Lymphocytic Choriomeningitis Virus Infections in Hungary between 2017–2023—Investigation of the First Congenital Infections
Abstract
:1. Introduction
2. Materials and Methods
- -
- Microcephaly, macrocephaly, or hydrocephalus;
- -
- Periventricular calcifications;
- -
- Chorioretinitis;
- -
- Other central nervous system disorders.
- -
- Detection of LCMV-specific IgG antibodies in high titers in the serum sample, in addition to the presence of IgM antibodies.
- -
- Seroconversion or a four-fold increase in the LCMV-specific antibody titers in paired sera.
- -
- Detection of LCMV-specific IgM antibodies in CSF samples.
- -
- Detection of LCMV-specific IgM or IgA antibodies in the infant’s sample.
- -
- Detection of at least four-fold higher anti-LCMV IgG titers in the infant’s serum sample, compared to the IgG titer of the maternal serum.
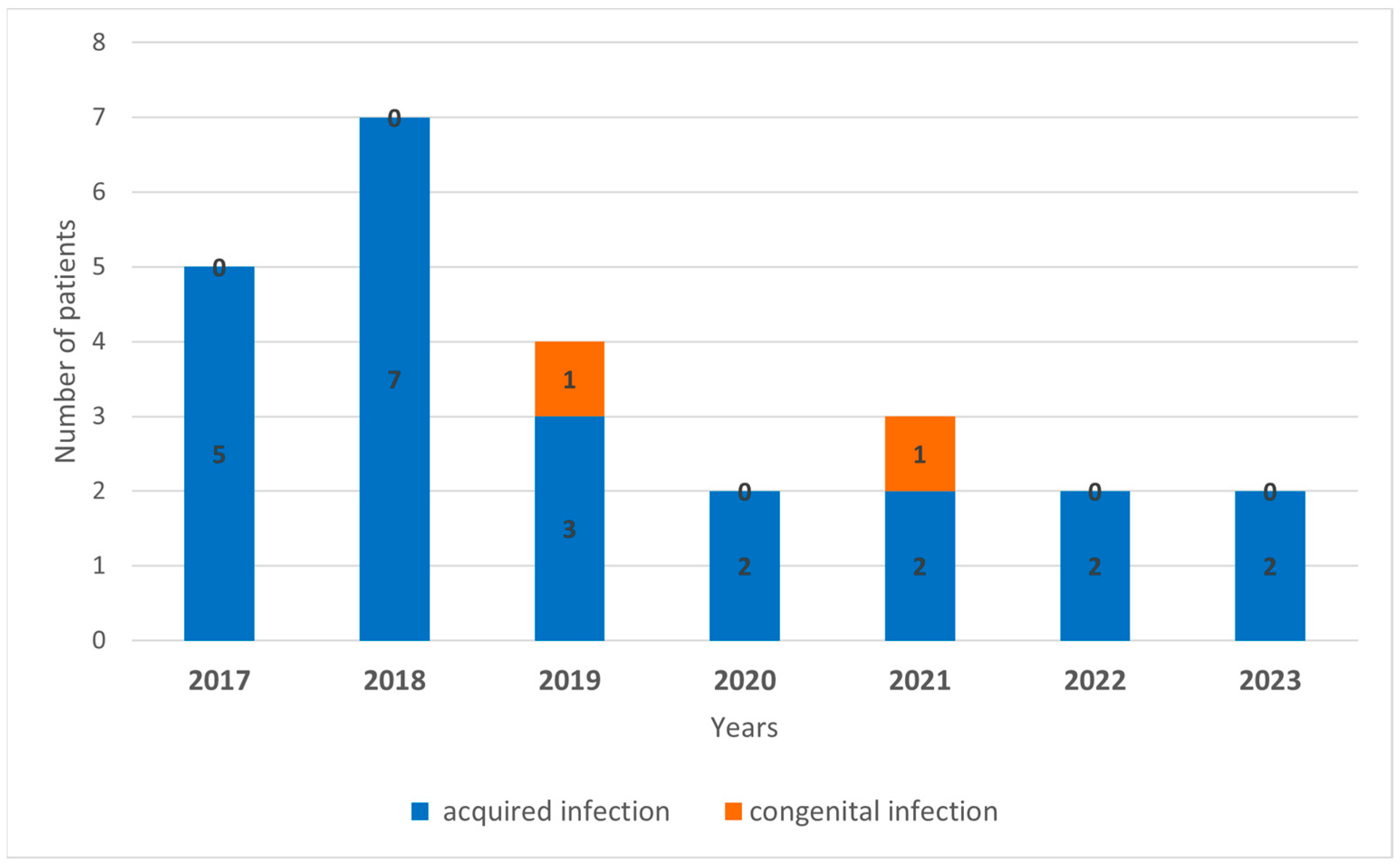
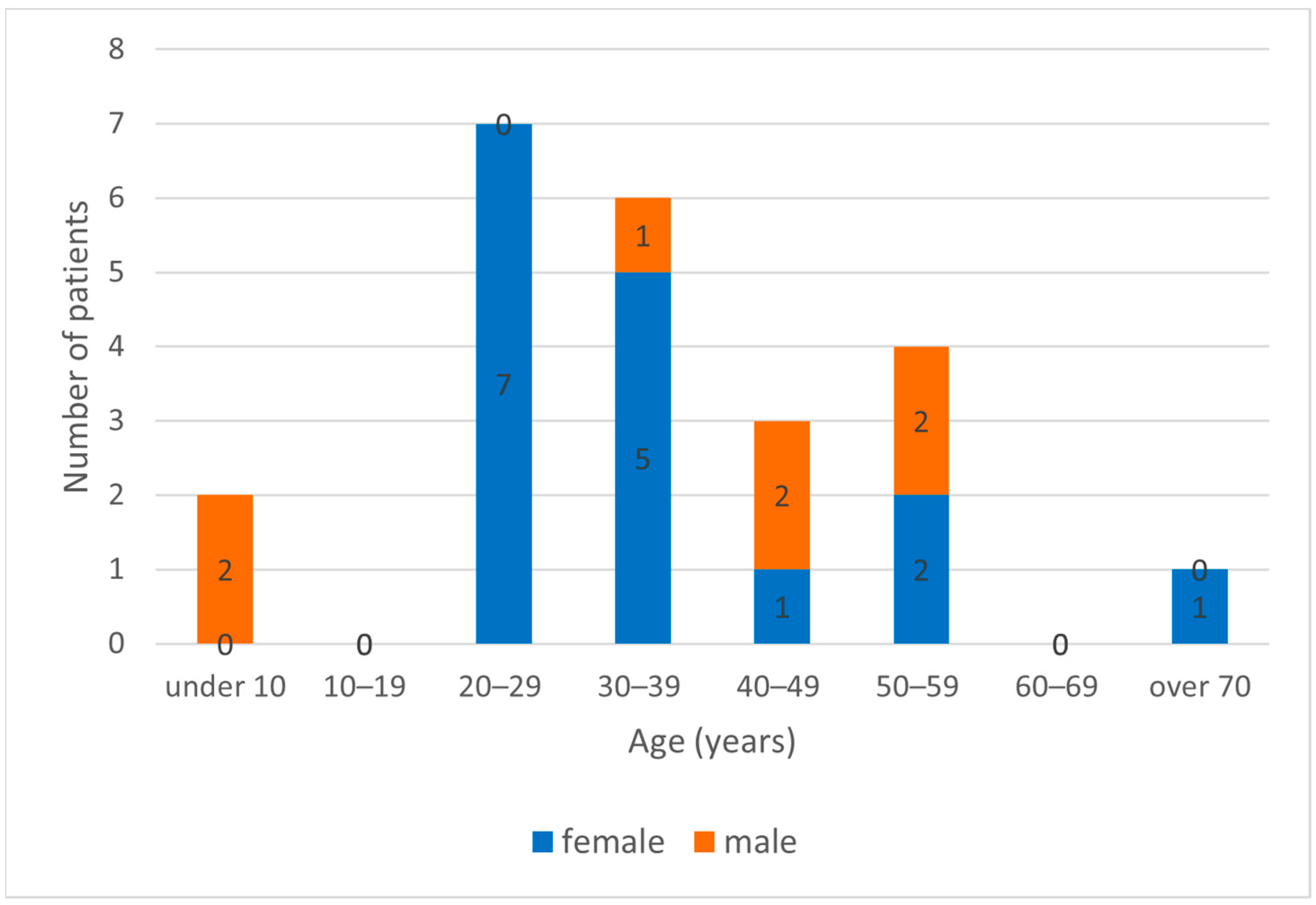
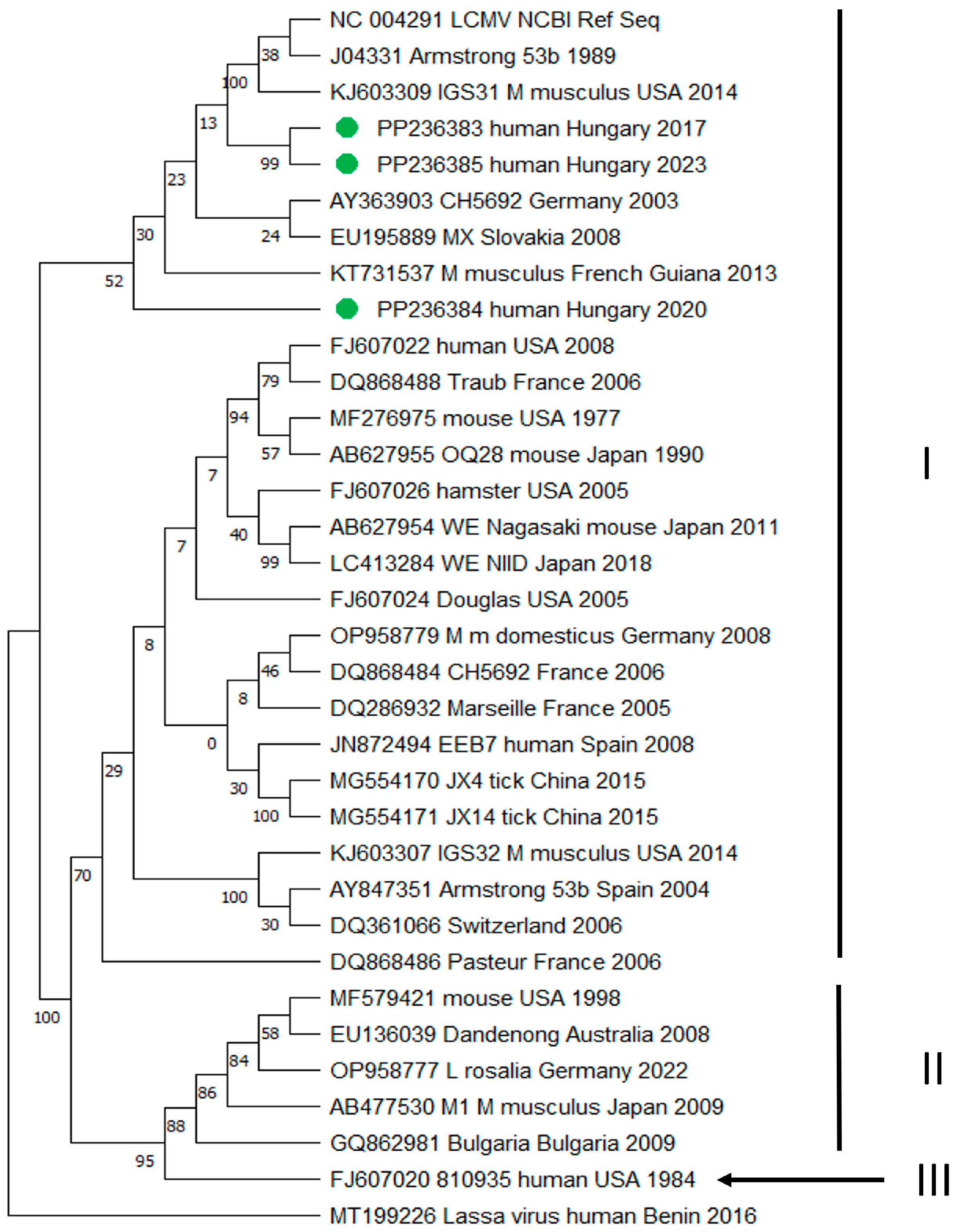
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lapošová, K.; Pastoreková, S.; Tomášková, J. Lymphocytic choriomeningitis virus: Invisible but not innocent. Acta Virol. 2013, 57, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Albariño, C.; Palacios, G.; Khristova, M.L.; Erickson, B.R.; Carroll, S.A.; Comer, J.A.; Hui, J.; Briese, T.; St George, K.; Ksiazek, T.G.; et al. High diversity and ancient common ancestry of lymphocytic choriomeningitis virus. Emerg. Infect. Dis. 2010, 16, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Vilibic-Cavlek, T.; Savic, V.; Ferenc, T.; Mrzljak, A.; Barbic, L.; Bogdanic, M.; Stevanovic, V.; Tabain, I.; Ferencak, I.; Zidovec-Lepej, S. Lymphocytic Choriomeningitis-Emerging Trends of a Neglected Virus: A Narrative Review. Trop. Med. Infect. Dis. 2021, 6, 88. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, C.; Lillie, R.D. Experimental lymphocytic choriomeningitis of monkeys and mice produced by a virus encountered in studies of the 1933 St. Louis encephalitis epidemic. Public Health Rep. 1934, 49, 1019–1027. [Google Scholar] [CrossRef]
- Barton, L.; Mets, M.B. Congenital lymphocytic choriomeningitis virus infection: Decade of rediscovery. Clin. Infect. Dis. 2001, 33, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, D.; Kourtis, A.P.; Bell, M.; Rasmussen, S.A. Lymphocytic choriomeningitis virus: An emerging obstetric pathogen? Am. J. Obstet. Gynecol. 2006, 194, 1532–1536. [Google Scholar] [CrossRef]
- Ackermann, R.; Körver, G.; Turss, R.; Wönne, R.; Hochgesand, P. Prenatal infection with the virus of lymphocytic choriomeningitis: Report of two cases. Dtsch. Med. Wochenschr. 1974, 99, 629–632. (author’s transl)(In German) [Google Scholar] [CrossRef] [PubMed]
- Gregg, M. Recent outbreaks of lymphocytic choriomeningitis in the United States of America. Bull. World Health Organ. 1975, 52, 549–553. [Google Scholar]
- Hotchin, J.; Sikora, E.; Kinch, W.; Hinman, A.; Woodall, J. Lymphocytic choriomeningitis in a hamster colony causes infection of hospital personnel. Science 1974, 185, 1173–1174. [Google Scholar] [CrossRef]
- Bonthius, D. Lymphocytic choriomeningitis virus: An underrecognized cause of neurologic disease in the fetus, child, and adult. Semin. Pediatr. Neurol. 2012, 19, 89–95. [Google Scholar] [CrossRef]
- Bonthius, D. Lymphocytic choriomeningitis virus: A prenatal and postnatal threat. Adv. Pediatr. 2009, 56, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Ferenc, T.; Vujica, M.; Mrzljak, A.; Vilibic-Cavlek, T. Lymphocytic choriomeningitis virus: An under-recognized congenital teratogen. World J. Clin. Cases 2022, 10, 8922–8931. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; Levy, P.T.; Leonard, K.B.; Smyser, C.; Tychsen, L.; Cole, F.S. Congenital lymphocytic choriomeningitis virus: When to consider the diagnosis. J. Child. Neurol. 2014, 29, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.; Johnson, D.; Neumann, M.; Ksiazek, T.G.; Rollin, P.; Keech, R.V.; Bonthius, D.J.; Hitchon, P.; Grose, C.F.; Bell, W.E.; et al. Congenital lymphocytic choriomeningitis virus syndrome: A disease that mimics congenital toxoplasmosis or Cytomegalovirus infection. Pediatrics 1997, 100, E9. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Brief report: Lymphocytic choriomeningitis virus transmitted through solid organ transplantation--Massachusetts, 2008. MMWR Morb. Mortal. Wkly. Rep. 2008, 57, 799–801. [Google Scholar]
- Fischer, S.; Graham, M.B.; Kuehnert, M.J.; Kotton, C.N.; Srinivasan, A.; Marty, F.M.; Comer, J.A.; Guarner, J.; Paddock, C.D.; DeMeo, D.L.; et al. Transmission of lymphocytic choriomeningitis virus by organ transplantation. N. Engl. J. Med. 2006, 354, 2235–2249. [Google Scholar] [CrossRef]
- Macneil, A.; Ströher, U.; Farnon, E.; Campbell, S.; Cannon, D.; Paddock, C.D.; Drew, C.P.; Kuehnert, M.; Knust, B.; Gruenenfelder, R.; et al. Solid organ transplant-associated lymphocytic choriomeningitis, United States, 2011. Emerg. Infect. Dis. 2012, 18, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Mrzljak, A.; Novak, R.; Pandak, N.; Tabain, I.; Franusic, L.; Barbic, L.; Bogdanic, M.; Savic, V.; Mikulic, D.; Pavicic-Saric, J.; et al. Emerging and neglected zoonoses in transplant population. World J. Transplant. 2020, 10, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Ivancsó, J.; Koroknai, A.; Csonka, N.; Fejes, M.; Fürjész, D.; Kelen, M.; Megyeri, T.; Váradi, K.; Szűcs, I. First Hungarian description of congenital lymphocytic choriomeningitis virus infection. Gyermekgyógyászat 2020, 71, 323–327. (In Hungarian) [Google Scholar]
- Balogh, Z.; Egyed, L.; Ferenczi, E.; Ban, E.; Szomor, K.N.; Takacs, M.; Berencsi, G. Experimental infection of goats with tick-borne encephalitis virus and the possibilities to prevent virus transmission by raw goat milk. Intervirology 2012, 55, 194–200. [Google Scholar] [CrossRef]
- Park, J.; Peters, C.J.; Rollin, P.E.; Ksiazek, T.G.; Gray, B.; Waites, K.B.; Stephensen, C.B. Development of a reverse transcription-polymerase chain reaction assay for diagnosis of lymphocytic choriomeningitis virus infection and its use in a prospective surveillance study. J. Med. Virol. 1997, 51, 107–114. [Google Scholar] [CrossRef]
- Vieth, S.; Drosten, C.; Lenz, O.; Vincent, M.; Omilabu, S.; Hass, M.; Becker-Ziaja, B.; ter Meulen, J.; Nichol, S.T.; Schmitz, H.; et al. RT-PCR assay for detection of Lassa virus and related Old World arenaviruses targeting the L gene. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Simon, M. Lymphocytic choriomeningitis virus infections in Hungary during 1973–77. Orvosi Hetil. Orv Hetil 1978, 119, 1535–1541. (In Hungarian) [Google Scholar]
- European Centre for Disease Control and Prevention (ECDC). Directory of EVD-LabNet. Available online: https://qap.ecdc.europa.eu/public/extensions/EVD_LabNet/EVD_LabNet.html#main-tab (accessed on 12 March 2024).
- Bonthius, D.; Wright, R.; Tseng, B.; Barton, L.; Marco, E.; Karacay, B.; Larsen, P.D. Congenital lymphocytic choriomeningitis virus infection: Spectrum of disease. Ann. Neurol. 2007, 62, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Stephensen, C.; Blount, S.R.; Lanford, R.E.; Holmes, K.V.; Montali, R.J.; Fleenor, M.E.; Shaw, J.F. Prevalence of serum antibodies against lymphocytic choriomeningitis virus in selected populations from two U.S. cities. J. Med. Virol. 1992, 38, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Childs, J.; Glass, G.E.; Ksiazek, T.G.; Rossi, C.A.; Oro, J.G.; Leduc, J.W. Human-rodent contact and infection with lymphocytic choriomeningitis and Seoul viruses in an inner-city population. Am. J. Trop. Med. Hyg. 1991, 44, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Childs, J.; Glass, G.E.; Korch, G.W.; Ksiazek, T.G.; Leduc, J.W. Lymphocytic choriomeningitis virus infection and house mouse (Mus musculus) distribution in urban Baltimore. Am. J. Trop. Med. Hyg. 1992, 47, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Marrie, T.; Saron, M.F. Seroprevalence of lymphocytic choriomeningitis virus in Nova Scotia. Am. J. Trop. Med. Hyg. 1998, 58, 47–49. [Google Scholar] [CrossRef] [PubMed]
- Riera, L.; Castillo, E.; Del Carmen Saavedra, M.; Priotto, J.; Sottosanti, J.; Polop, J.; Ambrosio, A.M. Serological study of the lymphochoriomeningitis virus (LCMV) in an inner city of Argentina. J. Med. Virol. 2005, 76, 285–289. [Google Scholar] [CrossRef]
- Lledó, L.; Gegúndez, M.I.; Saz, J.V.; Bahamontes, N.; Beltrán, M. Lymphocytic choriomeningitis virus infection in a province of Spain: Analysis of sera from the general population and wild rodents. J. Med. Virol. 2003, 70, 273–275. [Google Scholar] [CrossRef]
- Dobec, M.; Dzelalija, B.; Punda-Polic, V.; Zoric, I. High prevalence of antibodies to lymphocytic choriomeningitis virus in a murine typhus endemic region in Croatia. J. Med. Virol. 2006, 78, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Reiserová, L.; Kaluzová, M.; Kaluz, S.; Willis, A.C.; Závada, J.; Závodská, E.; Závadová, Z.; Ciampor, F.; Pastorek, J.; Pastoreková, S. Identification of MaTu-MX agent as a new strain of lymphocytic choriomeningitis virus (LCMV) and serological indication of horizontal spread of LCMV in human population. Virology 1999, 257, 73–83. [Google Scholar] [CrossRef]
- Juncker-Voss, M.; Prosl, H.; Lussy, H.; Enzenberg, U.; Auer, H.; Lassnig, H.; Müller, M.; Nowotny, N. Screening for antibodies against zoonotic agents among employees of the Zoological Garden of Vienna, Schönbrunn, Austria. Berl. Munch. Tierarztl. Wochenschr. 2004, 117, 404–409. (In German) [Google Scholar]
- Knust, B.; Ströher, U.; Edison, L.; Albariño, C.G.; Lovejoy, J.; Armeanu, E.; House, J.; Cory, D.; Horton, C.; Fowler, K.; et al. Lymphocytic choriomeningitis virus in employees and mice at multipremises feeder-rodent operation, United States, 2012. Emerg. Infect. Dis. 2014, 20, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Kallio-Kokko, H.; Laakkonen, J.; Rizzoli, A.; Tagliapietra, V.; Cattadori, I.; Perkins, S.E.; Hudson, P.J.; Cristofolini, A.; Versini, W.; Vapalahti, O.; et al. Hantavirus and arenavirus antibody prevalence in rodents and humans in Trentino, Northern Italy. Epidemiol. Infect. 2006, 134, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Fevola, C.; Kuivanen, S.; Smura, T.; Vaheri, A.; Kallio-Kokko, H.; Hauffe, H.C.; Vapalahti, O.; Jääskeläinen, A.J. Seroprevalence of lymphocytic choriomeningitis virus and Ljungan virus in Finnish patients with suspected neurological infections. J. Med. Virol. 2018, 90, 429–435. [Google Scholar] [CrossRef]
- Alburkat, H.; Jääskeläinen, A.J.; Barakat, A.M.; Hasony, H.J.; Sironen, T.; Al-Hello, H.; Smura, T.; Vapalahti, O. Lymphocytic Choriomeningitis Virus Infections and Seroprevalence, Southern Iraq. Emerg. Infect. Dis. 2020, 26, 3002–3006. [Google Scholar] [CrossRef] [PubMed]
- Vilibic-Cavlek, T.; Oreski, T.; Korva, M.; Kolaric, B.; Stevanovic, V.; Zidovec-Lepej, S.; Tabain, I.; Jelicic, P.; Miklausic-Pavic, B.; Savic, V.; et al. Prevalence and Risk Factors for Lymphocytic Choriomeningitis Virus Infection in Continental Croatian Regions. Trop. Med. Infect. Dis. 2021, 6, 67. [Google Scholar] [CrossRef] [PubMed]
- Komrower, G.; Williams, B.L.; Stones, P.B. Lymphocytic choriomeningitis in the newborn; probable transplacental infection. Lancet 1955, 268, 697–698. [Google Scholar] [CrossRef]
- Lehmann-Grube, F.; Kallay, M.; Ibscher, B.; Schwartz, R. Serologic diagnosis of human infections with lymphocytic choriomeningitis virus: Comparative evaluation of seven methods. J. Med. Virol. 1979, 4, 125–136. [Google Scholar] [CrossRef]
- Delaine, M.; Weingertner, A.S.; Nougairede, A.; Lepiller, Q.; Fafi-Kremer, S.; Favre, R.; Charrel, R. Microcephaly Caused by Lymphocytic Choriomeningitis Virus. Emerg. Infect. Dis. 2017, 23, 1548–1550. [Google Scholar] [CrossRef] [PubMed]
- Meritet, J.; Krivine, A.; Lewin, F.; Poissonnier, M.H.; Poizat, R.; Loget, P.; Rozenberg, F.; Lebon, P. A case of congenital lymphocytic choriomeningitis virus (LCMV) infection revealed by hydrops fetalis. Prenat. Diagn. 2009, 29, 626–627. [Google Scholar] [CrossRef] [PubMed]
- Kinori, M.; Schwartzstein, H.; Zeid, J.L.; Kurup, S.P.; Mets, M.B. Congenital lymphocytic choriomeningitis virus-an underdiagnosed fetal teratogen. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2018, 22, 79–81.e1. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, A.; Cerveró, L.; Bataller, A.; Tortajada, J.L.; Huguet, J.; Crow, Y.J.; Ali, M.; Higuet, L.J.; Martínez-Frías, M.L. Genetic syndromes mimic congenital infections. J. Pediatr. 2005, 146, 701–705. [Google Scholar] [CrossRef]
- Tevaearai, F.; Moser, L.; Pomar, L. Prenatal Diagnosis of Congenital Lymphocytic Choriomeningitis Virus Infection: A Case Report. Viruses 2022, 14, 2586. [Google Scholar] [CrossRef]

| Primer Designation | Sequences 5′-3′ |
|---|---|
| S segment nested RT-PCR | |
| NP16 | CGCACAGTGGATCCTAGGC |
| NP17-1 | GCTGACYTCAGARAAGTCCAACC |
| NP21-1 | CCTAGGCATTTGATTGCGC |
| modified NP20-1 | ARAAGRYTRGTTGCRTCYTT * |
| L segment semi-nested RT-PCR | |
| LCML3160-plus | GCAAAGCTTGAATTTCAARTTTGA |
| LCML3722-minus | CTCGACAAGTTTATGCATRTGCCA |
| LVL3754-minus | CACATCATTGGTCCCCATTTACTRTGATC |
| Patients No. | Sample ID/Year | Specimen Type | Gender | Age (Year) | Symptoms | IgG (Titer) | IgM/ IgA | Amplified Partial Gene (Segment) | Genbank Accession Nr. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2915/2017 | serum | Female | 32 | Meningitis (fever, headache, vomiting, nuchal rigidity) | ≥1:640 | +/+ | nucleocapsid (S) | SD |
| 2 | 3824/2017 | CSF | Female | 37 | Meningitis (fever, headache, cerebral edema) | positive | +/+ | RNA polymerase (L) | PP236383 |
| 3 | 4681/2017 | serum | Male | 52 | Meningoencephalitis, paraparesis | ≥1:160 | +/+ | nucleocapsid (S) | SD |
| 4 | 75/2018 | serum | Female | 53 | Meningitis (fever, headache, elevated CSF protein levels) | ≥1:10 | +/+ | nucleocapsid (S) | SD |
| 5 | 222/2018 | serum | Female | 23 | Meningitis (fever, headache, vomiting, photophobia) | ≥1:10 | +/+ | nucleocapsid (S) | SD |
| 6 | 931/2018 | CSF | Female | 55 | Meningoencephalitis (fever, headache, confusion) | positive | +/+ | nucleocapsid (S) | SD |
| 7 | 1689/2018 | CSF | Female | 43 | Meningitis (fever, headache, nuchal rigidity) | positive | +/+ | nucleocapsid (S) | SD |
| 8 | 1539/2020 | CSF | Male | 5 | Meningitis (fever, headache, vomiting, nuchal rigidity) | positive | +/+ | RNA polymerase (L) | PP236384 |
| 9 | 3026/2022 | urine | Female | 38 | Meningitis (fever, headache, nausea, vertigo, liver function abnormality) | n.t. | n.t. | RNA polymerase (L) | SD |
| 10 | 2576/2023 | CSF | Male | 48 | Meningoencephalitis (fever, headache, confusion) | positive | +/+ | RNA polymerase (L) | PP236385 |
| Case No. | Time of Diagnosis | Patient’s Age | Symptoms | Additional Anamnestic Data | Clinical Specimens | IgG (Titer) | IgM | IgA |
|---|---|---|---|---|---|---|---|---|
| 1 | October 2019 | 6-week-old premature baby | chorioretinitis, microcephaly, intrauterine dystrophy | TORCH and Zika serology did not confirm intrauterine infection. The mother had flu-like symptoms during the first trimester of the pregnancy. There were rodents around the house. | Newborn’s serum I. | ≥1:640 | borderline # | positive |
| Newborn’s serum II. (19 days later) | ≥1:20,480 | borderline # | borderline # | |||||
| Maternal serum | 1:160 | negative | borderline # | |||||
| 2 | March 2021 | 6-month-old child | microcephaly | Intrauterine toxoplasma infection was not confirmed from child’s serum I. Intrauterine rubella, CMV, and HSV infections were not confirmed from child’s serum II. | Infant’s serum I. (1.5 months old) | 1:2560 | negative | negative |
| Infant’s serum II. (6 months old) | 1:640 | negative | negative | |||||
| Maternal serum I. | 1:160 | negative | negative | |||||
| Maternal serum II. | 1:40 | negative | negative |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koroknai, A.; Nagy, A.; Nagy, O.; Csonka, N.; Mezei, E.; Szomor, K.; Takács, M. Lymphocytic Choriomeningitis Virus Infections in Hungary between 2017–2023—Investigation of the First Congenital Infections. Diagnostics 2024, 14, 1436. https://doi.org/10.3390/diagnostics14131436
Koroknai A, Nagy A, Nagy O, Csonka N, Mezei E, Szomor K, Takács M. Lymphocytic Choriomeningitis Virus Infections in Hungary between 2017–2023—Investigation of the First Congenital Infections. Diagnostics. 2024; 14(13):1436. https://doi.org/10.3390/diagnostics14131436
Chicago/Turabian StyleKoroknai, Anita, Anna Nagy, Orsolya Nagy, Nikolett Csonka, Eszter Mezei, Katalin Szomor, and Mária Takács. 2024. "Lymphocytic Choriomeningitis Virus Infections in Hungary between 2017–2023—Investigation of the First Congenital Infections" Diagnostics 14, no. 13: 1436. https://doi.org/10.3390/diagnostics14131436





