Quantitative Edge Analysis Can Differentiate Pancreatic Carcinoma from Normal Pancreatic Parenchyma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
- Patients with adenocarcinoma of the tail of the pancreas;
- Diagnoses of adenocarcinoma confirmed at pathology of the resected specimen or at biopsy in case of unresectable neoplasms;
- Patients who underwent MDCT at our institute for the initial staging of the pancreatic neoplasm with images available for review on PACS.
- Adenocarcinoma of the head or body of the pancreas;
- No pathologically proven diagnosis of the adenocarcinoma;
- No MDCT images available for review.
2.2. Image Acquisition
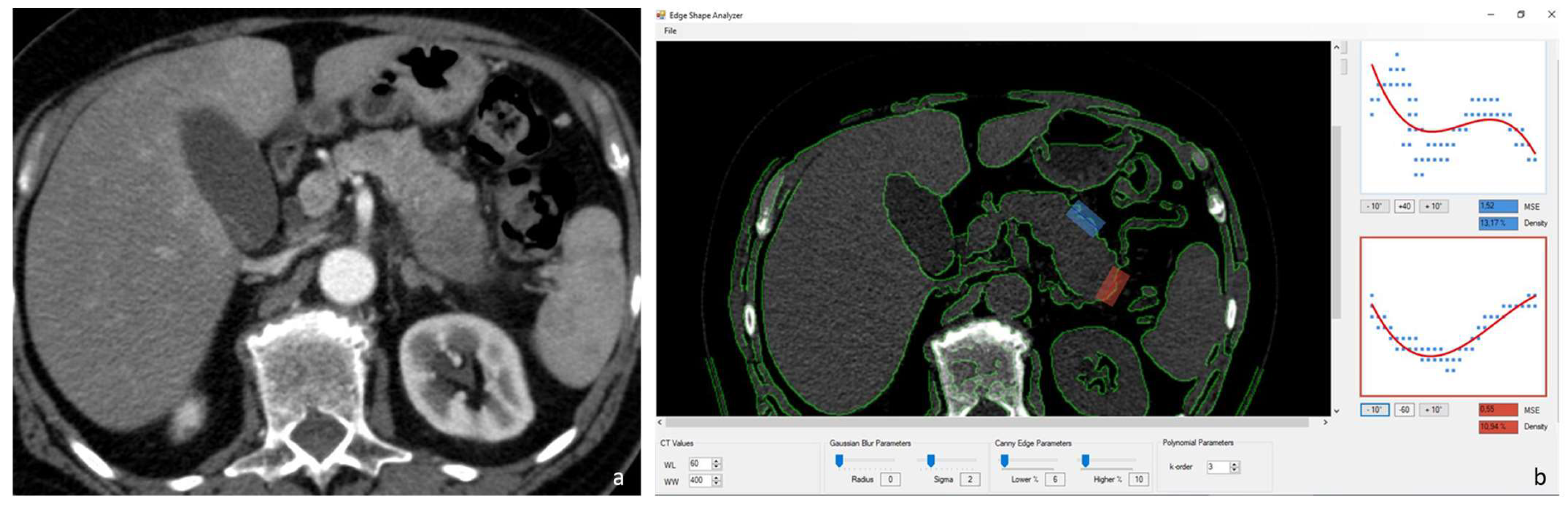
2.3. Image Analysis–Pancreatic Edge Analysis Software
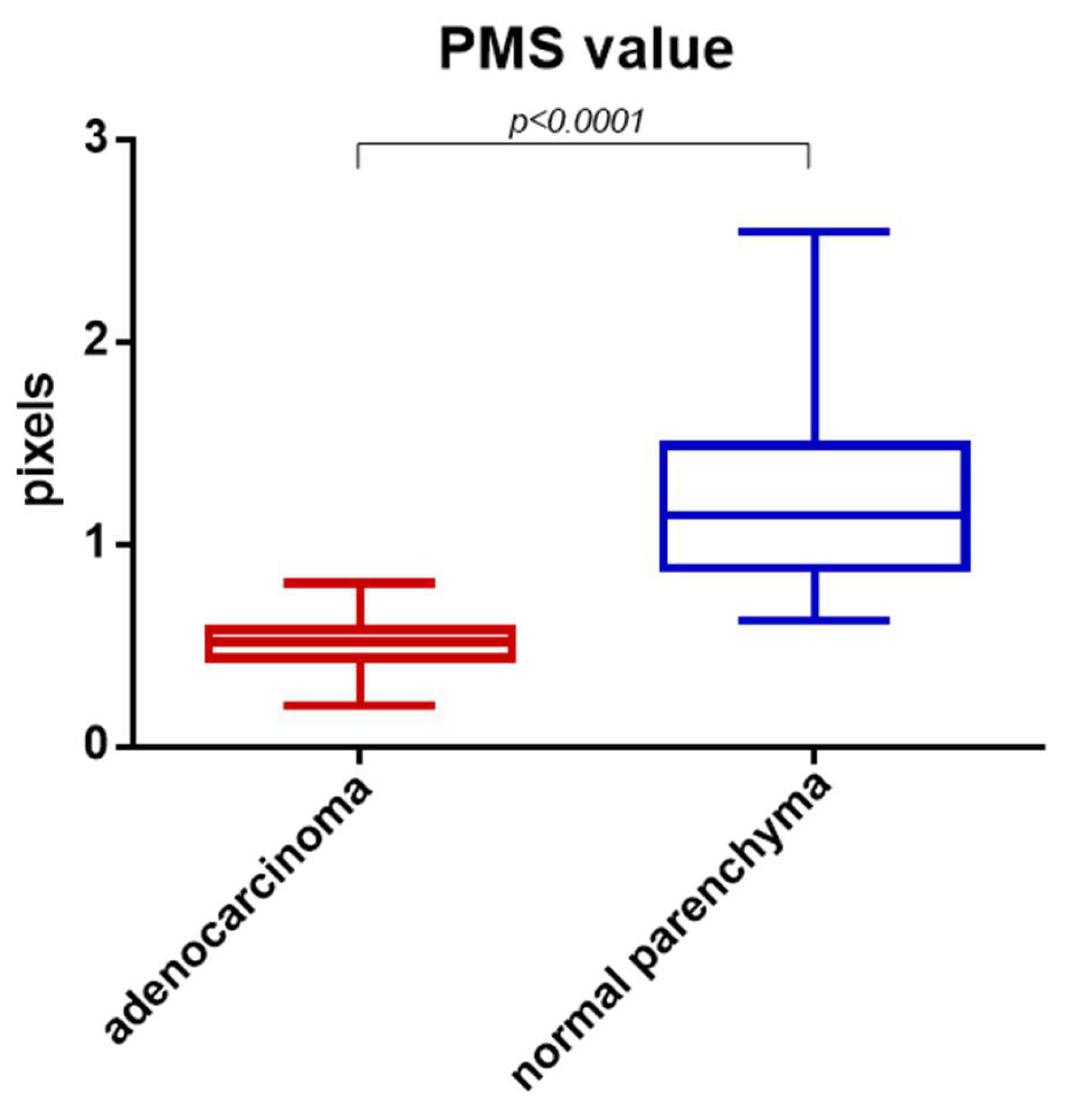
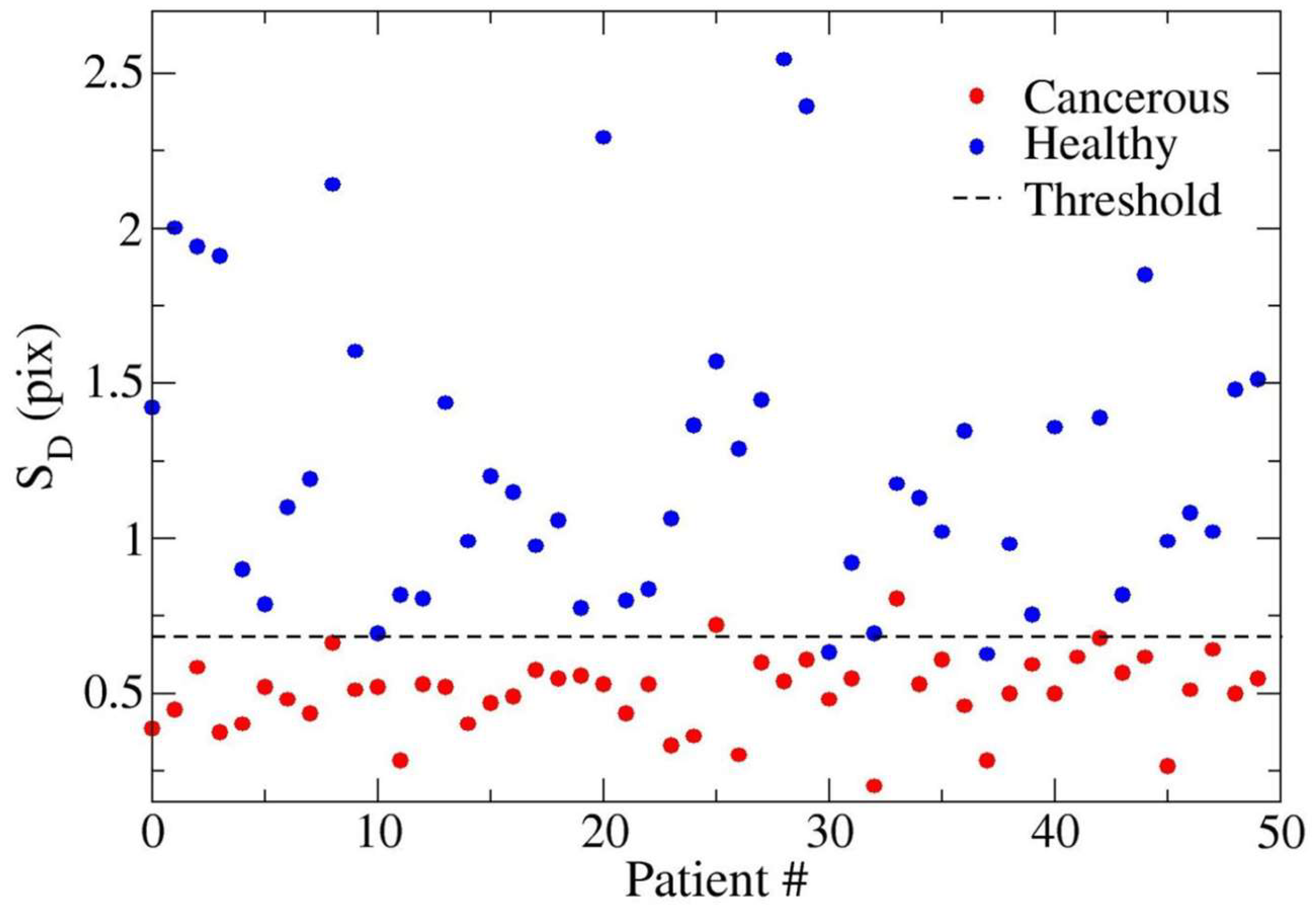
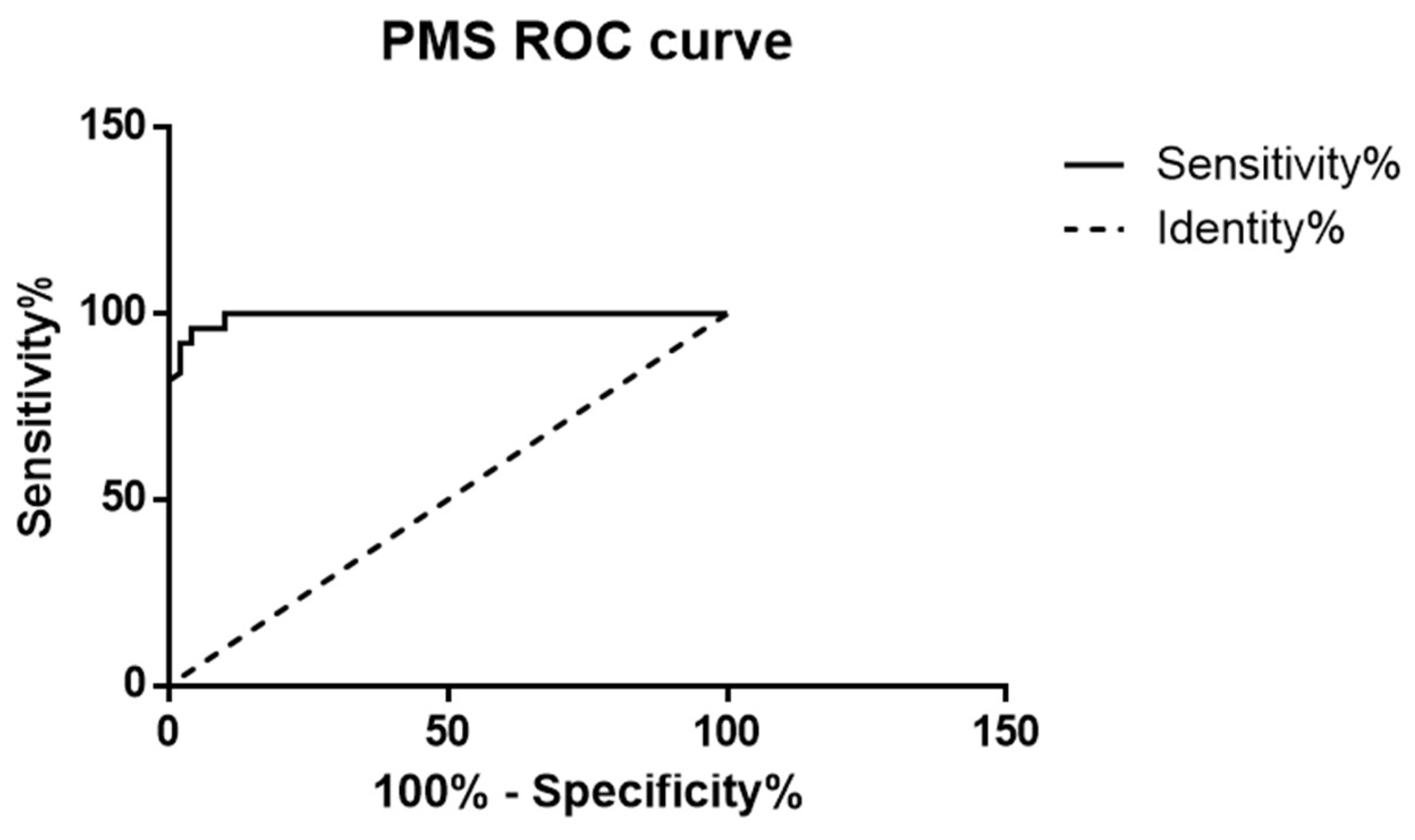
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prescott, J.W. Quantitative imaging biomarkers: The application of advanced image processing and analysis to clinical and preclinical decision making. J. Digit. Imaging 2013, 26, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Foran, D.J.; Chen, W.; Yang, L. Automated image interpretation and computer-assisted diagnostics. Stud. Health Technol. Inform. 2013, 185, 77–108. [Google Scholar] [CrossRef]
- Chan, H.-P.; Samala, R.K.; Hadjiiski, L.M.; Zhou, C. Deep Learning in Medical Image Analysis. Adv. Exp. Med. Biol. 2020, 1213, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Pickhardt, P.J.; Malecki, K.; Kloke, J.; Lubner, M.G. Accuracy of liver surface nodularity quantifcation on MDCT as a noninvasive biomarker for staging hepatic fibrosis. Am. J. Roentgenol. 2016, 207, 1194–1199. [Google Scholar] [CrossRef]
- Goshima, S.; Bae, K.T. Liver surface nodularity as a biomarker for detection and evaluation of cirrhosis. Radiology 2017, 283, 921–922. [Google Scholar] [CrossRef]
- Sartoris, R.; Rautou, P.E.; Elkrief, L.; Pollorsi, G.; Durand, F.; Valla, D.; Spahr, L.; Terraz, S.; Soubrane, O.; Cauchy, F.; et al. Quantification of liver surface nodularity at CT: Utility for detection of portal hypertension. Radiology 2018, 289, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Branch, C.R.; Zand, K.; Subramony, C.; Zhang, H.; Thaggard, K.; Hosch, R.; Bryan, J.; Vasanji, A.; Griswold, M.; et al. Liver surface nodularity quantification from routine ct images as a biomarker for detection and evaluation of cirrhosis. Radiology 2016, 280, 771–781. [Google Scholar] [CrossRef]
- Smith, A.D.; Zand, K.A.; Florez, E.; Sirous, R.; Shlapak, D.; Souza, F.; Roda, M.; Bryan, J.; Vasanji, A.; Griswold, M.; et al. Liver surface nodularity score allows Prediction of cirrhosis Decompensation and Death. Radiology 2017, 283, 711–722. [Google Scholar] [CrossRef]
- Hobeika, C.; Cauchy, F.; Sartoris, R.; Beaufrère, A.; Yoh, T.; Vilgrain, V.; Rautou, P.E.; Paradis, V.; Bouattour, M.; Ronot, M.; et al. Relevance of liver surface nodularity for preoperative risk assessment in patients with resectable hepatocellular carcinoma. Br. J. Surg. 2020, 107, 878–888. [Google Scholar] [CrossRef]
- Yoh, T.; Perrot, A.; Beaufrère, A.; Hobeika, C.; Sartoris, R.; Paradis, V.; Vilgrain, V.; Soubrane, O.; Cauchy, F.; Ronot, M. Liver surface nodularity: A novel predictor of post-hepatectomy liver failure in patients with colorectal liver metastases following chemotherapy. Eur. Radiol. 2021, 31, 5830–5839. [Google Scholar] [CrossRef]
- Dioguardi Burgio, M.; Sartoris, R.; Beaufrere, A.; Grégory, J.; Guiu, B.; Guillot, C.; Rautou, P.-E.; Castera, L.; Bouattour, M.; Paradis, V.; et al. Liver surface nodularity on non-contrast MRI identifies advanced fibrosis in patients with NAFLD. Eur. Radiol. 2022, 32, 1781–1791. [Google Scholar] [CrossRef]
- Lubner, M.G.; Jones, D.; Said, A.; Kloke, J.; Lee, S.; Pickhardt, P.J. Accuracy of liver surface nodularity quantification on MDCT for staging hepatic fibrosis in patients with hepatitis C virus. Abdom. Radiol. 2018, 43, 2980–2986. [Google Scholar] [CrossRef]
- Schattenberg, J.M.; Emrich, T. Refining imaging tools to detect advanced fibrosis: Could liver surface nodularity address an unmet need in the NAFLD epidemic? Eur. Radiol. 2022, 32, 1757–1759. [Google Scholar] [CrossRef]
- Souhami, A.; Sartoris, R.; Rautou, P.-E.; Cauchy, F.; Bouattour, M.; Durand, F.; Giannelli, V.; Gigante, E.; Castera, L.; Valla, D.; et al. Similar performance of liver stiffness measurement and liver surface nodularity for the detection of portal hypertension in patients with hepatocellular carcinoma. JHEP Rep. Innov. Hepatol. 2020, 2, 100147. [Google Scholar] [CrossRef]
- Elkassem, A.A.; Allen, B.C.; Lirette, S.T.; Cox, K.L.; Remer, E.M.; Pickhardt, P.J.; Lubner, M.G.; Sirlin, C.B.; Dondlinger, T.; Schmainda, M.; et al. Multiinstitutional Evaluation of the Liver Surface Nodularity Score on CT for Staging Liver Fibrosis and Predicting Liver-Related Events in Patients with Hepatitis C. AJR. Am. J. Roentgenol. 2022, 218, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Chen, J.; Zhou, J.; Jiang, Z.; Xiang, D.; Xing, W. Association between renal surface nodularity and increased adverse vascular event risk in patients with arterial hypertension. Clin. Exp. Hypertens. 2023, 45, 2228518. [Google Scholar] [CrossRef]
- Previtali, C.; Sartoris, R.; Dioguardi Burgio, M.; Couvelard, A.; Rebours, V.; Sauvanet, A.; Vilgrain, V.; Ronot, M. Quantitative imaging analysis predicts pancreatic fatty infltration on routine CT. Insights Imaging 2022, 13 (Suppl. S2), 24. [Google Scholar]
- Ambrosetti, M.C.; Grecchi, A.; Ambrosetti, A.; Amodio, A.; Mansueto, G.; Montemezzi, S.; Zamboni, G.A. Quantitative Edge Analysis of Pancreatic Margins in Patients with Chronic Pancreatitis: A Correlation with Exocrine Function. Diagnostics 2023, 13, 2272. [Google Scholar] [CrossRef]
- Ambrosetti, M.-C.; Ambrosetti, A.; Perri, G.; Gasparini, C.; Marchegiani, G.; Salvia, R.; Montemezzi, S.; Mansueto, G.; Zamboni, G.A. Quantitative edge analysis of pancreatic margins in patients with head pancreatic tumors: Correlations between pancreatic margins and the onset of postoperative pancreatic fistula. Eur. Radiol. 2024, 34, 1515–1523. [Google Scholar] [CrossRef]
- D’Onofrio, M.; Capelli, P.; Pederzoli, P. Imaging and Pathology of Pancreatic Neoplasms; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Kulkarni, N.M.; Hough, D.M.; Tolat, P.P.; Soloff, E.V.; Kambadakone, A.R. Pancreatic adenocarcinoma: Cross-sectional imaging techniques. Abdom. Radiol. 2018, 43, 253–263. [Google Scholar] [CrossRef]
- Brennan, D.D.D.; Zamboni, G.A.; Raptopoulos, V.D.; Kruskal, J.B. Comprehensive preoperative assessment of pancreatic adenocarcinoma with 64-section volumetric CT. Radiographics 2007, 27, 1653–1666. [Google Scholar] [CrossRef] [PubMed]
- Weikert, T.; Cyriac, J.; Yang, S.; Nesic, I.; Parmar, V.; Stieltjes, B. A Practical Guide to Artificial Intelligence–Based Image Analysis in Radiology. Investig. Radiol. 2019, 55, 1–7. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrosetti, M.C.; Ambrosetti, A.; Bariani, M.; Malleo, G.; Mansueto, G.; Zamboni, G.A. Quantitative Edge Analysis Can Differentiate Pancreatic Carcinoma from Normal Pancreatic Parenchyma. Diagnostics 2024, 14, 1681. https://doi.org/10.3390/diagnostics14151681
Ambrosetti MC, Ambrosetti A, Bariani M, Malleo G, Mansueto G, Zamboni GA. Quantitative Edge Analysis Can Differentiate Pancreatic Carcinoma from Normal Pancreatic Parenchyma. Diagnostics. 2024; 14(15):1681. https://doi.org/10.3390/diagnostics14151681
Chicago/Turabian StyleAmbrosetti, Maria Chiara, Alberto Ambrosetti, Matilde Bariani, Giuseppe Malleo, Giancarlo Mansueto, and Giulia A. Zamboni. 2024. "Quantitative Edge Analysis Can Differentiate Pancreatic Carcinoma from Normal Pancreatic Parenchyma" Diagnostics 14, no. 15: 1681. https://doi.org/10.3390/diagnostics14151681




