Activated Factor VII–Antithrombin Complex, a Biomarker of Tissue Factor-Related Pathways in Different Clinical Settings: A Narrative Review from Cardiovascular Diseases to Cancer
Abstract
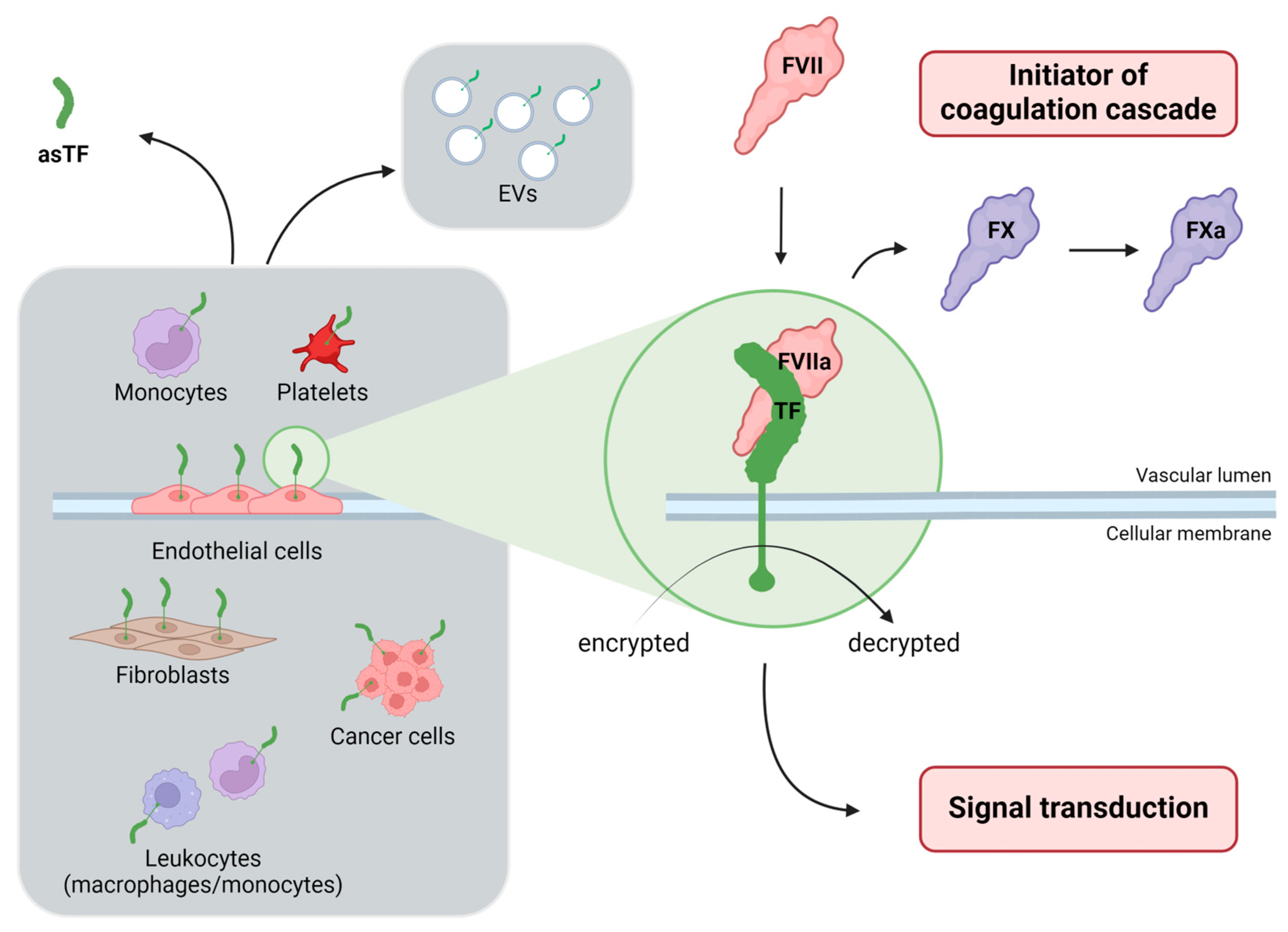
:1. The Crucial Roles of Tissue Factor: Haemostasis and Beyond
2. The Difficulty of Evaluating Tissue Factor Activity: Biomarkers Searching for an Elusive Molecule
3. Activated Factor VII-Antithrombin Complex as an Indirect Biomarker of Tissue Factor Pathway
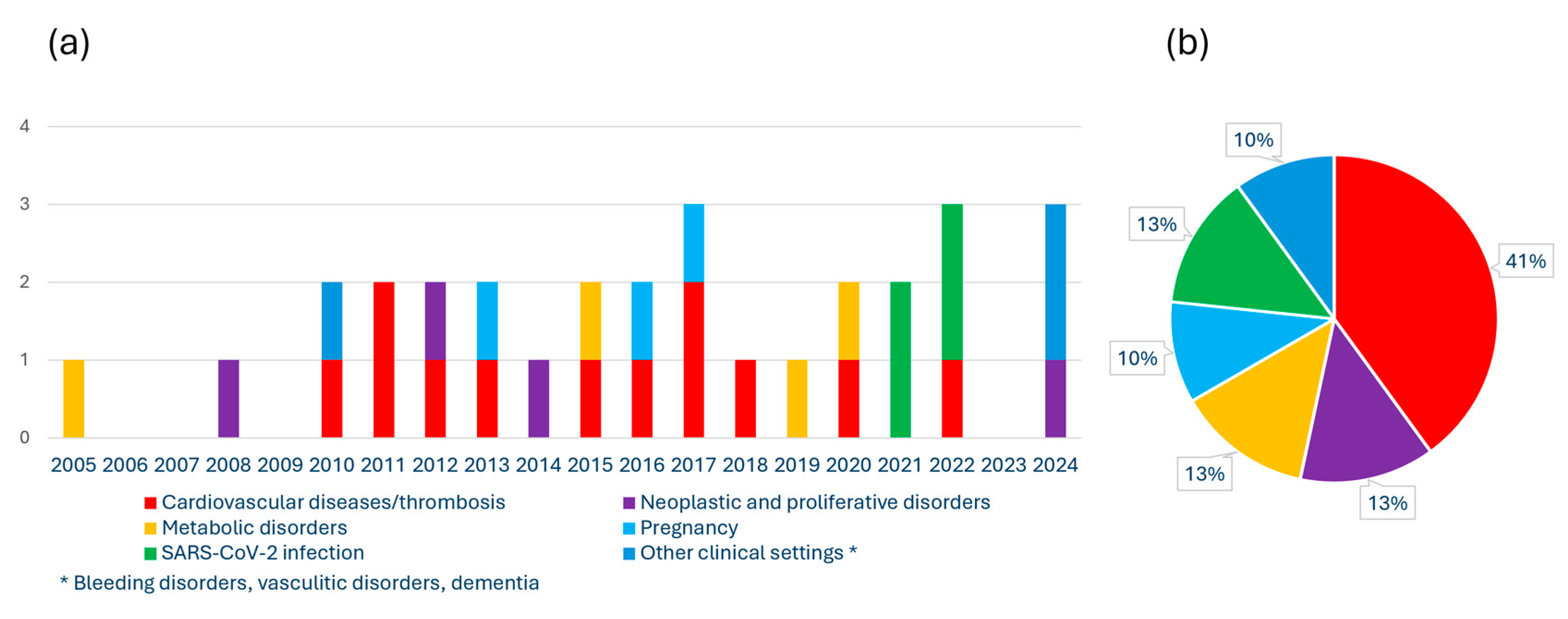
4. Plasma Concentration of Activated Factor VII-Antithrombin Complex in Different Clinical Settings in Humans
4.1. Thrombosis and Cardiovascular Disease
4.2. Neoplastic and Proliferative Disorders
4.3. Metabolic Disorders
4.4. SARS-CoV-2 Infection
| Thrombosis and Cardiovascular Disorders (CVD) | |||
| Type of Study | Study Population | FVIIa–AT-Related Main Results | Reference |
| Case-control study |
|
| Spiezia L et al., 2010 [25] |
| Case-control study |
|
| Spiezia L et al., 2011 [36] |
| Cohort study |
|
| Davidson SJ and Woodhams B, 2011 [35] |
| Case-control study and Cohort Study with nested case-control design | Stockholm Coronary Atherosclerosis Risk Factor (SCARF) study
Stockholm prospective study of 60-year-olds individuals
|
| Silveira A et al., 2012 [30] |
| Case-control study |
|
| Rossetto V et al., 2013 [28] |
| Case-control study |
| FVIIa–AT plasma concentration was measured using an in-house developed assay and was detected only in a minority of patients (n = 16, 11%) and controls (n = 58, 33%). In these individuals the related levels were substantially higher in patients with DVT than in controls. | Schut AM et al., 2015 [27] |
| Case-control study and Longitudinal Cohort study | Verona Heart Study (VHS)
|
| Martinelli N et al., 2016 [31] |
| Case-control study |
| FVIIa–AT levels were significantly lower in patients with IS than in controls. | Slomka A et al., 2017 [37] |
| Case-control study |
|
| Abu El-Makarem MA et al., 2017 [29] |
| Prospective Cohort studyGenome-wide association study (GWAS) of FVIIa and FVIIa–AT | Cardiovascular Health Study (CHS) in European-Americans adults aged 65-years and older
|
| Olson NC et al., 2018 [33] |
| Observational study |
|
| Baroni M et al., 2020 [32] |
| Longitudinal Cohort study |
|
| Paszek E et al., 2022 [34] |
| Neoplastic and Proliferative Disorders | |||
| Type of Study | Study Population | FVIIa–AT-Related Main Results | Reference |
| Case-control study |
|
| Negaard HFS et al., 2008 [38] |
| Retrospective case-control study |
|
| Spiezia L et al., 2012 [40] |
| Case-control study |
| FVIIa–AT levels were significantly elevated in patients with essential thrombocythemia, in particular those positive for the JAK2V617F mutation, as compared with controls. | Marchetti M et al., 2014 [39] |
| Case-control study and Longitudinal Cohort study |
|
| Martinelli N et al., 2024 [41] |
| Metabolic Disorders | |||
| Type of Study | Study Population | FVIIa–AT-Related Main Results | Reference |
| Experimental study |
|
| Johansson H et al., 2005 [45] |
| Case-control study |
|
| Campello E et al., 2015 [42] |
| Observational study | Verona Heart Study (VHS)
|
| Martinelli N et al., 2019 [44] |
| Controlled cross-over study | 20 obese patients, in whom high-fat and low-fat meals were served |
| Landgrebe LE et al., 2020 [43] |
| SARS-CoV-2 Infection | |||
| Type of Study | Study Population | FVIIa–AT-Related Main Results | Reference |
| Case-control study |
| FVIIa–AT levels were significantly higher in COVID-19 patients with severe disease than in controls, with intermediate levels in COVID-19 patients with moderate disease. | Francischetti IMB et al., 2021 [48] |
| Cohort study |
| FVIIa–AT plasma levels (measured using an in-house developed assay) were significantly decreased after vaccination. | Willems LH et al., 2021 [51] |
| Cross-sectional Cohort study |
| FVIIa–AT levels were increased in a third of the patients (35%) 6–20 weeks after recovery of acute COVID-19. | Willems LH et al., 2022 [50] |
| Case-control study |
| FVIIa–AT levels were higher in COVID-19 patients than in non-COVID-19 subjects, either with or without inflammation, while no difference was observed among non-COVID-19 subjects. | Martinelli N et al., 2022 [49] |
| Pregnancy and Preeclampsia | |||
| Type of Study | Study Population | FVIIa–AT-Related Main Results | Reference |
| Case-control study |
|
| Spiezia L. et al., 2013 [52] |
| Case-control study |
| FVIIa–AT levels were not different between severe preeclamptic and normotensive pregnant women. | Alpoim PN et al., 2016 [53] |
| Cohort study |
| Increased FVIIa–AT levels were found in early severe preeclampsia compared to late severe preeclampsia. | Dusse LMS et al., 2017 [54] |
| Other Clinical Settings | |||
| Type of Study | Study Population | FVIIa–AT-Related Main Results | Reference |
| Cohort study for a population pharmacokinetic model |
| The rFVII–AT complex formation was responsible for 65% of the total rFVIIa clearance. The formation of rFVIIa–AT complex was able to explain the difference observed between the rFVIIa:C and the rFVII:Ag concentration. | Agerso H et al., 2010 [55] |
| Longitudinal Cohort study |
|
| Busch MH et al., 2024 [56] |
| Prospective Cohort study | Cardiovascular Health Study (CHS) in adults—3185 older adults (aged 65-years and older) free of dementia in 1992/1993 | There was no evidence of linear associations between FVIIa–AT levels with any dementia risk. | Harrington LB et al., 2024 [57] |
4.5. Pregnancy and Preeclampsia
4.6. Other Clinical Settings
5. Correlations with Other Clinical and Laboratory Parameters
6. Strengths, Limitations, and Perspectives for Using FVIIa–AT to Explore the TF-Related Pathway
- (i)
- FVIIa–AT plasma levels can be informative on the global TF–FVIIa interaction but are unable to disentangle the different cellular origins of TF expression/activity (e.g., EVs, platelets, monocytes, activated endothelial cells, neoplastic cells, etc.) which may have significant pathophysiological and different clinical implications;
- (ii)
- The method for measuring FVIIa–AT complex concentration requires standardisation, for the pre-analytical and analytical phases, in order to ensure greater reproducibility and comparability of the results. Taking into account that activated platelets can express TF [60] and how platelets can be triggered/activated during blood drawn [61], the compliance with procedures to limit platelet activation during blood sampling and the appropriate preparation of platelet-poor plasma (PPP) samples would be crucial to avoid further confounding elements in FVIIa–AT evaluation;
- (iii)
- Up to now, all the studies in humans showed a large range of overall distribution of FVIIa–AT plasma levels, as well as a substantial overlap of values between pathological cases and healthy controls. Therefore, any potential for diagnostic sensitivity and specificity appears to be limited;
- (iv)
- The plasma levels of FVIIa–AT are strongly influenced by those of FVII/FVIIa, which have been indicated among the main determinants of FVIIa–AT concentration in many studies [25,31,33]. Consistently, several clinical conditions are recognized to be related to low levels of FVII/FVIIa by means of either reduced synthesis (e.g., genetic variants leading to low FVII expression, liver cirrhosis, VKAs therapy) or increased consumption (e.g., acute thrombosis, disseminated intravascular coagulation), and have been also associated with low levels of FVIIa–AT [25,28,29,30,33]. Such decrease, which is mediated predominantly by FVII levels (and not by TF–FVIIa interaction), could affect the value of the FVIIa–AT assay as an efficacious biomarker of TF activity and likely limits its prognostic role. For instance, in previous studies, FVIIa–AT discriminated between subjects with or without portal vein thrombosis only among non-cirrhotic subjects, while cirrhotic patients with or without portal vein thrombosis had similarly very low levels of FVIIa–AT [28,29];
- (v)
- Many traditional laboratory parameters, from renal function to plasma lipid profile, have been shown to predict FVIIa–AT variability, also consistently with the effects on FVII/FVIIa, thus raising the clinical suspicion that the FVIIa–AT-related prognostic role may be merely an epiphenomenon of the changes and imbalances of other traditional and crucial cardiovascular risk factors [31,43,44].

7. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mackman, N. Role of Tissue Factor in Hemostasis, Thrombosis, and Vascular Development. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1015–1022. [Google Scholar] [CrossRef]
- Butenas, S. Tissue Factor Structure and Function. Scientifica (Cairo) 2012, 2012, 964862. [Google Scholar] [CrossRef]
- Drake, T.A.; Morrissey, J.H.; Edgington, T.S. Selective Cellular Expression of Tissue Factor in Human Tissues. Implications for Disorders of Hemostasis and Thrombosis. Am. J. Pathol. 1989, 134, 1087–1097. [Google Scholar]
- Kaneko, T.; Fujii, S.; Matsumoto, A.; Goto, D.; Makita, N.; Hamada, J.; Moriuchi, T.; Kitabatake, A. Induction of Tissue Factor Expression in Endothelial Cells by Basic Fibroblast Growth Factor and Its Modulation by Fenofibric Acid. Thromb. J. 2003, 1, 6. [Google Scholar] [CrossRef]
- Steffel, J.; Lüscher, T.F.; Tanner, F.C. Tissue Factor in Cardiovascular Diseases. Circulation 2006, 113, 722–731. [Google Scholar] [CrossRef]
- Owens, A.P.; Mackman, N. Microparticles in Hemostasis and Thrombosis. Circ. Res. 2011, 108, 1284–1297. [Google Scholar] [CrossRef]
- Baroni, M.; Pizzirani, C.; Pinotti, M.; Ferrari, D.; Adinolfi, E.; Calzavarini, S.; Caruso, P.; Bernardi, F.; Di Virgilio, F. Stimulation of P2 (P2X 7) Receptors in Human Dendritic Cells Induces the Release of Tissue Factor-bearing Microparticles. FASEB J. 2007, 21, 1926–1933. [Google Scholar] [CrossRef]
- Koizume, S.; Miyagi, Y. Tissue Factor in Cancer-Associated Thromboembolism: Possible Mechanisms and Clinical Applications. Br. J. Cancer 2022, 127, 2099–2107. [Google Scholar] [CrossRef]
- Grover, S.P.; Mackman, N. Tissue Factor: An Essential Mediator of Hemostasis and Trigger of Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 709–725. [Google Scholar] [CrossRef]
- Bach, R.R. Tissue Factor Encryption. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 456–461. [Google Scholar] [CrossRef]
- Chen, V.M.; Hogg, P.J. Encryption and Decryption of Tissue Factor. J. Thromb. Haemost. 2013, 11, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Spiezia, L.; Campello, E.; Valle, F.D.; Woodhams, B.; Simioni, P. Factor VIIa-Antithrombin Complex: A Possible New Biomarker for Activated Coagulation. Clin. Chem. Lab. Med. 2017, 55, 484–488. [Google Scholar] [CrossRef]
- Bogdanov, V.Y.; Balasubramanian, V.; Hathcock, J.; Vele, O.; Lieb, M.; Nemerson, Y. Alternatively Spliced Human Tissue Factor: A Circulating, Soluble, Thrombogenic Protein. Nat. Med. 2003, 9, 458–462. [Google Scholar] [CrossRef]
- Böing, A.N.; Hau, C.M.; Sturk, A.; Nieuwland, R. Human Alternatively Spliced Tissue Factor Is Not Secreted and Does Not Trigger Coagulation. J. Thromb. Haemost. 2009, 7, 1423–1426. [Google Scholar] [CrossRef]
- van den Berg, Y.W.; van den Hengel, L.G.; Myers, H.R.; Ayachi, O.; Jordanova, E.; Ruf, W.; Spek, C.A.; Reitsma, P.H.; Bogdanov, V.Y.; Versteeg, H.H. Alternatively Spliced Tissue Factor Induces Angiogenesis through Integrin Ligation. Proc. Natl. Acad. Sci. USA 2009, 106, 19497–19502. [Google Scholar] [CrossRef] [PubMed]
- Kocatürk, B.; Van den Berg, Y.W.; Tieken, C.; Mieog, J.S.D.; de Kruijf, E.M.; Engels, C.C.; van der Ent, M.A.; Kuppen, P.J.; Van de Velde, C.J.; Ruf, W.; et al. Alternatively Spliced Tissue Factor Promotes Breast Cancer Growth in a Β1 Integrin-Dependent Manner. Proc. Natl. Acad. Sci. USA 2013, 110, 11517–11522. [Google Scholar] [CrossRef]
- Tavares, V.; Neto, B.V.; Marques, I.S.; Assis, J.; Pereira, D.; Medeiros, R. Cancer-Associated Thrombosis: What about MicroRNAs Targeting the Tissue Factor Coagulation Pathway? Biochim. Biophys. Acta (BBA)-Rev. Cancer 2024, 1879, 189053. [Google Scholar] [CrossRef]
- Morelli, V.M.; Snir, O.; Hindberg, K.D.; Hveem, K.; Brækkan, S.K.; Hansen, J.-B. High MicroRNA-145 Plasma Levels Are Associated with Decreased Risk of Future Incident Venous Thromboembolism: The HUNT Study. Blood 2024, 143, 1773–1781. [Google Scholar] [CrossRef]
- Bogdanov, V.Y.; Versteeg, H.H. “Soluble Tissue Factor” in the 21st Century: Definitions, Biochemistry, and Pathophysiological Role in Thrombus Formation. Semin. Thromb. Hemost. 2015, 41, 700–707. [Google Scholar] [CrossRef]
- Mackman, N.; Sachetto, A.T.A.; Hisada, Y. Measurement of Tissue Factor-Positive Extracellular Vesicles in Plasma: Strengths and Weaknesses of Current Methods. Curr. Opin. Hematol. 2022, 29, 266–274. [Google Scholar] [CrossRef]
- Godal, H.C.; Rygh, M.; Laake, K. Progressive Inactivation of Purified Factor VII by Heparin and Antithrombin III. Thromb. Res. 1974, 5, 773–775. [Google Scholar] [CrossRef]
- Lawson, J.H.; Butenas, S.; Ribarik, N.; Mann, K.G. Complex-Dependent Inhibition of Factor VIIa by Antithrombin III and Heparin. J. Biol. Chem. 1993, 268, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.V.; Nordfang, O.; Hoang, A.D.; Pendurthi, U.R. Mechanism of Antithrombin III Inhibition of Factor VIIa/Tissue Factor Activity on Cell Surfaces. Comparison with Tissue Factor Pathway Inhibitor/Factor Xa-Induced Inhibition of Factor VIIa/Tissue Factor Activity. Blood 1995, 85, 121–129. [Google Scholar] [CrossRef]
- Morrissey, J.H. Assay for Measuring Factor VIIa-Antithrombin Complexes. WO 03/004694, 16 January 2003. [Google Scholar]
- Spiezia, L.; Rossetto, V.; Campello, E.; Gavasso, S.; Woodhams, B.; Tormene, D.; Simioni, P. Factor VIIa-Antithrombin Complexes in Patients with Arterial and Venous Thrombosis. Thromb. Haemost. 2010, 103, 1188–1192. [Google Scholar] [CrossRef]
- Scharling, B.; Nielsen, G.G.; Klitgaard, T.; Skovsted, T.A.; Møss, J.; Segel, S.; Larsen, L.F. Comparison of Coagulant Activity of Factor VII and Activated Factor VII Activity Assays When Used for Determination of Recombinant Activated Factor VII Levels in Plasma. Blood Coagul. Fibrinolysis 2007, 18, 677–684. [Google Scholar] [CrossRef]
- Schut, A.M.; Meijers, J.C.M.; Lisman-van Leeuwen, Y.; van Montfoort, M.L.; Roest, M.; de Groot, P.G.; Urbanus, R.T.; Coppens, M.; Lisman, T. Decreased Plasma Levels of Activated Factor VII in Patients with Deep Vein Thrombosis. J. Thromb. Haemost. 2015, 13, 1320–1324. [Google Scholar] [CrossRef]
- Rossetto, V.; Spiezia, L.; Senzolo, M.; Rodriguez, K.; Gavasso, S.; Woodhams, B.; Simioni, P. Factor VIIa-Antithrombin Complexes in Patients with Non-Neoplastic Portal Vein Thrombosis with and without Cirrhosis. Int. J. Lab. Hematol. 2013, 35, 101–105. [Google Scholar] [CrossRef]
- Abu El-Makarem, M.A.; El-Akad, A.F.; Elian, M.M.; Sherif, T.M.; El-shaheed, R.A.; Abd EL Fatah, A.S.; Sayed, D.M.; Bakry, R.M.; Mahmoud, A.M. Non-Neoplastic Portal Vein Thrombosis in HCV Cirrhosis Patients: Is It an Immuno-Inflammatory Disorder? Ann Hepatol 2017, 16, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Silveira, A.; Scanavini, D.; Boquist, S.; Ericsson, C.-G.; Hellénius, M.-L.; Leander, K.; de Faire, U.; Ohrvik, J.; Woodhams, B.; Morrissey, J.H.; et al. Relationships of Plasma Factor VIIa-Antithrombin Complexes to Manifest and Future Cardiovascular Disease. Thromb. Res. 2012, 130, 221–225. [Google Scholar] [CrossRef]
- Martinelli, N.; Girelli, D.; Baroni, M.; Guarini, P.; Sandri, M.; Lunghi, B.; Tosi, F.; Branchini, A.; Sartori, F.; Woodhams, B.; et al. Activated Factor VII-Antithrombin Complex Predicts Mortality in Patients with Stable Coronary Artery Disease: A Cohort Study. J. Thromb. Haemost. 2016, 14, 655–666. [Google Scholar] [CrossRef]
- Baroni, M.; Martinelli, N.; Lunghi, B.; Marchetti, G.; Castagna, A.; Stefanoni, F.; Pinotti, M.; Woodhams, B.; Olivieri, O.; Bernardi, F. Aptamer-Modified FXa Generation Assays to Investigate Hypercoagulability in Plasma from Patients with Ischemic Heart Disease. Thromb. Res. 2020, 189, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Olson, N.C.; Raffield, L.M.; Lange, L.A.; Lange, E.M.; Longstreth, W.T.; Chauhan, G.; Debette, S.; Seshadri, S.; Reiner, A.P.; Tracy, R.P. Associations of Activated Coagulation Factor VII and Factor VIIa-Antithrombin Levels with Genome-Wide Polymorphisms and Cardiovascular Disease Risk. J. Thromb. Haemost. 2018, 16, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Paszek, E.; Pociask, E.; Ząbczyk, M.; Butenas, S.; Undas, A. Activated Factor XI Is Associated with Increased Factor VIIa—Antithrombin Complexes in Stable Coronary Artery Disease: Impact on Cardiovascular Outcomes. Eur. J. Clin. Investig. 2022, 52, e13857. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.J.; Woodhams, B. Factor VIIa-Antithrombin Complexes during Cardiac Surgery Using Cardiopulmonary Bypass. Int. J. Lab. Hematol. 2011, 33, 614–616. [Google Scholar] [CrossRef] [PubMed]
- Spiezia, L.; Campello, E.; Gentilomo, C.; Gavasso, S.; Woodhams, B.; Laverda, A.M.; Simioni, P. Factor VIIa-Antithrombin Complexes in Children with Ischemic Stroke. Thromb. Res. 2011, 128, 303–304. [Google Scholar] [CrossRef]
- Słomka, A.; Świtońska, M.; Sinkiewicz, W.; Żekanowska, E. Assessing Circulating Factor VIIa-Antithrombin Complexes in Acute Ischemic Stroke: A Pilot Study. Clin. Appl. Thromb. Hemost. 2017, 23, 351–359. [Google Scholar] [CrossRef]
- Negaard, H.F.S.; Iversen, P.O.; Østenstad, B.; Iversen, N.; Holme, P.A.; Sandset, P.M. Hypercoagulability in Patients with Haematological Neoplasia: No Apparent Initiation by Tissue Factor. Thromb. Haemost. 2008, 99, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, M.; Tartari, C.J.; Russo, L.; Panova-Noeva, M.; Leuzzi, A.; Rambaldi, A.; Finazzi, G.; Woodhams, B.; Falanga, A. Phospholipid-Dependent Procoagulant Activity Is Highly Expressed by Circulating Microparticles in Patients with Essential Thrombocythemia. Am. J. Hematol. 2014, 89, 68–73. [Google Scholar] [CrossRef]
- Spiezia, L.; Campello, E.; Bon, M.; Gavasso, S.; Woodhams, B.; Simioni, P. Factor VIIa-Antithrombin Complexes Plasma Levels in Cancer Patients with and without Thrombosis. Thromb. Res. 2012, 129, 818–819. [Google Scholar] [CrossRef]
- Martinelli, N.; Moruzzi, S.; Udali, S.; Castagna, A.; Di Santo, L.; Ambrosani, F.; Baroni, M.; Pattini, P.; Pizzolo, F.; Ruzzenente, A.; et al. Tissue Factor Pathway-Related Biomarkers in Liver Cancer: Activated Factor VII-Antithrombin Complex and Tissue Factor MRNA Levels Are Associated with Mortality. Res. Pract. Thromb. Haemost. 2024, 8, 102310. [Google Scholar] [CrossRef]
- Campello, E.; Zabeo, E.; Radu, C.M.; Spiezia, L.; Gavasso, S.; Fadin, M.; Woodhams, B.; Vettor, R.; Simioni, P. Hypercoagulability in Overweight and Obese Subjects Who Are Asymptomatic for Thrombotic Events. Thromb. Haemost. 2015, 113, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Landgrebe, L.E.; Juhl, C.B.; Andersen, V.; Moitinho-Silva, L.; Bang, C.; Bladbjerg, E.M. Postprandial Factor VII Activation Does Not Increase Plasma Concentrations of Prothrombin Fragment 1 + 2 in Patients with Morbid Obesity. Thromb. Res. 2020, 196, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, N.; Baroni, M.; Castagna, A.; Lunghi, B.; Stefanoni, F.; Tosi, F.; Croce, J.; Udali, S.; Woodhams, B.; Girelli, D.; et al. Apolipoprotein C-III Strongly Correlates with Activated Factor VII-Anti-Thrombin Complex: An Additional Link between Plasma Lipids and Coagulation. Thromb. Haemost. 2019, 119, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.; Lukinius, A.; Moberg, L.; Lundgren, T.; Berne, C.; Foss, A.; Felldin, M.; Källen, R.; Salmela, K.; Tibell, A.; et al. Tissue Factor Produced by the Endocrine Cells of the Islets of Langerhans Is Associated with a Negative Outcome of Clinical Islet Transplantation. Diabetes 2005, 54, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Sachetto, A.T.A.; Mackman, N. Monocyte Tissue Factor Expression: Lipopolysaccharide Induction and Roles in Pathological Activation of Coagulation. Thromb. Haemost. 2023, 123, 1017–1033. [Google Scholar] [CrossRef] [PubMed]
- Lehner, G.F.; Tobiasch, A.K.; Perschinka, F.; Mayerhöfer, T.; Waditzer, M.; Haller, V.; Zassler, B.; Maier, S.; Ulmer, H.; Joannidis, M. Associations of Tissue Factor and Tissue Factor Pathway Inhibitor with Organ Dysfunctions in Septic Shock. Sci. Rep. 2024, 14, 14468. [Google Scholar] [CrossRef]
- Francischetti, I.M.B.; Toomer, K.; Zhang, Y.; Jani, J.; Siddiqui, Z.; Brotman, D.J.; Hooper, J.E.; Kickler, T.S. Upregulation of Pulmonary Tissue Factor, Loss of Thrombomodulin and Immunothrombosis in SARS-CoV-2 Infection. EClinicalMedicine 2021, 39, 101069. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, N.; Rigoni, A.M.; De Marchi, S.; Osti, N.; Donini, M.; Montagnana, M.; Castagna, A.; Pattini, P.; Udali, S.; De Franceschi, L.; et al. High Plasma Levels of Activated Factor VII-Antithrombin Complex Point to Increased Tissue Factor Expression in Patients with SARS-CoV-2 Pneumonia: A Potential Link with COVID-19 Prothrombotic Diathesis. Diagnostics 2022, 12, 2792. [Google Scholar] [CrossRef]
- Willems, L.H.; Nagy, M.; Ten Cate, H.; Spronk, H.M.H.; Groh, L.A.; Leentjens, J.; Janssen, N.A.F.; Netea, M.G.; Thijssen, D.H.J.; Hannink, G.; et al. Sustained Inflammation, Coagulation Activation and Elevated Endothelin-1 Levels without Macrovascular Dysfunction at 3 Months after COVID-19. Thromb. Res. 2022, 209, 106–114. [Google Scholar] [CrossRef]
- Willems, L.H.; Nagy, M.; Ten Cate, H.; Spronk, H.M.H.; Jacobs, L.M.C.; Kranendonk, J.; van Leeuwen, M.; Meijer, D.; Middeldorp, S.; Groh, L.A.; et al. ChAdOx1 Vaccination, Blood Coagulation, and Inflammation: No Effect on Coagulation but Increased Interleukin-6. Res. Pr. Thromb. Haemost. 2021, 5, e12630. [Google Scholar] [CrossRef]
- Spiezia, L.; Visentin, S.; Radu, C.; Bon, M.; Woodhams, B.; Cosmi, E.; Simioni, P. Association between Increased FVIIa-Antithrombin Complex/FVIIa Ratio and Pre-Eclampsia. J. Matern. Fetal Neonatal Med. 2013, 26, 1352–1354. [Google Scholar] [CrossRef]
- Alpoim, P.N.; Godoi, L.C.; Pinheiro, M.D.B.; Freitas, L.G.; Carvalho, M.d.G.; Dusse, L.M. The Unexpected Beneficial Role of Smoking in Preeclampsia. Clin. Chim. Acta 2016, 459, 105–108. [Google Scholar] [CrossRef]
- Dusse, L.M.S.; Godoi, L.C.; Alpoim, P.N.; Gomes, K.B.; Sousa, L.P.; Perucci, L.O.; Lwaleed, B.; Carvalho, M.G. FVIIa-Antithrombin Levels in Early and Late Preeclampsia. Clin. Chim. Acta 2017, 474, 67–69. [Google Scholar] [CrossRef]
- Agersø, H.; Brophy, D.F.; Pelzer, H.; Martin, E.J.; Carr, M.; Hedner, U.; Ezban, M. Recombinant Human Factor VIIa (RFVIIa) Cleared Principally by Antithrombin Following Intravenous Administration in Hemophilia Patients. J. Thromb. Haemost. 2011, 9, 333–338. [Google Scholar] [CrossRef]
- Busch, M.H.; Ysermans, R.; Aendekerk, J.P.; Timmermans, S.A.M.E.G.; Potjewijd, J.; Damoiseaux, J.G.M.C.; Spronk, H.M.H.; Ten Cate, H.; Reutelingsperger, C.P.; Nagy, M.; et al. The Intrinsic Coagulation Pathway Plays a Dominant Role in Driving Hypercoagulability in ANCA-Associated Vasculitis. Blood Adv. 2024, 8, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Harrington, L.B.; Ehlert, A.N.; Thacker, E.L.; Jenny, N.S.; Lopez, O.; Cushman, M.; Olson, N.C.; Fitzpatrick, A.; Mukamal, K.J.; Jensen, M.K. Levels of Procoagulant Factors and Peak Thrombin Generation in Relation to Dementia Risk in Older Adults: The Cardiovascular Health Study. Thromb. Res. 2024, 235, 148–154. [Google Scholar] [CrossRef]
- Huang, M.-J.; Wei, R.; Wang, Y.; Su, T.; Di, P.; Li, Q.; Yang, X.; Li, P.; Chen, X. Blood Coagulation System in Patients with Chronic Kidney Disease: A Prospective Observational Study. BMJ Open 2017, 7, e014294. [Google Scholar] [CrossRef] [PubMed]
- Fazavana, J.G.; Muczynski, V.; Proulle, V.; Wohner, N.; Christophe, O.D.; Lenting, P.J.; Denis, C.V. LDL Receptor-related Protein 1 Contributes to the Clearance of the Activated Factor VII–Antithrombin Complex. J. Thromb. Haemost. 2016, 14, 2458–2470. [Google Scholar] [CrossRef]
- Brambilla, M.; Becchetti, A.; Rovati, G.E.; Cosentino, N.; Conti, M.; Canzano, P.; Giesen, P.L.A.; Loffreda, A.; Bonomi, A.; Cattaneo, M.; et al. Cell Surface Platelet Tissue Factor Expression: Regulation by P2Y 12 and Link to Residual Platelet Reactivity. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 2042–2057. [Google Scholar] [CrossRef]
- Mani, H.; Kirchmayr, K.; Kläffling, C.; Schindewolf, M.; Luxembourg, B.; Linnemann, B.; Lindhoff-Last, E. Influence of Blood Collection Techniques on Platelet Function. Platelets 2004, 15, 315–318. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Dagenais, G.R.; Hart, R.G.; Shestakovska, O.; Diaz, R.; Alings, M.; Lonn, E.M.; Anand, S.S.; et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Versteeg, H.H.; Schaffner, F.; Kerver, M.; Petersen, H.H.; Ahamed, J.; Felding-Habermann, B.; Takada, Y.; Mueller, B.M.; Ruf, W. Inhibition of Tissue Factor Signaling Suppresses Tumor Growth. Blood 2008, 111, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Graf, C.; Wilgenbus, P.; Pagel, S.; Pott, J.; Marini, F.; Reyda, S.; Kitano, M.; Macher-Göppinger, S.; Weiler, H.; Ruf, W. Myeloid Cell–Synthesized Coagulation Factor X Dampens Antitumor Immunity. Sci. Immunol. 2019, 4, eaaw8405. [Google Scholar] [CrossRef] [PubMed]
- Haist, M.; Stege, H.; Pemler, S.; Heinz, J.; Fleischer, M.I.; Graf, C.; Ruf, W.; Loquai, C.; Grabbe, S. Anticoagulation with Factor Xa Inhibitors Is Associated with Improved Overall Response and Progression-Free Survival in Patients with Metastatic Malignant Melanoma Receiving Immune Checkpoint Inhibitors—A Retrospective, Real-World Cohort Study. Cancers 2021, 13, 5103. [Google Scholar] [CrossRef]
- Coleman, R.L.; Lorusso, D.; Gennigens, C.; González-Martín, A.; Randall, L.; Cibula, D.; Lund, B.; Woelber, L.; Pignata, S.; Forget, F.; et al. Efficacy and Safety of Tisotumab Vedotin in Previously Treated Recurrent or Metastatic Cervical Cancer (InnovaTV 204/GOG-3023/ENGOT-Cx6): A Multicentre, Open-Label, Single-Arm, Phase 2 Study. Lancet Oncol. 2021, 22, 609–619. [Google Scholar] [CrossRef]
- Heitz, N.; Greer, S.C.; Halford, Z. A Review of Tisotumab Vedotin-Tftv in Recurrent or Metastatic Cervical Cancer. Ann. Pharmacother. 2023, 57, 585–596. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moruzzi, S.; Castagna, A.; Spizzo, M.; Udali, S.; Pattini, P.; Pizzolo, F.; Friso, S.; Martinelli, N. Activated Factor VII–Antithrombin Complex, a Biomarker of Tissue Factor-Related Pathways in Different Clinical Settings: A Narrative Review from Cardiovascular Diseases to Cancer. Diagnostics 2024, 14, 1711. https://doi.org/10.3390/diagnostics14161711
Moruzzi S, Castagna A, Spizzo M, Udali S, Pattini P, Pizzolo F, Friso S, Martinelli N. Activated Factor VII–Antithrombin Complex, a Biomarker of Tissue Factor-Related Pathways in Different Clinical Settings: A Narrative Review from Cardiovascular Diseases to Cancer. Diagnostics. 2024; 14(16):1711. https://doi.org/10.3390/diagnostics14161711
Chicago/Turabian StyleMoruzzi, Sara, Annalisa Castagna, Marianna Spizzo, Silvia Udali, Patrizia Pattini, Francesca Pizzolo, Simonetta Friso, and Nicola Martinelli. 2024. "Activated Factor VII–Antithrombin Complex, a Biomarker of Tissue Factor-Related Pathways in Different Clinical Settings: A Narrative Review from Cardiovascular Diseases to Cancer" Diagnostics 14, no. 16: 1711. https://doi.org/10.3390/diagnostics14161711
APA StyleMoruzzi, S., Castagna, A., Spizzo, M., Udali, S., Pattini, P., Pizzolo, F., Friso, S., & Martinelli, N. (2024). Activated Factor VII–Antithrombin Complex, a Biomarker of Tissue Factor-Related Pathways in Different Clinical Settings: A Narrative Review from Cardiovascular Diseases to Cancer. Diagnostics, 14(16), 1711. https://doi.org/10.3390/diagnostics14161711






