Identification of Pathogenic Pathways for Recurrence of Focal Segmental Glomerulosclerosis after Kidney Transplantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection and Exome Sequencing
2.3. Expression Analysis
2.4. Immunoblotting
2.5. Protein Array Screening
2.6. Pathway and Gene Enrichment Analysis
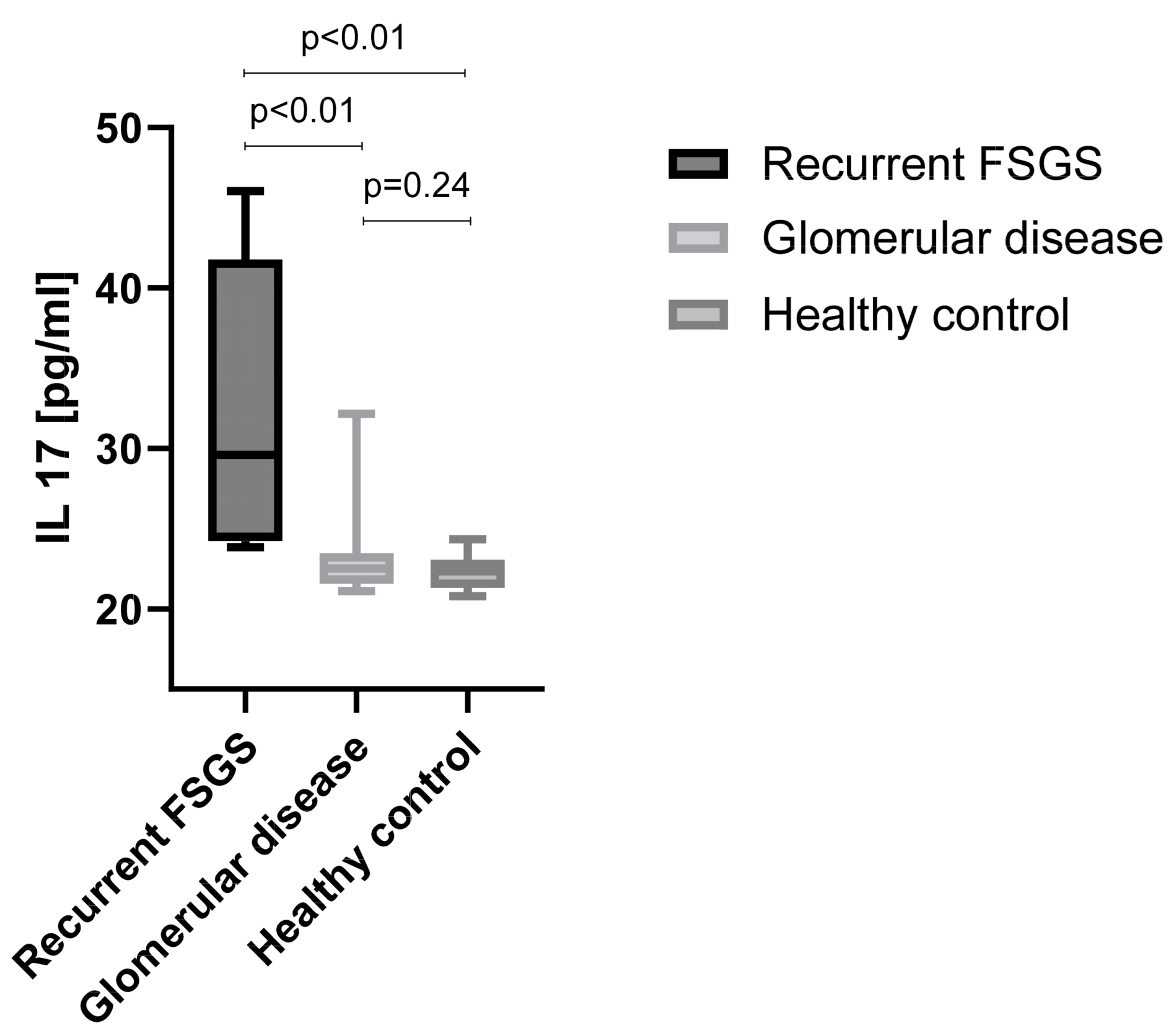
2.7. IL-17 Analysis by ELISA
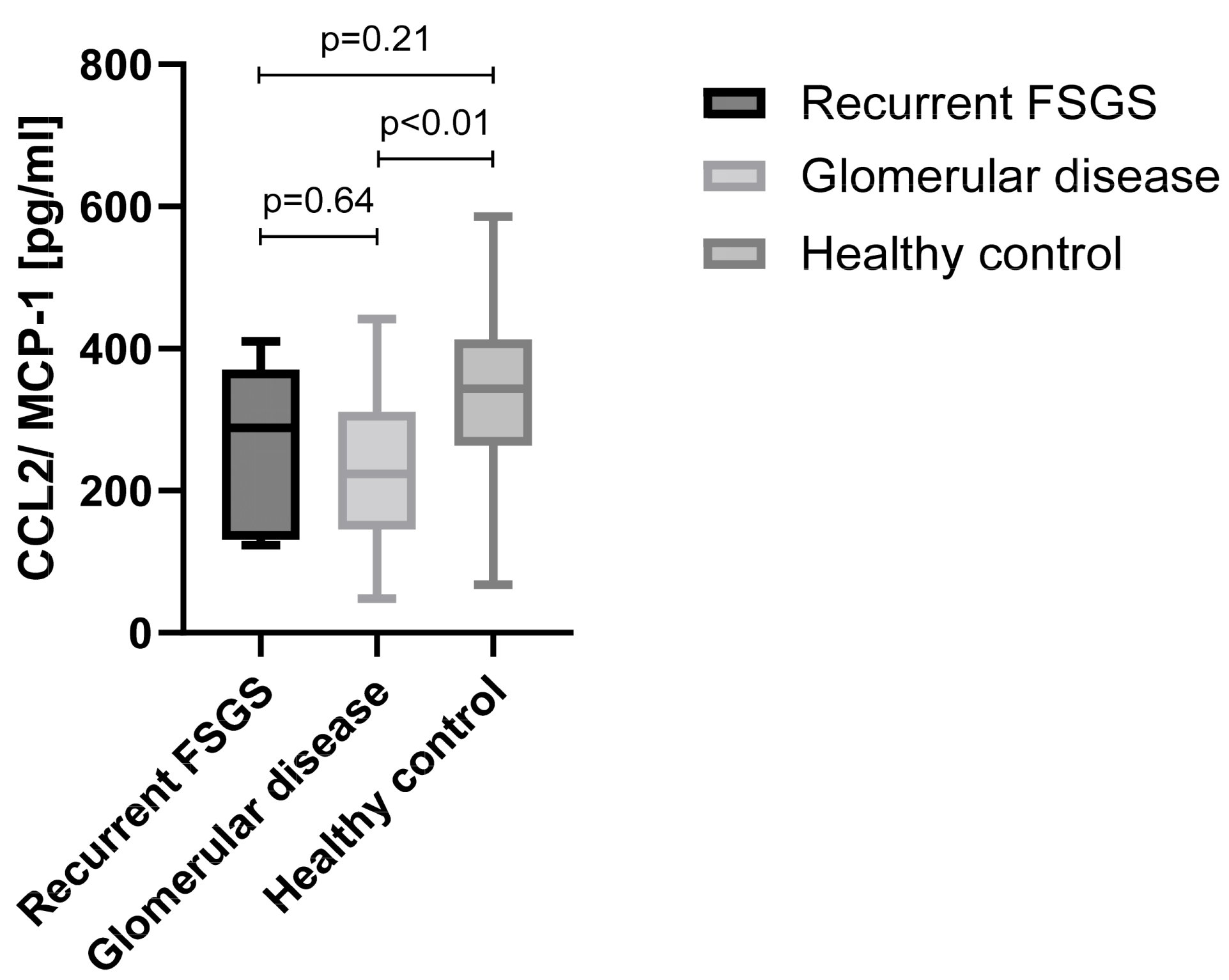
2.8. CCL2/MCP-1 Analysis by ELISA
2.9. Routine Laboratory Parameters and Statistical Analysis
3. Results
3.1. Patient Demographics and Clinical Course of Study Patients
3.2. Exome Sequencing
3.3. Expression Analysis
3.4. Protein Array Screening
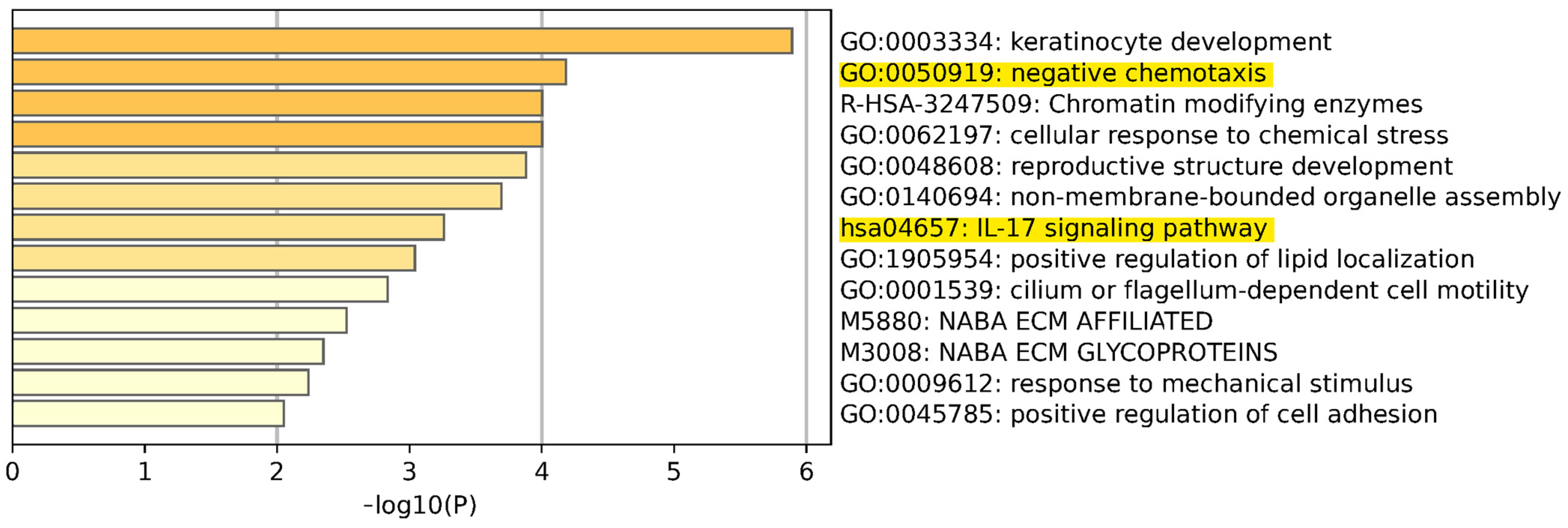
3.5. Pathway and Gene Enrichment Analysis
3.6. IL-17 Signaling Pathway
3.7. Chemotaxis Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uffing, A.; Pérez-Sáez, M.J.; Mazzali, M.; Manfro, R.C.; Bauer, A.C.; de Sottomaior Drumond, F.; O’Shaughnessy, M.M.; Cheng, X.S.; Chin, K.K.; Ventura, C.G.; et al. Recurrence of FSGS after Kidney Transplantation in Adults. Clin. J. Am. Soc. Nephrol. 2020, 15, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Kestilä, M.; Lenkkeri, U.; Männikkö, M.; Lamerdin, J.; McCready, P.; Putaala, H.; Ruotsalainen, V.; Morita, T.; Nissinen, M.; Herva, R.; et al. Positionally cloned gene for a novel glomerular protein—Nephrin—Is mutated in congenital nephrotic syndrome. Mol. Cell 1998, 1, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Holzman, L.B.; St John, P.L.; Kovari, I.A.; Verma, R.; Holthofer, H.; Abrahamson, D.R. Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int. 1999, 56, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, L.; Huang, L.; Yang, Z.; Li, G.; Wang, L. A comprehensive analysis of NPHS1 gene mutations in patients with sporadic focal segmental glomerulosclerosis. BMC Med. Genet. 2019, 20, 111. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.X.; Ahola, H.; Palmen, T.; Solin, M.L.; Luimula, P.; Holthöfer, H. Recurrence of nephrotic syndrome after transplantation in CNF is due to autoantibodies to nephrin. Exp. Nephrol. 2001, 9, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Reindl-Schwaighofer, R.; Heinzel, A.; Gualdoni, G.A.; Mesnard, L.; Claas, F.H.J.; Oberbauer, R. Novel insights into non-HLA alloimmunity in kidney transplantation. Transpl. Int. 2020, 33, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Gallon, L.; Leventhal, J.; Skaro, A.; Kanwar, Y.; Alvarado, A. Resolution of Recurrent Focal Segmental Glomerulosclerosis after Retransplantation. N. Engl. J. Med. 2012, 366, 1648–1649. [Google Scholar] [CrossRef] [PubMed]
- Kienzl-Wagner, K.; Waldegger, S.; Schneeberger, S. Disease Recurrence-The Sword of Damocles in Kidney Transplantation for Primary Focal Segmental Glomerulosclerosis. Front. Immunol. 2019, 10, 1669. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; El Hindi, S.; Li, J.; Fornoni, A.; Goes, N.; Sageshima, J.; Maiguel, D.; Karumanchi, S.A.; Yap, H.-K.; Saleem, M.; et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat. Med. 2011, 17, 952–960. [Google Scholar] [CrossRef]
- Winnicki, W.; Sunder-Plassmann, G.; Sengölge, G.; Handisurya, A.; Herkner, H.; Kornauth, C.; Bielesz, B.; Wagner, L.; Kikić, Ž.; Pajenda, S.; et al. Diagnostic and Prognostic Value of Soluble Urokinase-type Plasminogen Activator Receptor (suPAR) in Focal Segmental Glomerulosclerosis and Impact of Detection Method. Sci. Rep. 2019, 9, 13783. [Google Scholar] [CrossRef]
- Gyetko, M.R.; Todd, R.F., 3rd; Wilkinson, C.C.; Sitrin, R.G. The urokinase receptor is required for human monocyte chemotaxis in vitro. J. Clin. Investig. 1994, 93, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Resnati, M.; Pallavicini, I.; Wang, J.M.; Oppenheim, J.; Serhan, C.N.; Romano, M.; Blasi, F. The fibrinolytic receptor for urokinase activates the G protein-coupled chemotactic receptor FPRL1/LXA4R. Proc. Natl. Acad. Sci. USA 2002, 99, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Sol, M.; Kamps, J.A.A.M.; van den Born, J.; van den Heuvel, M.C.; van der Vlag, J.; Krenning, G.; Hillebrands, J.-L. Glomerular Endothelial Cells as Instigators of Glomerular Sclerotic Diseases. Front. Pharmacol. 2020, 11, 573557. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.; Mohammadi, S.; Davila-Velderrain, J.; Goods, B.A.; Cadwell, T.D.; Xing, Y.; Stemmer-Rachamimov, A.; Shalek, A.K.; Love, J.C.; Kellis, M.; et al. Single-cell transcriptomic atlas of the human retina identifies cell types associated with age-related macular degeneration. Nat. Commun. 2019, 10, 4902. [Google Scholar] [CrossRef] [PubMed]
- de Sain-van der Velden, M.G.; Rabelink, T.J.; Reijngoud, D.J.; Gadellaa, M.M.; Voorbij, H.A.; Stellaard, F.; Kaysen, G.A. Plasma alpha 2 macroglobulin is increased in nephrotic patients as a result of increased synthesis alone. Kidney Int. 1998, 54, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Goulas, T.; Garcia-Ferrer, I.; Marrero, A.; Marino-Puertas, L.; Duquerroy, S.; Gomis-Rüth, F.X. Structural and functional insight into pan-endopeptidase inhibition by α2-macroglobulins. Biol. Chem. 2017, 398, 975–994. [Google Scholar] [CrossRef]
- Stevenson, F.T.; Greene, S.; Kaysen, G.A. Serum α2-macroglobulin and α1-inhibitor 3 concentrations are increased in hypoalbuminemia by post-transcriptional mechanisms. Kidney Int. 1998, 53, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, C.; Endlich, N.; Kriz, W.; Endlich, K. Podocytes are sensitive to fluid shear stress in vitro. Am. J. Physiol.-Ren. Physiol. 2006, 291, F856–F865. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Hochberg, Y.; Benjamini, Y. More powerful procedures for multiple significance testing. Stat. Med. 1990, 9, 811–818. [Google Scholar] [CrossRef]
- Cohen, J. A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- ISO 9001:2008; Quality Management Systems—Requirements. International Organisation for Standardisation (ISO): Geneva, Switzerland.
- ISO 15189:2008; Medical Laboratories—Requirements for Quality and Compentence. International Organisation for Standardisation (ISO): Geneva, Switzerland.
- Reiter, T.; Pajenda, S.; O’Connell, D.; Lynch, C.; Kapps, S.; Agis, H.; Schmidt, A.; Wagner, L.; Leung, N.; Winnicki, W. Renal expression of light chain binding proteins. Front. Med. 2021, 7, 609582. [Google Scholar] [CrossRef]
- Park, H.; Li, Z.X.; Yang, X.O.; Chang, S.H.; Nurieva, R.; Wang, Y.H.; Wang, Y.; Hood, L.; Zhu, Z.; Tian, Q.; et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005, 6, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Mosca, M.; Hong, J.; Hadeler, E.; Hakimi, M.; Liao, W.; Bhutani, T. The Role of IL-17 Cytokines in Psoriasis. Immunotargets Ther. 2021, 10, 409–418. [Google Scholar] [CrossRef]
- Wang, J.X.; He, L.X.; Li, W.H.; Lv, S.B. A Role of IL-17 in Rheumatoid Arthritis Patients Complicated with Atherosclerosis. Front. Pharmacol. 2022, 13, 828933. [Google Scholar] [CrossRef]
- Wagner, C.A.; Roqué, P.J.; Goverman, J.M. Pathogenic T cell cytokines in multiple sclerosis. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef]
- Zhao, J.J.; Lu, Q.L.; Liu, Y.; Shi, Z.; Hu, L.J.; Zeng, Z.; Tu, Y.F.; Xiao, Z.Q.; Xu, Q.R. Th17 Cells in Inflammatory Bowel Disease: Cytokines, Plasticity, and Therapies. J. Immunol. Res. 2021, 2021, 8816041. [Google Scholar] [CrossRef] [PubMed]
- Izati, A.F.; Wong, K.K.; Maraina, C.H.C. IL-23/IL-17 axis in the pathogenesis and treatment of systemic lupus erythematosus and rheumatoid arthritis. Malays. J. Pathol. 2020, 42, 333–347. [Google Scholar]
- Paquissi, F.C.; Abensur, H. The Th17/IL-17 Axis and Kidney Diseases, with Focus on Lupus Nephritis. Front. Med. 2021, 8, 654912. [Google Scholar] [CrossRef]
- Espinoza, J.L.; Takami, A.; Nakata, K.; Onizuka, M.; Kawase, T.; Akiyama, H.; Miyamura, K.; Morishima, Y.; Fukuda, T.; Kodera, Y.; et al. A Genetic Variant in the IL-17 Promoter Is Functionally Associated with Acute Graft-Versus-Host Disease after Unrelated Bone Marrow Transplantation. PLoS ONE 2011, 6, e26229. [Google Scholar] [CrossRef]
- Zhai, S.; Sun, B.; Zhang, Y.; Zhao, L.; Zhang, L. IL-17 aggravates renal injury by promoting podocyte injury in children with primary nephrotic syndrome. Exp. Ther. Med. 2020, 20, 409–417. [Google Scholar] [CrossRef]
- Schmidt, T.; Luebbe, J.; Kilian, C.; Riedel, J.H.; Hiekmann, S.; Asada, N.; Ginsberg, P.; Robben, L.; Song, N.; Kaffke, A.; et al. IL-17 Receptor C Signaling Controls CD4+ TH17 Immune Responses and Tissue Injury in Immune-Mediated Kidney Diseases. J. Am. Soc. Nephrol. 2021, 32, 3081–3098. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Q.; Wang, L.J.; Li, C.C.; Yang, H.P.; Wang, X.L.; Tao, H. The Role of Th17/IL-17 in the Pathogenesis of Primary Nephrotic Syndrome in Children. Kidney Blood Press. Res. 2013, 37, 332–345. [Google Scholar] [CrossRef]
- Ozaltin, F.; Ibsirlioglu, T.; Taskiran, E.Z.; Baydar, D.E.; Kaymaz, F.; Buyukcelik, M.; Kilic, B.D.; Balat, A.; Iatropoulos, P.; Asan, E.; et al. Disruption of PTPRO causes childhood-onset nephrotic syndrome. Am. J. Hum. Genet. 2011, 89, 139–147. [Google Scholar] [CrossRef]
- Lu, C.C.; Wang, G.H.; Lu, J.; Chen, P.P.; Zhang, Y.; Hu, Z.B.; Ma, K.L. Role of Podocyte Injury in Glomerulosclerosis. Adv. Exp. Med. Biol. 2019, 1165, 195–232. [Google Scholar] [CrossRef]
- Kashgary, A.; Sontrop, J.M.; Li, L.H.; Al-Jaishi, A.A.; Habibullah, Z.N.; Alsolaimani, R.; Clark, W.F. The role of plasma exchange in treating post-transplant focal segmental glomerulosclerosis: A systematic review and meta-analysis of 77 case-reports and case-series. BMC Nephrol. 2016, 17, 104. [Google Scholar] [CrossRef]
- Allard, L.; Kwon, T.; Krid, S.; Bacchetta, J.; Garnier, A.; Novo, R.; Deschenes, G.; Salomon, R.; Roussey, G.; Allain-Launay, E. Treatment by immunoadsorption for recurrent focal segmental glomerulosclerosis after paediatric kidney transplantation: A multicentre French cohort. Nephrol. Dial. Transpl. 2018, 33, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Lionaki, S.; Vlachopanos, G.; Georgalis, A.; Liapis, G.; Skalioti, C.; Zavos, G.; Boletis, J.N. Individualized scheme of immunoadsorption for the recurrence of idiopathic focal segmental glomerulosclerosis in the graft: A single center experience. Ren. Fail. 2015, 37, 777–783. [Google Scholar] [CrossRef]
- Bennani, H.N.; Bonzi, J.Y.; Noble, J.; Terrec, F.; Motte, L.; Imerzoukene, F.; Bugnazet, M.; Giovannini, D.; Janbon, B.; Malvezzi, P.; et al. Immunoadsorption for Recurrent Primary Focal Segmental Glomerulosclerosis on Kidney Allografts: A Single-Center Experience and Literature Review. Blood Purif. 2020, 49, 322–333. [Google Scholar] [CrossRef]
- Alasfar, S.; Matar, D.; Montgomery, R.A.; Desai, N.; Lonze, B.; Vujjini, V.; Estrella, M.M.; Dieck, J.M.; Khneizer, G.; Sever, S.; et al. Rituximab and Therapeutic Plasma Exchange in Recurrent Focal Segmental Glomerulosclerosis Postkidney Transplantation. Transplantation 2018, 102, e115–e120. [Google Scholar] [CrossRef]
- Reynolds, B.; Lamb, A.; Jones, C.A.; Yadav, P.; Tyerman, K.S.; Geddes, C.C. UK experience of ofatumumab in recurrence of focal segmental glomerulosclerosis post-kidney transplant. Pediatr. Nephrol. 2022, 37, 199–207. [Google Scholar] [CrossRef]
- Hansrivijit, P.; Puthenpura, M.M.; Ghahramani, N. Efficacy of abatacept treatment for focal segmental glomerulosclerosis and minimal change disease: A systematic review of case reports, case series, and observational studies. Clin. Nephrol. 2020, 94, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Alhamad, T.; Dieck, J.M.; Younus, U.; Matar, D.; Alasfar, S.; Vujjini, V.; Wall, D.; Kanawati, B.; Reiser, J.; Brennan, D.C.; et al. ACTH Gel in Resistant Focal Segmental Glomerulosclerosis After Kidney Transplantation. Transplantation 2019, 103, 202–209. [Google Scholar] [CrossRef]
- Francis, A.; Trnka, P.; McTaggart, S.J. Long-Term Outcome of Kidney Transplantation in Recipients with Focal Segmental Glomerulosclerosis. Clin. J. Am. Soc. Nephrol. 2016, 11, 2041–2046. [Google Scholar] [CrossRef] [PubMed]
- Uffing, A.; Hullekes, F.; Riella, L.V.; Hogan, J.J. Recurrent Glomerular Disease after Kidney Transplantation: Diagnostic and Management Dilemmas. Clin. J. Am. Soc. Nephrol. 2021, 16, 1730–1742. [Google Scholar] [CrossRef]
- Suresh, C.P.; Saha, A.; Kaur, M.; Kumar, R.; Dubey, N.K.; Basak, T.; Tanwar, V.S.; Bhardwaj, G.; Sengupta, S.; Batra, V.V.; et al. Differentially expressed urinary biomarkers in children with idiopathic nephrotic syndrome. Clin. Exp. Nephrol. 2016, 20, 273–283. [Google Scholar] [CrossRef]
- Typiak, M.; Audzeyenka, I.; Dubaniewicz, A. Presence and possible impact of Fcγ receptors on resident kidney cells in health and disease. Immunol. Cell Biol. 2022, 100, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Foss, S.; Bottermann, M.; Jonsson, A.; Sandlie, I.; James, L.C.; Andersen, J.T. TRIM21—From Intracellular Immunity to Therapy. Front. Immunol. 2019, 10, 2049. [Google Scholar] [CrossRef]
- Bruno, V.; Mühlig, A.K.; Oh, J.; Licht, C. New insights into the immune functions of podocytes: The role of complement. Mol. Cell. Pediatr. 2023, 10, 3. [Google Scholar] [CrossRef]
- Brown, E.J.; Schlöndorff, J.S.; Becker, D.J.; Tsukaguchi, H.; Tonna, S.J.; Uscinski, A.L.; Higgs, H.N.; Henderson, J.M.; Pollak, M.R. Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat. Genet. 2010, 42, 72–76. [Google Scholar] [CrossRef]
- Feng, D.; Notbohm, J.; Benjamin, A.; He, S.; Wang, M.; Ang, L.-H.; Bantawa, M.; Bouzid, M.; Del Gado, E.; Krishnan, R.; et al. Disease-causing mutation in α-actinin-4 promotes podocyte detachment through maladaptation to periodic stretch. Proc. Natl. Acad. Sci. USA 2018, 115, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Mele, C.; Iatropoulos, P.; Donadelli, R.; Calabria, A.; Maranta, R.; Cassis, P.; Buelli, S.; Tomasoni, S.; Piras, R.; Krendel, M.; et al. MYO1E mutations and childhood familial focal segmental glomerulosclerosis. N. Engl. J. Med. 2011, 365, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Heeringa, S.F.; Chernin, G.; Chaki, M.; Zhou, W.; Sloan, A.J.; Ji, Z.; Xie, L.X.; Salviati, L.; Hurd, T.W.; Vega-Warner, V.; et al. COQ6 mutations in human patients produce nephrotic syndrome with sensorineural deafness. J. Clin. Investig. 2011, 121, 2013–2024. [Google Scholar] [CrossRef] [PubMed]
- Berkovic, S.F.; Dibbens, L.M.; Oshlack, A.; Silver, J.D.; Katerelos, M.; Vears, D.F.; Lüllmann-Rauch, R.; Blanz, J.; Zhang, K.W.; Stankovich, J.; et al. Array-based gene discovery with three unrelated subjects shows SCARB2/LIMP-2 deficiency causes myoclonus epilepsy and glomerulosclerosis. Am. J. Hum. Genet. 2008, 82, 673–684. [Google Scholar] [CrossRef]
- Turcan, I.; Pasmooij, A.M.G.; Van den Akker, P.C.; Lemmink, H.; Sinke, R.J.; Jonkman, M.F. Association of Epidermolysis Bullosa Simplex with Mottled Pigmentation and Mutations. JAMA Dermatol. 2016, 152, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.; Ambur, A.; Foss, M.; Nathoo, R. Expanding the spectrum of epidermolysis bullosa simplex: Syndromic epidermolysis bullosa simplex with nephropathy and epilepsy secondary to CD151 tetraspanin defect—A case report and review of the literature. JAAD Case Rep. 2022, 23, 136–140. [Google Scholar] [CrossRef]
- Has, C.; Fischer, J. Inherited epidermolysis bullosa: New diagnostics and new clinical phenotypes. Exp. Dermatol. 2019, 28, 1146–1152. [Google Scholar] [CrossRef]

| A2M | CASP14 | EXPH5 | MUC5B | RGPD3 |
| ABCA12 | CATSPERB | FAM171B | NCOR2 | RNF213 |
| ABCC10 | CD36 | FER | OBSCN | SIL1 |
| ABI3BP | DIDO1 | GPR155 | PKD1L1 | SLIT2 |
| AMOTL2 | DNAH11 | GPR179 | PLEC | SORCS2 |
| ANKRD11 | DNAH3 | KMT2C | PLXNA4 | SSPO |
| ARHGEF4 | DNAH8 | MAPK15 | PNKD | TAT |
| ARID1B | DPP4 | MAPK6 | PPP1R16A | TRIM21 |
| ASPM | DPYS | MEGF6 | PRRC2C | TRRAP |
| C1orf170 | ERN1 | MUC16 | PTCH1 | WHSC1L1 |
| ZIM3 |
| ID | Transcript | Symbol | Function | pph2 | Variant Alleles | Identifier | Omim |
|---|---|---|---|---|---|---|---|
| A | Lys691Asn | A2M | missense | benign | 1 | rs369389194 | 103,950 |
| B | Glu1165Gly | A2M | missense | probably damaging | 1 | 103,950 | |
| C | Leu18Arg | A2M | missense | benign | 1 | 103,950 |
| ID | Intensity | MW Da |
|---|---|---|
| HIST1H3A | 276 | 15,404 |
| TRIM21 | 205 | 54,170 |
| FAM84A | 204 | 32,491 |
| MAP9 | 196 | 74,234 |
| DGKK | 186 | 141,829 |
| Nol3 | 121 | 22,629 |
| CCDC70 | 118 | 28,767 |
| HCLS1 | 105 | 54,014 |
| ZYX | 98 | 61,277 |
| BSND | 93 | 35,197 |
| PPM1E | 92 | 83,952 |
| BAG3 | 87 | 61,595 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pajenda, S.; Gerges, D.; Wagner, L.; O’Connell, D.; Aiad, M.; Imre, R.; Mechtler, K.; Zimprich, A.; Schmidt, A.; Sengoelge, G.; et al. Identification of Pathogenic Pathways for Recurrence of Focal Segmental Glomerulosclerosis after Kidney Transplantation. Diagnostics 2024, 14, 1591. https://doi.org/10.3390/diagnostics14151591
Pajenda S, Gerges D, Wagner L, O’Connell D, Aiad M, Imre R, Mechtler K, Zimprich A, Schmidt A, Sengoelge G, et al. Identification of Pathogenic Pathways for Recurrence of Focal Segmental Glomerulosclerosis after Kidney Transplantation. Diagnostics. 2024; 14(15):1591. https://doi.org/10.3390/diagnostics14151591
Chicago/Turabian StylePajenda, Sahra, Daniela Gerges, Ludwig Wagner, David O’Connell, Monika Aiad, Richard Imre, Karl Mechtler, Alexander Zimprich, Alice Schmidt, Guerkan Sengoelge, and et al. 2024. "Identification of Pathogenic Pathways for Recurrence of Focal Segmental Glomerulosclerosis after Kidney Transplantation" Diagnostics 14, no. 15: 1591. https://doi.org/10.3390/diagnostics14151591






