0.26 | Oshima et al., 2020 [20] |
| Deoxyhemoglobin molecule | 0.2 | Schenck, 1992 [19] |
| Red blood cells (deoxygenated) | −6.52 | |
| Deoxygenated blood (Hct = 0.45) | −7.9 | |
| Ferritin molecule (4500 iron atoms) | 520 | |
| Brain Tissue in vivo (relative to frontal deep WM) ** | ||
| Caudate nucleus | 0.044 | Deistung et al., 2013 [22] |
| Putamen | 0.038 | |
| Red nucleus | 0.100 | |
| Substantia nigra | 0.111 | |
| Globus pallidus | 0.131 | |
| Gray matter | 0.020 | |
| White matter | −0.030 | |
| MS lesions (relative to CSF) * | ||
| Rim+ lesion | 0.006/0.002 | Yao et al., 2018 [24] Kaunzner et al., 2019 [25] |
| Rim− lesion | −0.007/−0.015 | |
| Susceptibility at the rim in rim+ lesions | 0.013/0.020 | |
| Susceptibility at the core of rim+ lesions | 0.006/0.005 | |
| Central Vein Sign (CVS) | Paramagnetic Rim Lesion (PRLs) | |
|---|---|---|
| Definition | Presence of a vein in the center of an MS plaque, visible in T2*/SWI image Lesion requisites for CVS confirmation:
| Paramagnetic rim around an MS lesion
|
| Pathological meaning | Captures the venocentric growth of MS lesions | Paramagnetic rim = mainly driven by iron-laden macrophages (activated microglia). Demyelination also increases the local susceptibility of the tissue and can collaborate with the paramagnetic effect |
| Main application in MS | Advanced diagnostic
| Prognostic tool
|
| Secondary applications | Prognostic tool
|
|
| Limitations |
|
|
| Future directions |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rimkus, C.d.M.; Otsuka, F.S.; Nunes, D.M.; Chaim, K.T.; Otaduy, M.C.G. Central Vein Sign and Paramagnetic Rim Lesions: Susceptibility Changes in Brain Tissues and Their Implications for the Study of Multiple Sclerosis Pathology. Diagnostics 2024, 14, 1362. https://doi.org/10.3390/diagnostics14131362
Rimkus CdM, Otsuka FS, Nunes DM, Chaim KT, Otaduy MCG. Central Vein Sign and Paramagnetic Rim Lesions: Susceptibility Changes in Brain Tissues and Their Implications for the Study of Multiple Sclerosis Pathology. Diagnostics. 2024; 14(13):1362. https://doi.org/10.3390/diagnostics14131362
Chicago/Turabian StyleRimkus, Carolina de Medeiros, Fábio Seiji Otsuka, Douglas Mendes Nunes, Khallil Taverna Chaim, and Maria Concepción Garcia Otaduy. 2024. "Central Vein Sign and Paramagnetic Rim Lesions: Susceptibility Changes in Brain Tissues and Their Implications for the Study of Multiple Sclerosis Pathology" Diagnostics 14, no. 13: 1362. https://doi.org/10.3390/diagnostics14131362
APA StyleRimkus, C. d. M., Otsuka, F. S., Nunes, D. M., Chaim, K. T., & Otaduy, M. C. G. (2024). Central Vein Sign and Paramagnetic Rim Lesions: Susceptibility Changes in Brain Tissues and Their Implications for the Study of Multiple Sclerosis Pathology. Diagnostics, 14(13), 1362. https://doi.org/10.3390/diagnostics14131362






