Shear-Wave Elastography Gradient Analysis of Newly Diagnosed Breast Tumours: A Critical Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Committee Approval
2.2. Study Participants Screening and Inclusion
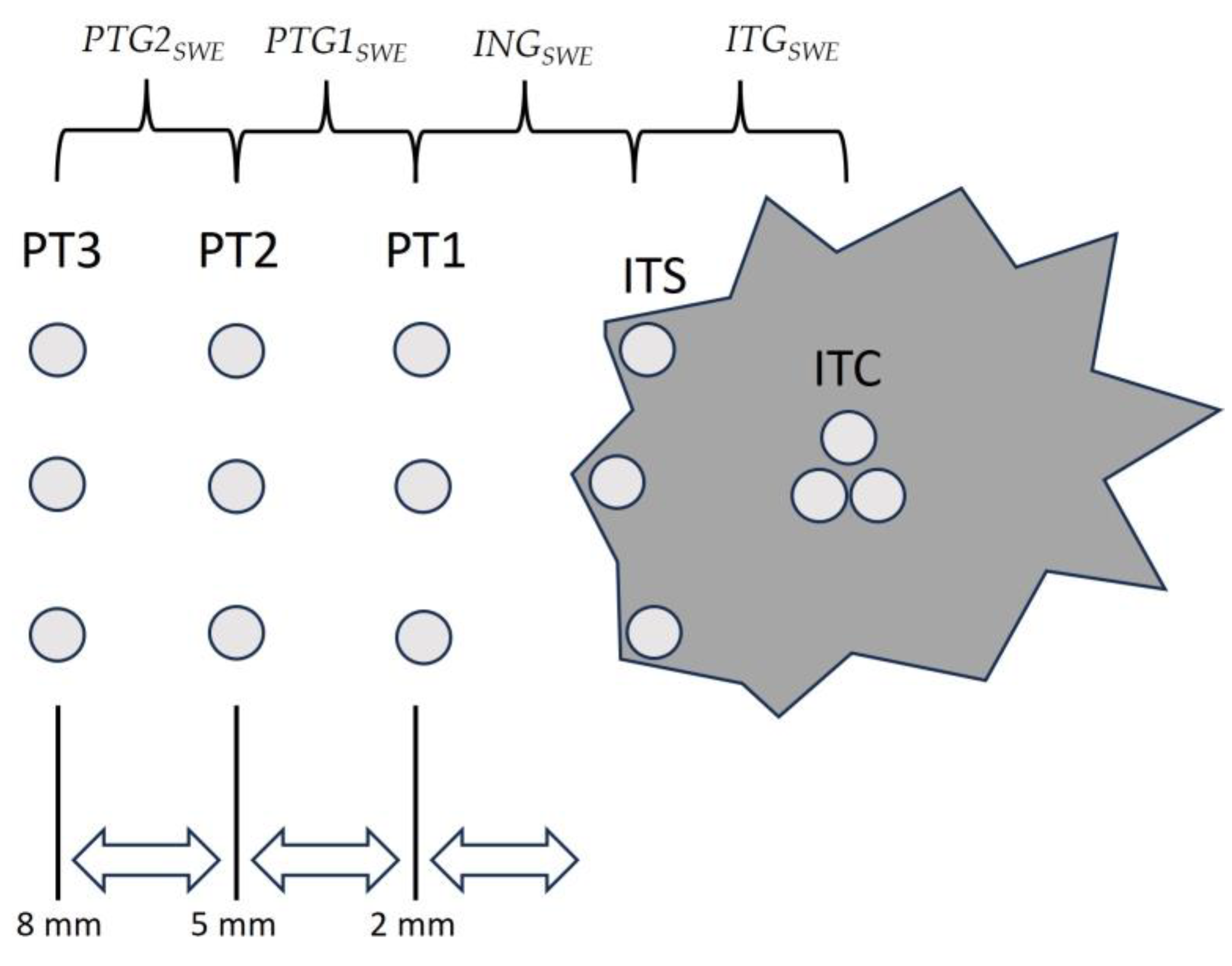
2.3. Ultrasound Equipment, Shear-Wave Elastography Measurements, and Gradient Calculations
2.4. Core-Needle Biopsy Procedure
2.5. Histologic Evaluation
2.6. Statistical Analysis
3. Results
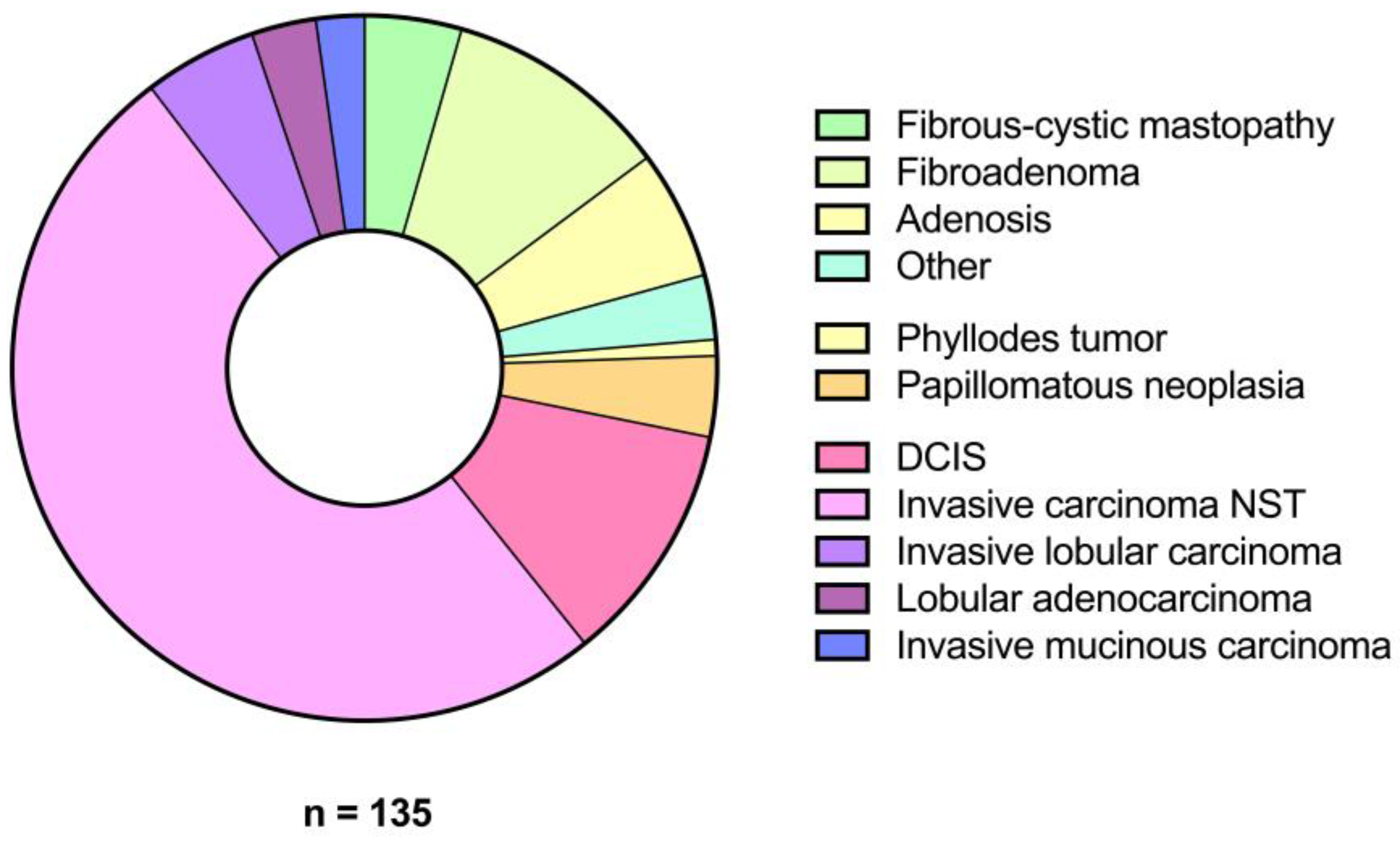
3.1. Patient Demographics and Lesion Characteristics
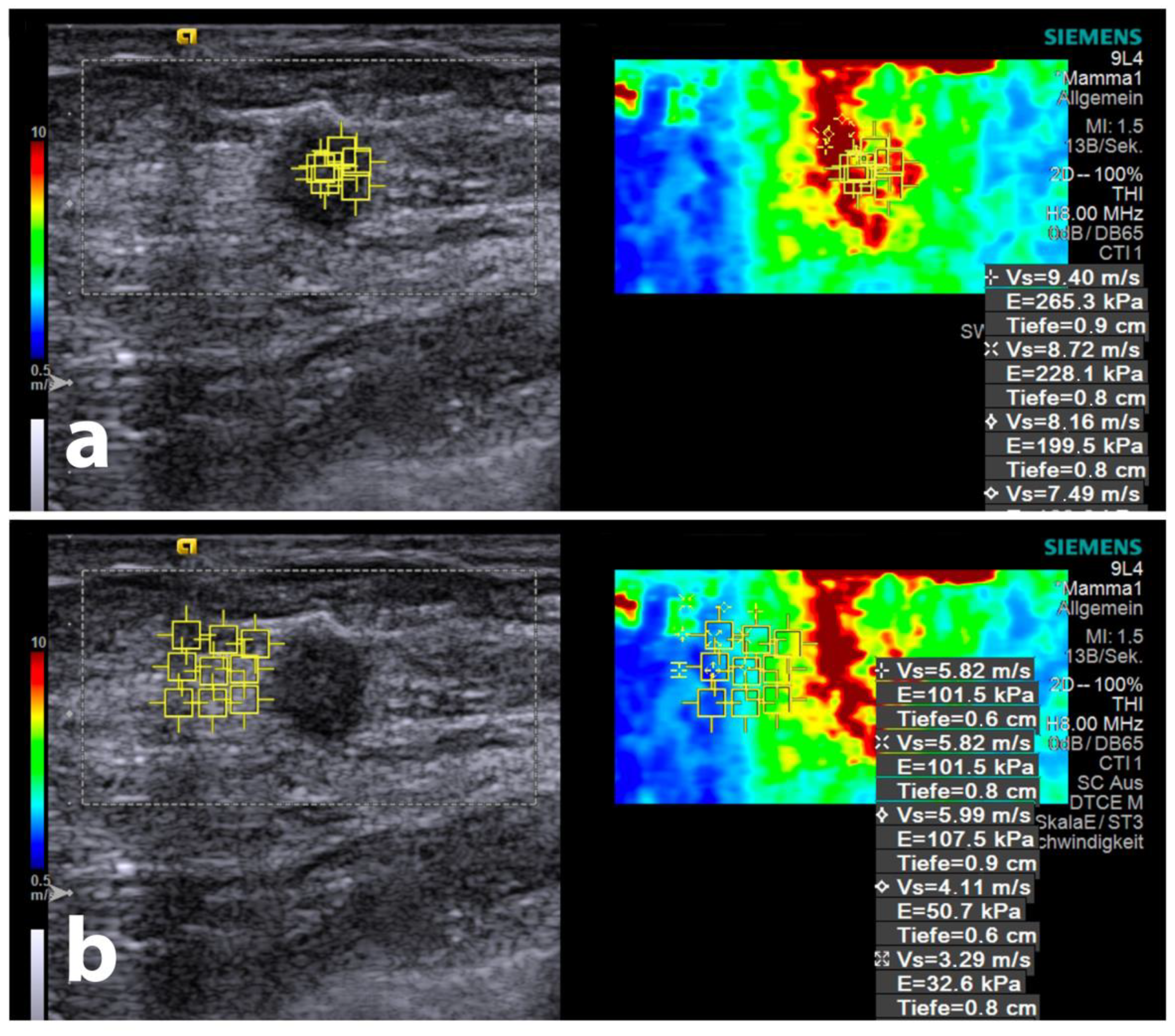
3.2. SWE Velocities
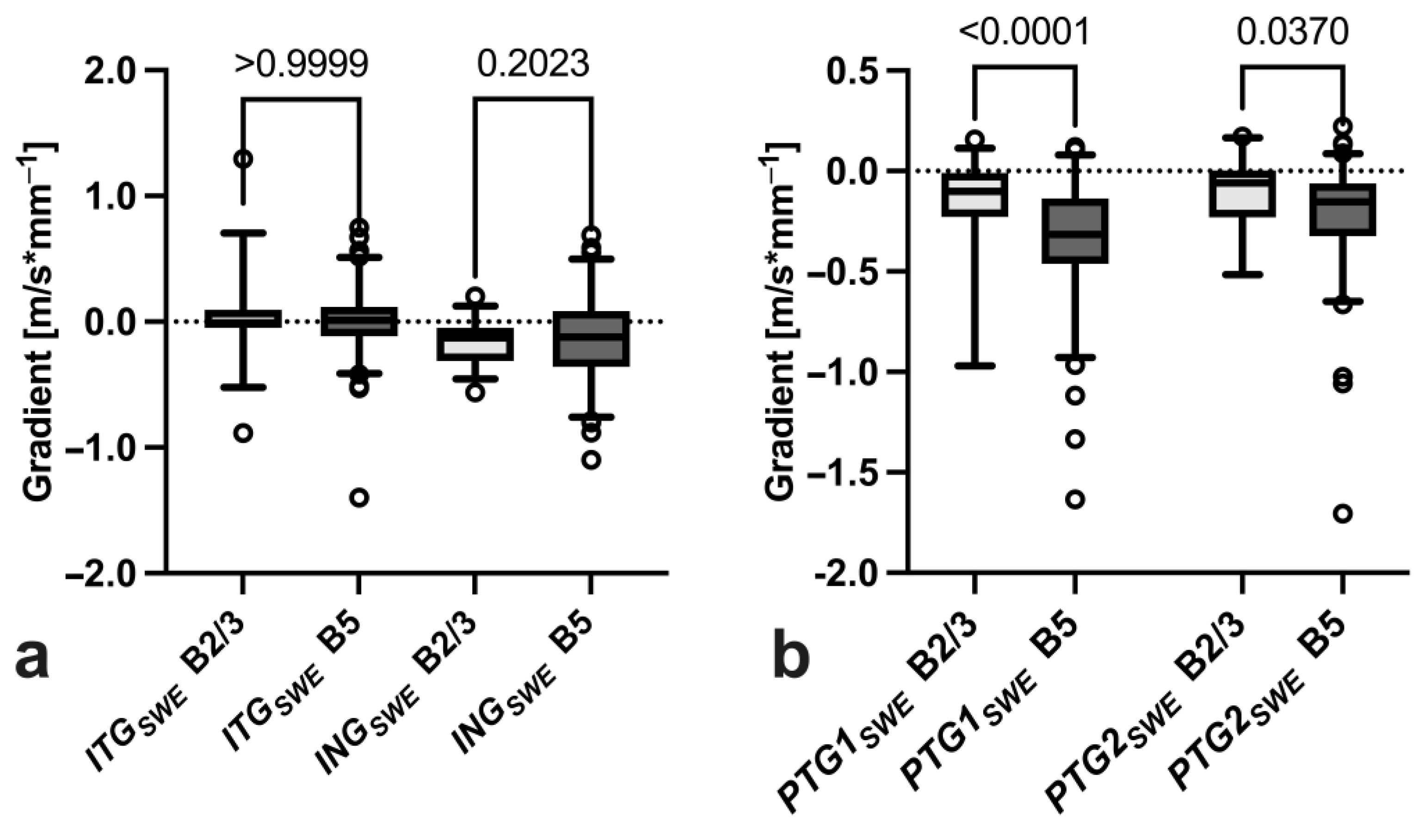
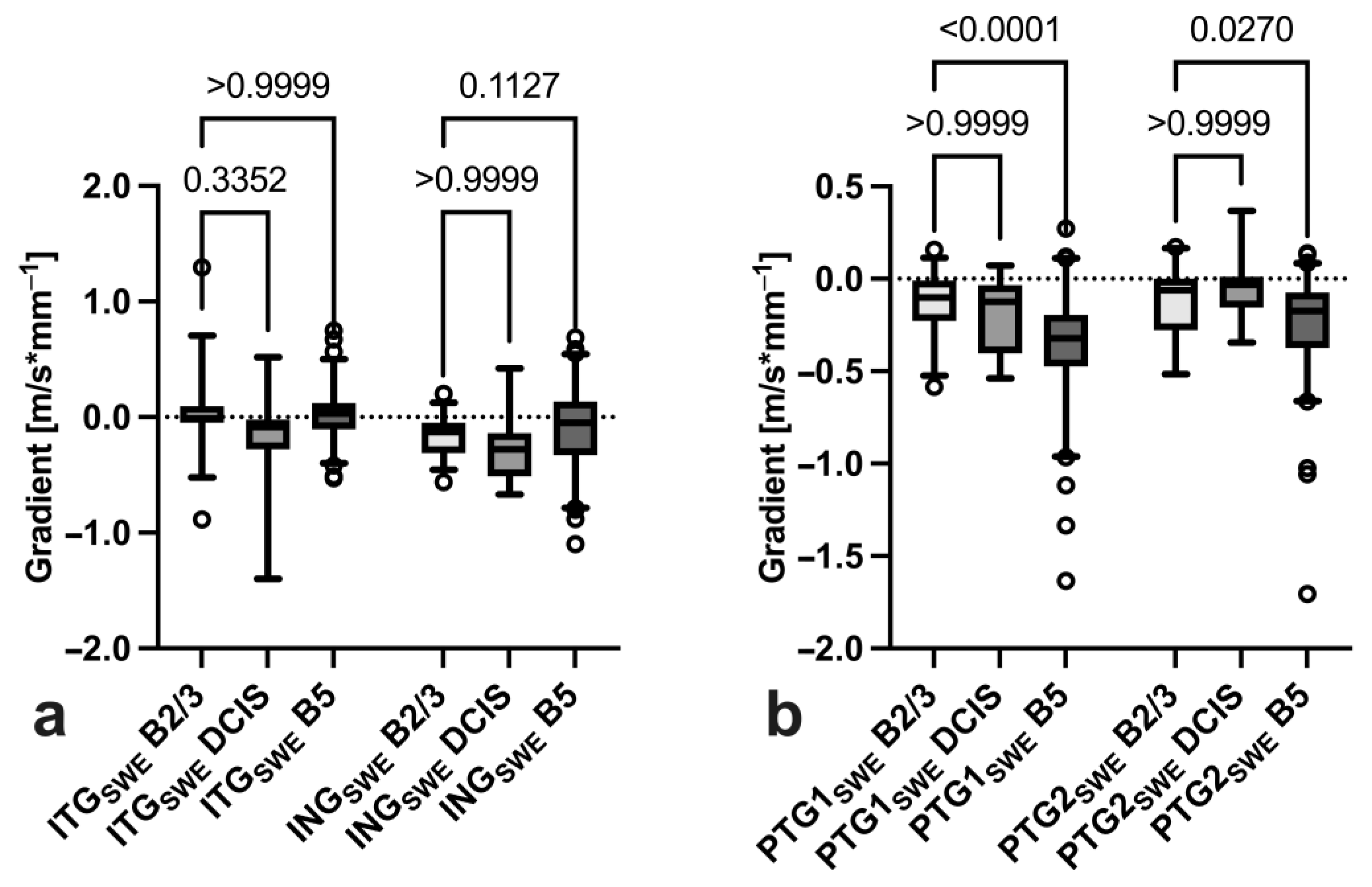
3.3. Gradient Analysis
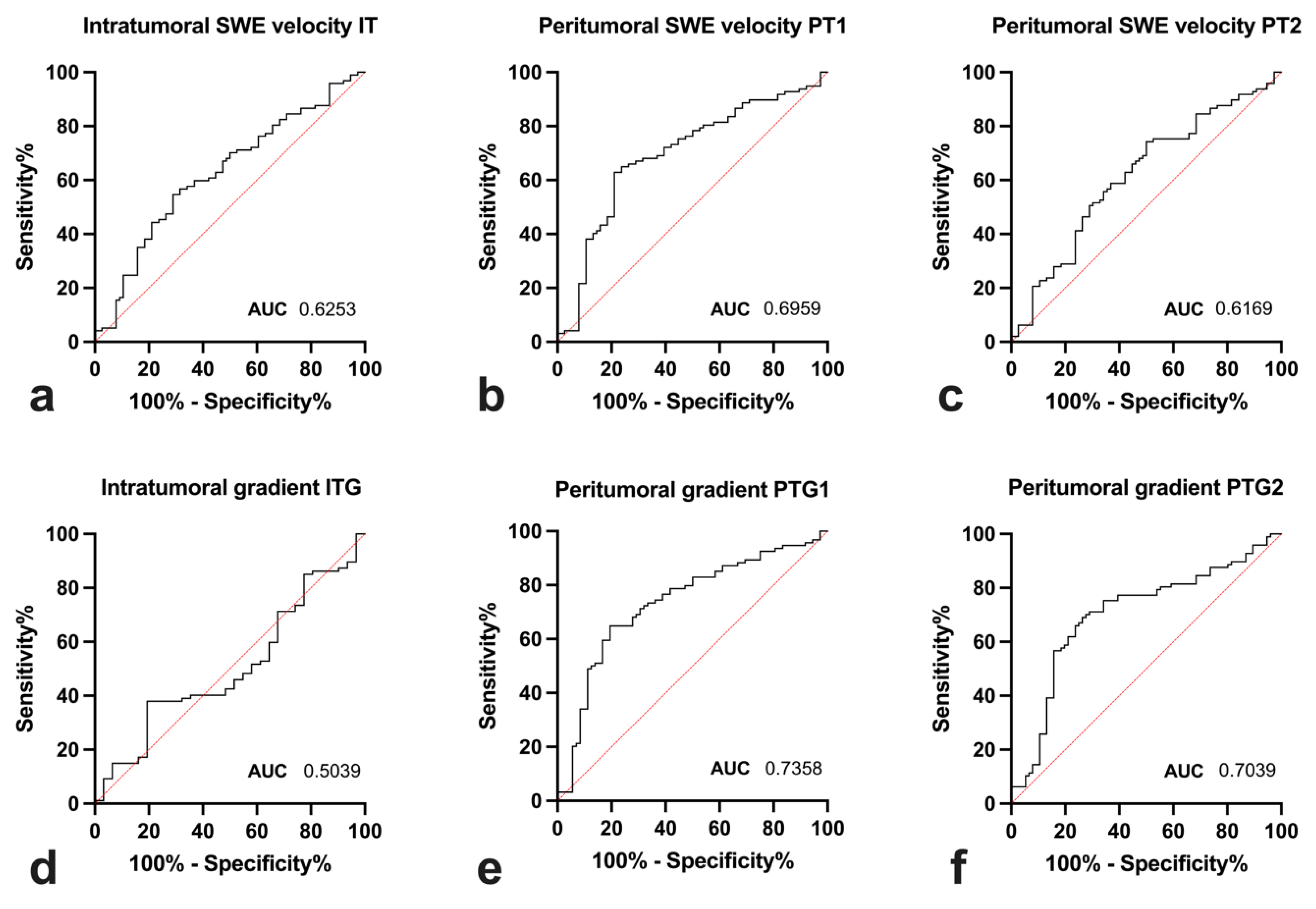
3.4. Diagnostic Utility
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast Cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Marmot, M.; Altman, D.G.; Cameron, D.A.; Dewar, J.A.; Thompson, S.G.; Wilcox, M. The Benefits and Harms of Breast Cancer Screening: An Independent Review. Lancet 2012, 380, 1778–1786. [Google Scholar] [CrossRef] [PubMed]
- Weißenhofer, S. Zweiter Evaluationsbericht Zum Österreichischen Brustkrebs-Früherkennungsprogramm. Available online: https://jasmin.goeg.at/id/eprint/1265/2/Zweiter%20Evaluationsbericht%20BKFP%202014-2017.pdf (accessed on 3 March 2023).
- Barr, R.G.; Nakashima, K.; Amy, D.; Cosgrove, D.; Farrokh, A.; Schafer, F.; Bamber, J.C.; Castera, L.; Choi, B.I.; Chou, Y.H.; et al. WFUMB Guidelines and Recommendations for Clinical Use of Ultrasound Elastography: Part 2: Breast. Ultrasound Med. Biol. 2015, 41, 1148–1160. [Google Scholar] [CrossRef] [PubMed]
- Bamber, J.; Cosgrove, D.; Dietrich, C.F.; Fromageau, J.; Bojunga, J.; Calliada, F.; Cantisani, V.; Correas, J.M.; D’Onofrio, M.; Drakonaki, E.E.; et al. EFSUMB Guidelines and Recommendations on the Clinical Use of Ultrasound Elastographypart 1: Basic Principles and Technology. Ultraschall Der Med. 2013, 34, 169–184. [Google Scholar] [CrossRef]
- Golatta, M.; Pfob, A.; Büsch, C.; Bruckner, T.; Alwafai, Z.; Balleyguier, C.; Clevert, D.A.; Duda, V.; Goncalo, M.; Gruber, I.; et al. The Potential of Shear Wave Elastography to Reduce Unnecessary Biopsies in Breast Cancer Diagnosis: An International, Diagnostic, Multicenter Trial. Ultraschall Der Med. 2021, 44, 162–168. [Google Scholar] [CrossRef]
- Golatta, M.; Pfob, A.; Büsch, C.; Bruckner, T.; Alwafai, Z.; Balleyguier, C.; Clevert, D.A.; Duda, V.; Goncalo, M.; Gruber, I.; et al. The Potential of Combined Shear Wave and Strain Elastography to Reduce Unnecessary Biopsies in Breast Cancer Diagnostics—An International, Multicentre Trial. Eur. J. Cancer 2022, 161, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pintican, R.M.; Chiorean, A.; Duma, M.; Feier, D.; Szep, M.; Eniu, D.; Goidescu, I.; Dudea, S. Are Mutation Carrier Patients Different from Non-Carrier Patients? Genetic, Pathology, and US Features of Patients with Breast Cancer. Cancers 2022, 14, 2759. [Google Scholar] [CrossRef]
- Zhou, J.Q.; Zhan, W.W.; Chang, C.; Zhang, X.X.; Jia, Y.; Dong, Y.J.; Zhou, C.; Sun, J.; Grant, E.G. Breast Lesions: Evaluation with Shear Wave Elastography, with Special Emphasis on the “Stiff Rim” Sign. Radiology 2014, 272, 63–72. [Google Scholar] [CrossRef]
- Park, H.S.; Shin, H.J.; Shin, K.C.; Cha, J.H.; Chae, E.Y.; Choi, W.J.; Kim, H.H. Comparison of Peritumoral Stromal Tissue Stiffness Obtained by Shear Wave Elastography between Benign and Malignant Breast Lesions. Acta Radiol. 2018, 59, 1168–1175. [Google Scholar] [CrossRef]
- Gruber, L.; Deeg, J.; Egle, D.; Soleiman, A.; Ladenhauf, V.; Luger, A.; Amort, B.; Daniaux, M. Peritumoural Strain Elastography of Newly Diagnosed Breast Tumours: Does Maximum Peritumoural Halo Depth Correlate with Tumour Differentiation and Grade? Diagnostics 2023, 13, 2064. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Otto, R.; Hackelöer, J. Guidelines for Ultrasound Guided Breast Biopsy. Ultraschall Der Med.-Eur. J. Ultrasound 2005, 26, 241–244. [Google Scholar] [CrossRef]
- Tan, P.H.; Ellis, I.; Allison, K.; Brogi, E.; Fox, S.B.; Lakhani, S.; Lazar, A.J.; Morris, E.A.; Sahin, A.; Salgado, R.; et al. The 2019 World Health Organization Classification of Tumours of the Breast. Histopathology 2020, 77, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.; Whelehan, P.; Thomson, K.; McLean, D.; Brauer, K.; Purdie, C.; Baker, L.; Jordan, L.; Rauchhaus, P.; Thompson, A. Invasive Breast Cancer: Relationship between Shear-Wave Elastographic Findings and Histologic Prognostic Factors. Radiology 2012, 263, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.; Whelehan, P.; Thomson, K.; McLean, D.; Brauer, K.; Purdie, C.; Jordan, L.; Baker, L.; Thompson, A. Quantitative Shear Wave Ultrasound Elastography: Initial Experience in Solid Breast Masses. Breast Cancer Res. 2010, 12, R104. [Google Scholar] [CrossRef]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.T.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.F.; Berse, B.; Jackman, R.W.; Tognazzi, K.; Guidi, A.J.; Dvorak, H.F.; Senger, D.R.; Connolly, J.L.; Schnitt, S.J. Expression of Vascular Permeability Factor (Vascular Endothelial Growth Factor) and Its Receptors in Breast Cancer. Hum. Pathol. 1995, 26, 86–91. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and Cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Sofopoulos, M.; Fortis, S.P.; Vaxevanis, C.K.; Sotiriadou, N.N.; Arnogiannaki, N.; Ardavanis, A.; Vlachodimitropoulos, D.; Perez, S.A.; Baxevanis, C.N. The Prognostic Significance of Peritumoral Tertiary Lymphoid Structures in Breast Cancer. Cancer Immunol. Immunother. 2019, 68, 1733–1745. [Google Scholar] [CrossRef]
- Berg, W.A.; Cosgrove, D.O.; Doré, C.J.; Schäfer, F.K.W.; Svensson, W.E.; Hooley, R.J.; Ohlinger, R.; Mendelson, E.B.; Balu-Maestro, C.; Locatelli, M.; et al. Shear-Wave Elastography Improves the Specificity of Breast US: The BE1 Multinational Study of 939 Masses. Radiology 2012, 262, 435–449. [Google Scholar] [CrossRef]
- Basara Akin, I.; Ozgul, H.A.; Guray Durak, M.; Balci, P. Evaluation of Elastographic Features in Complex Fibroadenomas With Radiologic-Pathologic Correlation. J. Ultrasound Med. 2021, 40, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xu, Y.; Zhao, Y.; Yin, J.; Chen, Z.; Huang, P. The Role of Tissue Elasticity in the Differential Diagnosis of Benign and Malignant Breast Lesions Using Shear Wave Elastography. BMC Cancer 2020, 20, 930. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.M.; Dinh, P.; Pathmanathan, N.; Graham, J.D. Ductal Carcinoma In Situ: Molecular Changes Accompanying Disease Progression. J. Mammary Gland. Biol. Neoplasia 2022, 27, 101. [Google Scholar] [CrossRef] [PubMed]
- Ryser, M.D.; Weaver, D.L.; Zhao, F.; Worni, M.; Grimm, L.J.; Gulati, R.; Etzioni, R.; Hyslop, T.; Lee, S.J.; Hwang, E.S. Cancer Outcomes in DCIS Patients Without Locoregional Treatment. J. Natl. Cancer Inst. 2019, 111, 952–960. [Google Scholar] [CrossRef]
- Worni, M.; Akushevich, I.; Greenup, R.; Sarma, D.; Ryser, M.D.; Myers, E.R.; Hwang, E.S. Trends in Treatment Patterns and Outcomes for Ductal Carcinoma In Situ. J. Natl. Cancer Inst. 2015, 107, djv263. [Google Scholar] [CrossRef] [PubMed]
- Lo, P.K.; Zhang, Y.; Yao, Y.; Wolfson, B.; Yu, J.; Han, S.Y.; Duru, N.; Zhou, Q. Tumor-Associated Myoepithelial Cells Promote the Invasive Progression of Ductal Carcinoma in Situ through Activation of TGFβ Signaling. J. Biol. Chem. 2017, 292, 11466–11484. [Google Scholar] [CrossRef]
- Ward, R.C.; Lourenco, A.P.; Mainiero, M.B. Ultrasound-Guided Breast Cancer Cryoablation. Am. J. Roentgenol. 2019, 213, 716–722. [Google Scholar] [CrossRef]
- Xia, L.Y.; Hu, Q.L.; Xu, W.Y. Efficacy and Safety of Radiofrequency Ablation for Breast Cancer Smaller Than 2 Cm: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 651646. [Google Scholar] [CrossRef]
- Mao, Y.J.; Lim, H.J.; Ni, M.; Yan, W.H.; Wong, D.W.C.; Cheung, J.C.W. Breast Tumour Classification Using Ultrasound Elastography with Machine Learning: A Systematic Scoping Review. Cancers 2022, 14, 367. [Google Scholar] [CrossRef]
- Dalton, L.W.; Pinder, S.E.; Elston, C.E.; Ellis, I.O.; Page, D.L.; Dupont, W.D.; Blamey, R.W. Histologic Grading of Breast Cancer: Linkage of Patient Outcome with Level of Pathologist Agreement. Mod. Pathol. 2000, 13, 730–735. [Google Scholar] [CrossRef]
- Frierson, H.F.; Wolber, R.A.; Berean, K.W.; Franquemont, D.W.; Gaffey, M.J.; Boyd, J.C.; Wilbur, D.C. Interobserver Reproducibility of the Nottingham Modification of the Bloom and Richardson Histologic Grading Scheme for Infiltrating Ductal Carcinoma. Am. J. Clin. Pathol. 1995, 103, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Olgun, D.Ç.; Korkmazer, B.; Kiliç, F.; Dikici, A.S.; Velidedeoǧlu, M.; Aydoǧan, F.; Kantarci, F.; Yilmaz, M.H. Use of Shear Wave Elastography to Differentiate Benign and Malignant Breast Lesions. Diagn. Interv. Radiol. 2014, 20, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.M.; Moon, W.K.; Cho, N.; Yi, A.; Koo, H.R.; Han, W.; Noh, D.Y.; Moon, H.G.; Kim, S.J. Clinical Application of Shear Wave Elastography (SWE) in the Diagnosis of Benign and Malignant Breast Diseases. Breast Cancer Res. Treat. 2011, 129, 89–97. [Google Scholar] [CrossRef]
- Tozaki, M.; Isobe, S.; Sakamoto, M. Combination of Elastography and Tissue Quantification Using the Acoustic Radiation Force Impulse (ARFI) Technology for Differential Diagnosis of Breast Masses. Jpn. J. Radiol. 2012, 30, 659–670. [Google Scholar] [CrossRef]
- Moon, W.K.; Huang, Y.S.; Lee, Y.W.; Chang, S.C.; Lo, C.M.; Yang, M.C.; Bae, M.S.; Lee, S.H.; Chang, J.M.; Huang, C.S.; et al. Computer-Aided Tumor Diagnosis Using Shear Wave Breast Elastography. Ultrasonics 2017, 78, 125–133. [Google Scholar] [CrossRef]
- Barr, R.G.; Zhang, Z. Shear-Wave Elastography of the Breast: Value of a Quality Measure and Comparison with Strain Elastography. Radiology 2015, 275, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Elseedawy, M.; Whelehan, P.; Vinnicombe, S.; Thomson, K.; Evans, A. Factors Influencing the Stiffness of Fibroadenomas at Shear Wave Elastography. Clin. Radiol. 2016, 71, 92–95. [Google Scholar] [CrossRef]
- Barr, R.G. Future of Breast Elastography. Ultrasonography 2019, 38, 93–105. [Google Scholar] [CrossRef]
- Shiina, T.; Nightingale, K.R.; Palmeri, M.L.; Hall, T.J.; Bamber, J.C.; Barr, R.G.; Castera, L.; Choi, B.I.; Chou, Y.H.; Cosgrove, D.; et al. WFUMB Guidelines and Recommendations for Clinical Use of Ultrasound Elastography: Part 1: Basic Principles and Terminology. Ultrasound Med. Biol. 2015, 41, 1126–1147. [Google Scholar] [CrossRef]
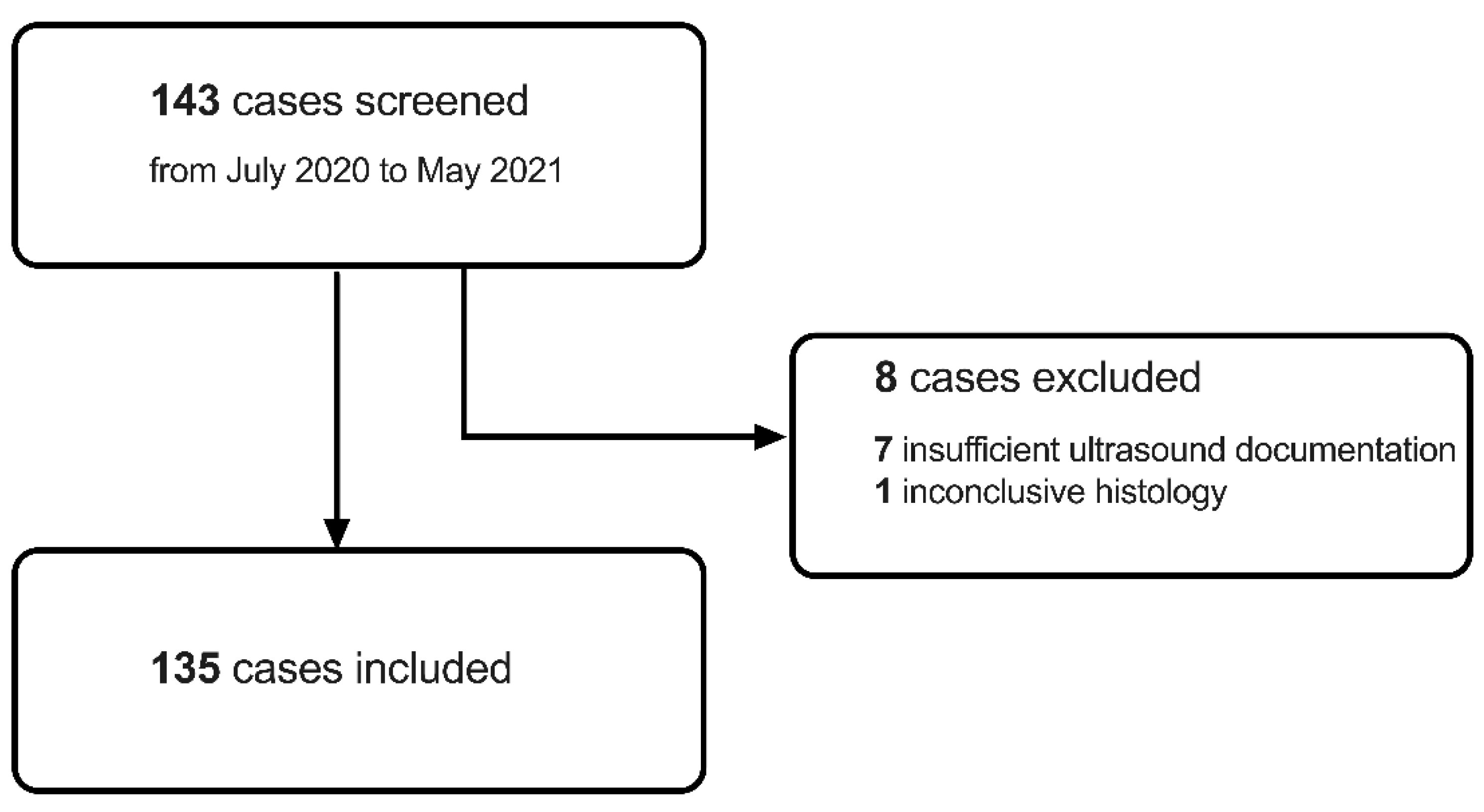

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Ultrasound-guided biopsy of newly diagnosed breast tumour | Patient age under 18 years |
| Sufficient ultrasound documentation including B-mode and shear-wave elastography imaging | Prior history of ipsilateral breast cancer |
| Available histological diagnosis | Insufficient ultrasound documentation |
| Inconclusive histology |
| Peritumoral SWE Speed PT1 | |||
|---|---|---|---|
| ≤3.76 m/s | >3.76 m/s | ||
| Peritumoral gradient PTG1 | ≥−0.238 m/s·mm−1 | 46.0% | 81.3% |
| <−0.238 m/s·mm−1 | 76.5% | 90.6% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deeg, J.; Swoboda, M.; Egle, D.; Wieser, V.; Soleiman, A.; Ladenhauf, V.; Galijasevic, M.; Amort, B.; Gruber, L. Shear-Wave Elastography Gradient Analysis of Newly Diagnosed Breast Tumours: A Critical Analysis. Diagnostics 2024, 14, 1657. https://doi.org/10.3390/diagnostics14151657
Deeg J, Swoboda M, Egle D, Wieser V, Soleiman A, Ladenhauf V, Galijasevic M, Amort B, Gruber L. Shear-Wave Elastography Gradient Analysis of Newly Diagnosed Breast Tumours: A Critical Analysis. Diagnostics. 2024; 14(15):1657. https://doi.org/10.3390/diagnostics14151657
Chicago/Turabian StyleDeeg, Johannes, Michael Swoboda, Daniel Egle, Verena Wieser, Afschin Soleiman, Valentin Ladenhauf, Malik Galijasevic, Birgit Amort, and Leonhard Gruber. 2024. "Shear-Wave Elastography Gradient Analysis of Newly Diagnosed Breast Tumours: A Critical Analysis" Diagnostics 14, no. 15: 1657. https://doi.org/10.3390/diagnostics14151657
APA StyleDeeg, J., Swoboda, M., Egle, D., Wieser, V., Soleiman, A., Ladenhauf, V., Galijasevic, M., Amort, B., & Gruber, L. (2024). Shear-Wave Elastography Gradient Analysis of Newly Diagnosed Breast Tumours: A Critical Analysis. Diagnostics, 14(15), 1657. https://doi.org/10.3390/diagnostics14151657







