Is There a Relationship between Salivary Cortisol and Temporomandibular Disorder: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources and Search Strategies
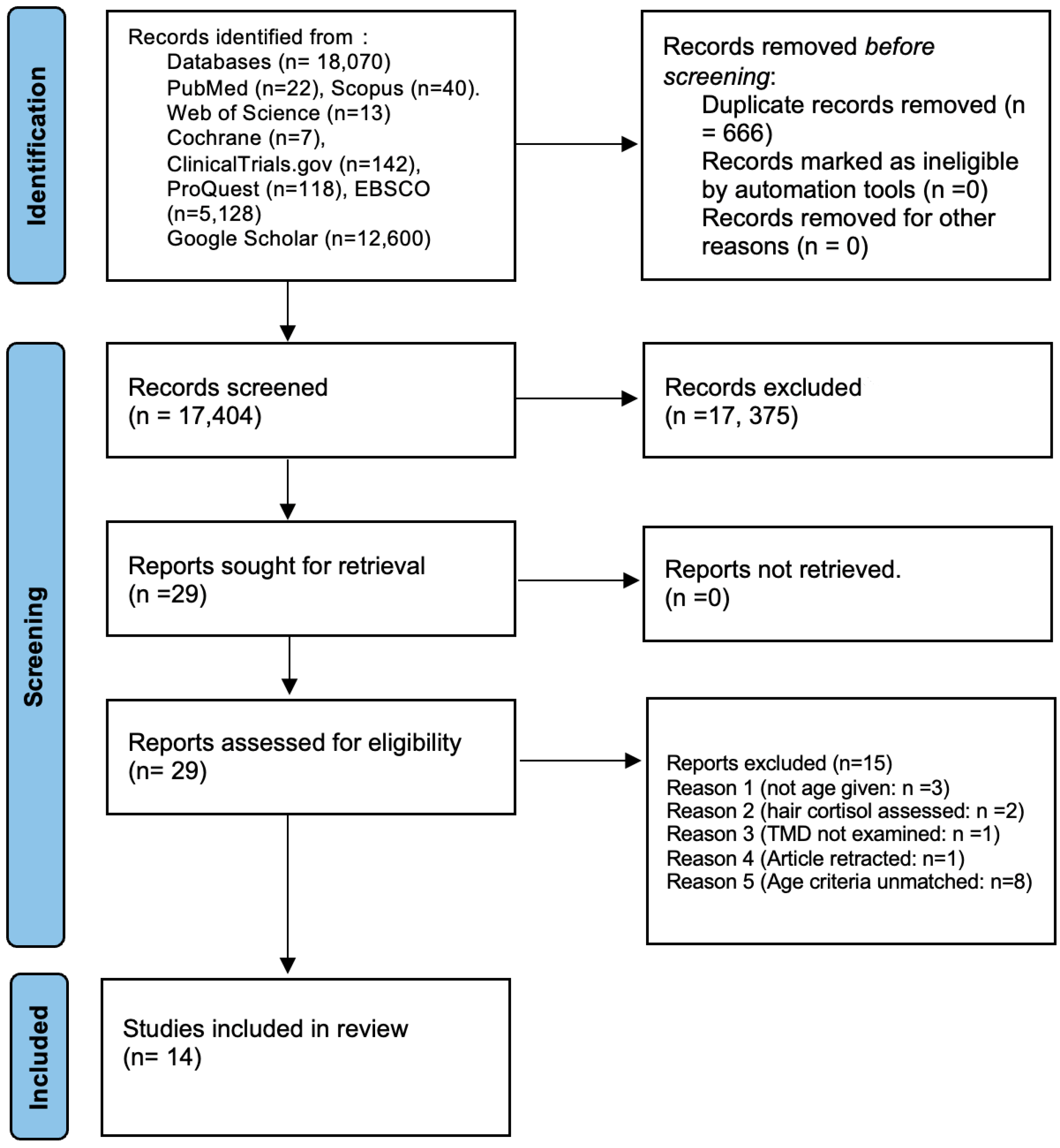
2.4. Study Selection
2.5. Data Extraction and Data Items
2.6. Data Synthesis (Meta-Analysis)
2.7. Risk of Bias in Individual Studies
3. Results
3.1. Characteristics of Included Studies (Table 2)
3.2. Salivary Parameters of the Participants of the Included Studies (Table 3)
3.3. Risk of Bias Assessment (Table 4, Table 5 and Table 6)
3.4. Certainty of Evidence (GRADE Analysis) (Table 7)
| Author, Year, Region | Study Design | Age Range/ Average (yrs) | Sample Size (Test/Control) | Study Population | Key Findings | Conclusion |
|---|---|---|---|---|---|---|
| Rosar et al., 2021, Brazil [30] | Cross-sectional | 19–30 | 43 (28/15) | TMD group Healthy group | Similar salivary cortisol levels found between groups on awakening and after 30 min | Cortisol levels were not associated with the number or duration of bruxism (TMD) episodes |
| Venkatesh et al., 2021, India [32] | Cross-sectional | 18–23 | 44 (22/22) | Test with TMD Controls without TMD | Salivary cortisol levels showed statistically significant difference between the TMD and control groups | Salivary cortisol can be used as a biological marker of stress in TMD |
| Goyal et al., 2020, India [28] | RCT | 24.05 ± 2.3 | 60 (20/20/20) | TMDs and positive depression levels TMDs and no depression Healthy control | Statistically significant higher value of salivary cortisol in TMD with depression, as compared to TMD without depression and control | Salivary cortisol could be a promising tool in identifying underlying psychological factors associated with TMDs |
| D’Avilla, 2019, Brazil [17] | Cross sectional | 25.3 ± 5.1 | 60 (45/15) | Group I: No TMD and clinically normal occlusion Group II: With TMD and malocclusion Group III: TMD and clinically normal occlusion Group IV: No TMD and with malocclusion | Salivary cortisol level was significantly higher in individuals with TMD (G2 and G3), independent of the presence/absence of malocclusion | Quality of life, pain, and emotional stress are associated with and impaired by the TMD condition, regardless of malocclusion presence |
| Bozovic et al., 2018, Bosnia and Herzegovina [33] | Case–control | 19.35 | 60 (30/30) | TMD group Healthy controls | Levels of salivary cortisol were found to be significantly higher in the study group compared to the control group | Salivary cortisol plays a vital role in TMD development |
| Chinthakanan et al., 2018, Thailand [34] | Case–control | 24 | 44 (21/23) | TMD group Control group | The salivary cortisol level of the TMD group was significantly greater than that of the control group | Patients with TMD demonstrated autonomic nervous system (ANS) imbalance and increased stress levels |
| Magri et al., 2018, Brazil [29] | RCT | 18–40 | 64 (41/23) | Laser group (TMD) Placebo group Without treatment group | Women with lower cortisol levels (below 10 ng/mL) were more responsive to active and placebo laser treatment than women with higher cortisol levels (above 10 ng/mL) | Most responsive cluster to active and placebo LLLT was women with low levels of anxiety, salivary levels below 10 ng/mL |
| Rosar et al., 2017, Brazil [27] | RCT | 19–30 | 43 (28/15) | Sleep bruxism group (Gsb) Control group (Gc) | Salivary cortisol showed a significant decrease between baseline and T1 in test, which was not observed in control | Short-term treatment with interocclusal splints had positive affect on salivary cortisol levels in subjects with sleep bruxism |
| Poorian et al., 2016, Iran [37] | Case–control | 19–40 | 41 (15/26) | TMD patients Healthy people | Salivary cortisol levels in TMD patients are significantly higher than in healthy people | Increase in salivary cortisol levels increases the probability of suffering from TMD |
| Tosato et al., 2015, Brazil [31] | Cross-sectional | 18–40 | 49 (26/25) | Women with TMD Healthy women | Moderate to strong correlations were found between salivary cortisol and EMG activities of the women with severe TMD | Increase in cortisol levels corresponded with greater muscle activity and TMD severity |
| Almeida et al., 2014, Brazil [16] | Case–control | 19–32 | 48 (25/23) | With TMD Without TMD | Results show no difference between groups | No relationship between saliva cortisol, TMD, and depression |
| Nilsson and Dahlstrom, 2010, Sweden [36] | Case–control | 18–24 | 60 (30/30) | RDC/TMD criteria I RDC/TMD criteria II Control group with no TMD | No statistically significant differences were found between any of the groups | Waking cortisol levels were not associated with symptoms of TMD and were not differentiated between the groups |
| Quartana et al., 2010, USA [38] | Case–control | 29.85 | 61 (39/22) | TMD patients Healthy controls | Pain index was not associated with cortisol levels | There was no association between markers of pain sensitivity and adrenocortical responses |
| Jones et al., 1997, Canada [35] | Case–control | 27.07 | 75 (36/39) | TMD group Control group | No significant differences found between TMD and control cortisol levels at baseline, but values were significantly higher in the TMD group at both 30 and 50 min | No relationship was found between psychological factors and hypersecretion of cortisol in TMD group |
| Study (Author, Year) | Saliva Collection | Salivary Cortisol Levels in Tests/Morning/Night | Salivary Cortisol Levels in Controls | Statistical Significance |
|---|---|---|---|---|
| Rosar et al., 2021 [30] | Stimulated saliva Collection time: immediately after waking up and 30 min after waking up | Upon waking: 0.19 ± 0.21, After 30 min: 0.24 ± 0.28 μg/dL | Upon waking: 0.16 ± 0.13, After 30 min: 0.16 ± 0.09 μg/dL | No p > 0.05 |
| Venkatesh et al., 2021 [32] | Stimulated saliva Collection time: 9:30 a.m. to 10:00 a.m. | 1.107 ± 0.17 | 0.696 ± 0.16 | Yes p < 0.001 |
| Goyal et al., 2020 [28] | Unstimulated saliva Collection time: twice between 7.00 and 8.00 h, and again between 20.00 and 22.00 h | Morning: TMD with depression: 52.45 ± 18.62 TMD without depression: 20.35 ± 10.59 Evening: TMD with depression: 28.13 ± 10.88 TMD without depression: 12.33 ± 6.15 | Morning: 12.85 ± 4.28 Evening: 8.51 ± 4.32 | Yes p = 0.0001 |
| D’Avilla, 2019 [17] | Stimulated whole saliva was collected | G2: 7.45 ± 4.93, G3: 7.87 ± 3.52, G4: 4.35 ± 2.59 μg/dL | 3.83 ± 2.72 μg/dL | Yes p < 0.05 |
| Bozovic et al., 2018 [33] | Stimulated saliva | 2.8 µg/dL | 0.6 µg/dL | Yes p < 0.001 |
| Chinthakanan et al., 2018 [34] | Unstimulated saliva Collection time: morning, over five minutes | 29.78 ± 2.67 ng/ml | 22.88 ± 1.38 ng/mL | Yes p < 0.05 |
| Magri et al., 2018 [29] | Unstimulated saliva Collection time: between 7 and 10 a.m. | Under 10 ng/mL: 5/7 Above 10 ng/mL: 15/14 | Under 10 ng/mL: 6 Above 10 ng/mL: 17 | Yes p < 0.05 |
| Rosar et al., 2017 [27] | Stimulated saliva Collection time: morning | Baseline: 5.9, T1: 2.6, T2: 2.5 | Baseline: 4.9, T1: 4.4, T2: 4.3 | Yes p < 0.05 |
| Poorian et al., 2016 [37] | Unstimulated saliva Collection time: between 9–11 a.m. | 29.0240 ± 5.27835 ng/ml | 8.8950 ± 9.58974 ng/mL | Yes p = 0.000 |
| Tosato et al., 2015 [31] | Unstimulated saliva Collection time: between 8 and 9 a.m. | Mild: 25.39, moderate: 116.7, severe: 250.1 µg/dL | Yes p < 0.05 for moderate and severe | |
| Almeida et al., 2014 [16] | Unstimulated saliva Collection time: between 9:00 and 9:25 a.m. | 0.272 µg/dL | 0.395 µg/dL | No p = 0.121 |
| Nilsson and Dahlstrom, 2010 [36] | Stimulated saliva | 10.53 ± 5.05/12.61 ± 8.17 nmol/L | 13.68 ± 9.96 nmol/L | No p > 0.05 |
| Quartana et al., 2010 [38] | Stimulated saliva Collection time: immediately prior to the start of pain testing, immediately following the pain testing procedures, and 20 min after the pain testing procedures | High PCS: BL: 0.8 Post-pain: 0.85 20 min after pain: 0.9 µg/dL | Low PCS: BL: 0.92 Post-pain: 0.75 20 min after pain: 0.7 µg/ml | No p > 0.05 |
| Jones et al., 1997 [35] | Unstimulated saliva Collection time: baseline (time, 0 min), peak secretion (time, 30 min), and after 20 min of rest (time, 50 min) | 0 min: 6.41, 30 min: 11.96, 50 min: 10.28 | 0 min: 5.89, 30 min: 7.63, 50 min: 6.39 | Yes p ˂ 0.01 |
| Authors/Year | Randomization Process | Deviation from Intended Intervention | Missing Outcome Data | Measurement of the Outcome | Selection of the Reported Results | Overall Bias |
|---|---|---|---|---|---|---|
| Goyal, 2020 [28] | Low | Low | Low | Some concern | Low | Low |
| Magri, 2017 [29] | Low | Low | Low | Low | Low | Low |
| Rosar, 2017 [27] | High | High | Low | High | High | High |
| Author, Year | Selection | Comparability | Exposure | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Is the Case Definition Adequate? | Representativeness of the Cases | Selection of Controls | Definition of Controls | Comparability of Cases and Controls Based on the Design or Analysis | Ascertainment of Exposure | Same Method of Ascertainment for Cases and Controls | Non-Response Rate | Risk of Bias | |
| Almeida et al., 2014 [16] | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | Medium (6) |
| Bozovic et al., 2018 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Low (8) |
| Chinthakanan et al., 2018 [34] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | Medium (6) |
| Jones et al., 1997 [35] | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | Medium (6) |
| Nilsson and Dahlstrom, 2010 [36] | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | Medium (5) |
| Poorian et al., 2016 [37] | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | High (3) |
| Quartana et al., 2010 [38] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Low (8) |
| Representativeness of the Sample | Sample Size | Non-Respondents | Ascertainment of the Exposure (Risk Factor) | The Subjects in Different Outcome Groups are Comparable, Based on the Study Design or Analysis; Confounding Factors are Controlled | Assessment of the Outcome | Statistical Test | Risk of Bias | |
|---|---|---|---|---|---|---|---|---|
| D’Avilla, 2019 [17] | 1 | 1 | 1 | 2 | 1 | 1 | 1 | Low (8) |
| Rosar et al., 2021 [30] | 1 | 1 | 1 | 2 | 1 | 1 | 1 | Low (8) |
| Tosato et al., 2015 [31] | 1 | 1 | 1 | 1 | 1 | 2 | 1 | Low (8) |
| Venkatesh et al., 2021 [32] | 1 | 1 | 0 | 1 | 0 | 1 | 1 | Medium (5) |
| No. of Studies | Certainty assessment | Effect | Certainty | Importance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | No. of Events | No. of Individuals | Rate (95% CI) | |||
| 3 | Randomized trials | not serious | serious a | serious b | very serious c | Strong association; all plausible residual confounding would reduce the demonstrated effect | We cannot provide examples extracted from our review since our review was not intentionally limited to a specific prognostic factor. Instead, our goal has been to explore salivary cortisol levels at different times of day that have been investigated to date as potential risks for the persistence of a variety of chronic pain conditions and their associated TMDs. However, this poor representation would happen, for instance, if we were interested in exploring the effects of various levels of salivary cortisol on types of TMD. The studies included were only investigating the prognostic effect of salivary cortisol on TMD at a specific age. | ⨁⨁◯◯ Low | IMPORTANT | ||
| 4 | Observational studies (cross-sectional) | serious d | serious e | not serious | serious f | All plausible residual confounding would suggest spurious effect, while no effect was observed | 151 | 196 | ⨁⨁◯◯ Low | IMPORTANT | |
| 7 | Observational studies (case–control) | serious g | not serious | serious h | serious i | Publication bias strongly suspected; strong association; all plausible residual confounding would suggest spurious effect, while no effect was observed | When conducting comprehensive systematic reviews of the effects of cortisol levels on TMD incidence among young adults, authors reported that the evidence of increasing salivary cortisol as a prognostic factor for chronic TMD pain has serious limitations. This evidence comes from four studies, and all of them have a moderate risk of bias. | ⨁⨁◯◯ Low | IMPORTANT | ||
4. Discussion
4.1. Association of Cortisol and TMD
4.2. Evidence from Randomized Controlled Trials
4.3. Evidence from Case–Control Studies
4.4. Evidence from Cross-Sectional Studies
4.5. Evidence from Systematic Reviews
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murphy, M.K.; MacBarb, R.F.; Wong, M.E. Temporomandibular Joint Disorders: A Review of Etiology, Clinical Management, and Tissue Engineering Strategies. Int. J. Oral Maxillofac. Implants 2013, 28, e393. [Google Scholar] [CrossRef] [PubMed]
- Giannakopoulos, N.N.; Keller, L.; Rammelsberg, P.; Kronmüller, K.-T.; Schmitter, M. Anxiety and depression in patients with chronic temporomandibular pain and in controls. J. Dent. 2010, 38, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, E.; Detamore, M.S.; Mercuri, L.G. Degenerative disorders of the temporomandibular joint: Etiology, diagnosis, and treatment. J. Dent. Res. 2008, 87, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Steinkeler, A. Epidemiology, diagnosis, and treatment of temporomandibular disorders. Dent. Clin. N. Am. 2013, 57, 465–479. [Google Scholar] [CrossRef] [PubMed]
- McNeill, C. Management of temporomandibular disorders: Concepts and controversies. J. Prosthet. Dent. 1997, 77, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Valesan, L.F.; Da-Cas, C.D.; Reus, J.C.; Denardin, A.C.S.; Garanhani, R.R.; Bonotto, D.; Januzzi, E.; de Souza, B.D.M. Prevalence of temporomandibular joint disorders: A systematic review and meta-analysis. Clin. Oral Investig. 2021, 25, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Chisnoiu, A.M.; Picos, A.M.; Popa, S.; Chisnoiu, P.D.; Lascu, L.; Picos, A.; Chisnoiu, R. Factors involved in the etiology of temporomandibular disorders—A literature review. Med. Pharm. Rep. 2015, 88, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Atsu, S.S.; Guner, S.; Palulu, N.; Bulut, A.C.; Kurkcuoglu, I. Oral parafunctions, personality traits, anxiety and their association with signs and symptoms of temporomandibular disorders in the adolescents. Afr. Health Sci. 2019, 19, 1801–1810. [Google Scholar] [CrossRef] [PubMed]
- Ohrbach, R.; Michelotti, A. The Role of Stress in the Etiology of Oral Parafunction and Myofascial Pain. Oral Maxillofac. Surg. Clin. 2018, 30, 369–379. [Google Scholar] [CrossRef]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [CrossRef]
- De Leeuw, R.; Bertoli, E.; Schmidt, J.E.; Carlson, C.R. Prevalence of traumatic stressors in patients with temporomandibular disorders. J. Oral Maxillofac. Surg. 2005, 63, 42–50. [Google Scholar] [CrossRef]
- Gameiro, G.H.; da Silva Andrade, A.; Nouer, D.F.; Ferraz de Arruda Veiga, M.C. How may stressful experiences contribute to the development of temporomandibular disorders? Clin. Oral Investig. 2006, 10, 261–268. [Google Scholar] [CrossRef]
- Cui, Q.; Liu, D.; Xiang, B.; Sun, Q.; Fan, L.; He, M.; Wang, Y.; Zhu, X.; Ye, H. Morning Serum Cortisol as a Predictor for the HPA Axis Recovery in Cushing’s Disease. Int. J. Endocrinol. 2021, 2021, 4586229. [Google Scholar] [CrossRef]
- El-Farhan, N.; Rees, D.A.; Evans, C. Measuring cortisol in serum, urine and saliva—Are our assays good enough? Ann. Clin. Biochem. 2017, 54, 308–322. [Google Scholar] [CrossRef]
- Kirschbaum, C.; Hellhammer, D.H. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology 1994, 19, 313–333. [Google Scholar] [CrossRef]
- Almeida, C.D.; Paludo, A.; Stechman-Eto, J.; Amenábar, J.M. Saliva cortisol levels and depression in individuals with temporomandibular disorder: Preliminary study. Rev. Dor 2014, 15, 169–172. [Google Scholar] [CrossRef]
- D’Avilla, B.M.; Pimenta, M.C.; Furletti, V.F.; Vedovello Filho, M.; Venezian, G.C.; Custodio, W. Comorbidity of TMD and malocclusion: Impacts on quality of life, masticatory capacity and emotional features. Braz. J. Oral Sci. 2019, 18, e191679. [Google Scholar] [CrossRef]
- Kobayashi, F.Y.; Gavião, M.B.D.; Marquezin, M.C.S.; Fonseca, F.L.A.; Montes, A.B.M.; Barbosa, T.S.; Castelo, P.M. Salivary stress biomarkers and anxiety symptoms in children with and without temporomandibular disorders. Braz. Oral Res. 2017, 31, e78. [Google Scholar] [CrossRef]
- Suprajith, T.; Wali, A.; Jain, A.; Patil, K.; Mahale, P.; Niranjan, V. Effect of Temporomandibular Disorders on Cortisol Concentration in the Body and Treatment with Occlusal Equilibrium. J. Pharm. Bioallied Sci. 2022, 14, S483–S485. [Google Scholar] [CrossRef]
- Fritzen, V.M.; Colonetti, T.; Cruz, M.V.; Ferraz, S.D.; Ceretta, L.; Tuon, L.; Da Rosa, M.I.; Ceretta, R.A. Levels of Salivary Cortisol in Adults and Children with Bruxism Diagnosis: A Systematic Review and Meta-Analysis. J. Evid.-Based Dent. Pract. 2022, 22, 101634. [Google Scholar] [CrossRef]
- Lu, L.; Yang, B.; Li, M.; Bao, B. Salivary cortisol levels and temporomandibular disorders—A systematic review and meta-analysis of 13 case-control studies. Trop. J. Pharm. Res. 2022, 21, 1341–1349. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Huguet, A.; Hayden, J.A.; Stinson, J.; McGrath, P.J.; Chambers, C.T.; Tougas, M.E.; Wozney, L. Judging the quality of evidence in reviews of prognostic factor research: Adapting the GRADE framework. Syst. Rev. 2013, 2, 71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Dubey, V.P.; Kievisiene, J.; Rauckiene-Michealsson, A.; Norkiene, S.; Razbadauskas, A.; Agostinis-Sobrinho, C. Bullying and Health Related Quality of Life among Adolescents-A Systematic Review. Children 2022, 9, 766. [Google Scholar] [CrossRef]
- Rosar, J.V.; Barbosa, T.S.; Dias, I.O.V.; Kobayashi, F.Y.; Costa, Y.M.; Gaviao, M.B.D.; Bonjardim, L.R.; Castelo, P.M. Effect of interocclusal appliance on bite force, sleep quality, salivary cortisol levels and signs and symptoms of temporomandibular dysfunction in adults with sleep bruxism. Arch. Oral Biol. 2017, 82, 62–70. [Google Scholar] [CrossRef]
- Goyal, G.; Gupta, D.; Pallagatti, S. Salivary Cortisol Could Be a Promising Tool in the Diagnosis of Temporomandibular Disorders Associated with Psychological Factors. J. Indian Acad. Oral Med. Radiol. 2021, 32, 354–359. [Google Scholar] [CrossRef]
- Magri, L.V.; Carvalho, V.A.; Rodrigues, F.C.C.; Bataglion, C.; Leite-Panissi, C.R.A. Non-specific effects and clusters of women with painful TMD responders and non-responders to LLLT: Double-blind randomized clinical trial. Lasers Med. Sci. 2018, 33, 385–392. [Google Scholar] [CrossRef]
- Rosar, J.V.; Marquezin, M.C.S.; Pizzolato, A.S.; Kobayashi, F.Y.; Bussadori, S.K.; Pereira, L.J.; Castelo, P.M. Identifying predictive factors for sleep bruxism severity using clinical and polysomnographic parameters: A principal component analysis. J. Clin. Sleep Med. 2021, 17, 949–956. [Google Scholar] [CrossRef]
- de Paiva Tosato, J.; Caria, P.H.; de Paula Gomes, C.A.; Berzin, F.; Politti, F.; de Oliveira Gonzalez, T.; Biasotto-Gonzalez, D.A. Correlation of stress and muscle activity of patients with different degrees of temporomandibular disorder. J. Phys. Ther. Sci. 2015, 27, 1227–1231. [Google Scholar] [CrossRef]
- Venkatesh, S.B.; Shetty, S.S.; Kamath, V. Prevalence of temporomandibular disorders and its correlation with stress and salivary cortisol levels among students. Pesqui. Bras. Odontopediatria Clín. Integr. 2021, 21, e0120. [Google Scholar] [CrossRef]
- Božović, Đ.; Ivković, N.; Račić, M.; Ristić, S. Salivary cortisol responses to acute stress in students with myofascial pain. Srpski Arhiv za Celokupno Lekarstvo 2018, 146, 20–25. [Google Scholar] [CrossRef]
- Chinthakanan, S.; Laosuwan, K.; Boonyawong, P.; Kumfu, S.; Chattipakorn, N.; Chattipakorn, S.C. Reduced heart rate variability and increased saliva cortisol in patients with TMD. Arch. Oral Biol. 2018, 90, 125–129. [Google Scholar] [CrossRef]
- Jones, D.A.; Rollman, G.B.; Brooke, R.I. The cortisol response to psychological stress in temporomandibular dysfunction. Pain 1997, 72, 171–182. [Google Scholar] [CrossRef]
- Nilsson, A.M.; Dahlstrom, L. Perceived symptoms of psychological distress and salivary cortisol levels in young women with muscular or disk-related temporomandibular disorders. Acta Odontol. Scand. 2010, 68, 284–288. [Google Scholar] [CrossRef]
- Poorian, B.; Dehghani, N.; Bemanali, M. Comparison of Salivary Cortisol Level in Temporomandibular Disorders and Healthy People. Int. J. Rev. Life Sci. 2015, 5, 1105–1113. [Google Scholar]
- Quartana, P.J.; Buenaver, L.F.; Edwards, R.R.; Klick, B.; Haythornthwaite, J.A.; Smith, M.T. Pain catastrophizing and salivary cortisol responses to laboratory pain testing in temporomandibular disorder and healthy participants. J. Pain 2010, 11, 186–194. [Google Scholar] [CrossRef]
- Anna, S.; Joanna, K.; Teresa, S.; Maria, G.; Aneta, W. The influence of emotional state on the masticatory muscles function in the group of young healthy adults. Biomed. Res. Int. 2015, 2015, 174013. [Google Scholar] [CrossRef]
- Apkarian, A.V.; Baliki, M.N.; Geha, P.Y. Towards a theory of chronic pain. Prog. Neurobiol. 2009, 87, 81–97. [Google Scholar] [CrossRef]
- Hannibal, K.E.; Bishop, M.D. Chronic stress, cortisol dysfunction, and pain: A psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys. Ther. 2014, 94, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Scrivani, S.J.; Keith, D.A.; Kaban, L.B. Temporomandibular disorders. N. Engl. J. Med. 2008, 359, 2693–2705. [Google Scholar] [CrossRef] [PubMed]
- Jasim, H.; Louca, S.; Christidis, N.; Ernberg, M. Salivary cortisol and psychological factors in women with chronic and acute oro-facial pain. J. Oral Rehabil. 2014, 41, 122–132. [Google Scholar] [CrossRef]
- Nadendla, L.K.; Meduri, V.; Paramkusam, G.; Pachava, K.R. Evaluation of salivary cortisol and anxiety levels in myofascial pain dysfunction syndrome. Korean J. Pain 2014, 27, 30–34. [Google Scholar] [CrossRef]
| PubMed | (hydrocortisone) “[MeSH Terms] OR “hydrocortisone”[All Fields] OR “cortisol”[All Fields]) AND (“Temporomandibular disorder”[MeSH Terms] OR “TMD”[All Fields]) OR (“temporomandibular disfunction”[MeSH Terms] OR (“Facial muscle pain”[All Fields] AND young Adults [All Fields]. |
| Scopus | (TITLE-ABS-KEY (“craniomandibular disorder*” OR “temporomandibular joint disorder*” OR “temporomandibular disorder*” OR tmjd OR tmd OR “tmj disorder*” OR ((facial OR jaw OR orofacial OR craniofacial OR trigem*) AND pain))) AND (TITLE-ABS-KEY (pcs OR “Salivary cortisol” OR Hydrocortisone* OR cortisol AND (Young adults)))) |
| Web of science | cortisol* OR hydrocortisone* AND Temporomandibular disorder* OR TMD* AND Young adults. |
| Google scholar | (cortisol OR Salivary cortisol AND Temporomandibular disorder OR TMD AND Young Adults). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlSahman, L.; AlBagieh, H.; AlSahman, R. Is There a Relationship between Salivary Cortisol and Temporomandibular Disorder: A Systematic Review. Diagnostics 2024, 14, 1435. https://doi.org/10.3390/diagnostics14131435
AlSahman L, AlBagieh H, AlSahman R. Is There a Relationship between Salivary Cortisol and Temporomandibular Disorder: A Systematic Review. Diagnostics. 2024; 14(13):1435. https://doi.org/10.3390/diagnostics14131435
Chicago/Turabian StyleAlSahman, Lujain, Hamad AlBagieh, and Roba AlSahman. 2024. "Is There a Relationship between Salivary Cortisol and Temporomandibular Disorder: A Systematic Review" Diagnostics 14, no. 13: 1435. https://doi.org/10.3390/diagnostics14131435
APA StyleAlSahman, L., AlBagieh, H., & AlSahman, R. (2024). Is There a Relationship between Salivary Cortisol and Temporomandibular Disorder: A Systematic Review. Diagnostics, 14(13), 1435. https://doi.org/10.3390/diagnostics14131435





