Adropin Predicts Asymptomatic Heart Failure in Patients with Type 2 Diabetes Mellitus Independent of the Levels of Natriuretic Peptides
Abstract
1. Introduction
2. Materials and Methods
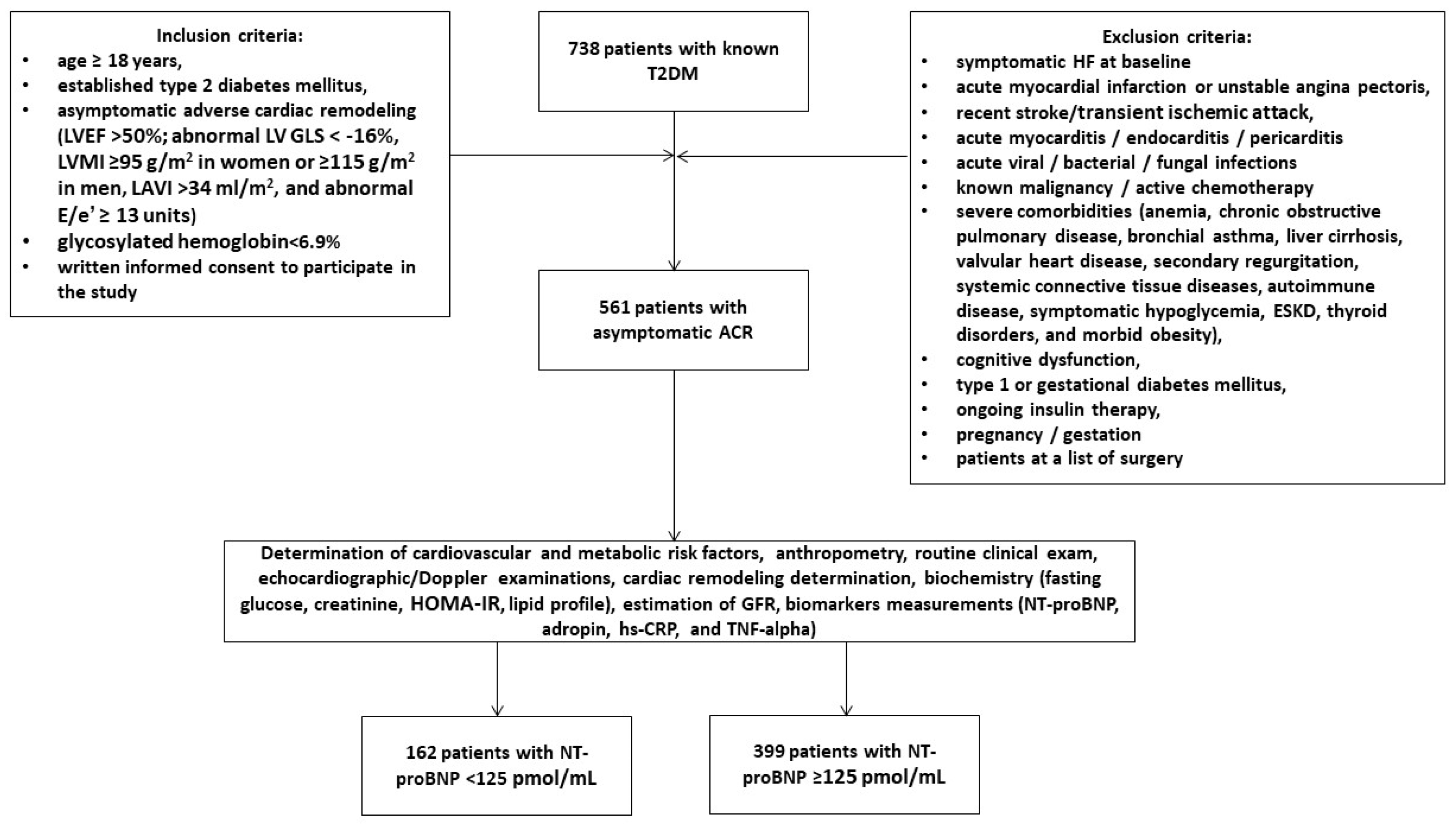
2.1. Patient Population and Study Design
2.2. Collection of Relevant Medical Data and Background Information
2.3. Echocardiographic Examination
2.4. Blood Sampling and Biomarker Analysis
2.5. Glomerular Filtration Rate and Insulin Resistance Determination
2.6. Statistics
3. Results
3.1. Spearman’s Correlations between the Levels of Biomarkers and Other Parameters
3.2. Predictors of Asymptomatic HFpEF: Univariate and Multivariate Logistic Regression Analyses
3.3. Comparison of the Predictive Models
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kosmala, W.; Marwick, T.H. Asymptomatic Left Ventricular Diastolic Dysfunction: Predicting Progression to Symptomatic Heart Failure. JACC Cardiovasc. Imaging 2020, 13 Pt 2, 215–227. [Google Scholar] [CrossRef]
- Coller, J.M.; Gong, F.F.; McGrady, M.; Shiel, L.; Liew, D.; Stewart, S.; Owen, A.J.; Krum, H.; Reid, C.M.; Prior, D.L.; et al. Risk factors for asymptomatic echocardiographic abnormalities that predict symptomatic heart failure. ESC Heart Fail. 2022, 9, 196–212. [Google Scholar] [CrossRef] [PubMed]
- Echouffo-Tcheugui, J.B.; Erqou, S.; Butler, J.; Yancy, C.W.; Fonarow, G.C. Assessing the Risk of Progression from Asymptomatic Left Ventricular Dysfunction to Overt Heart Failure: A Systematic Overview and Meta-Analysis. JACC Heart Fail. 2016, 4, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, e263–e421. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diag-nosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Kobayashi, M.; Huttin, O.; Magnusson, M.; Ferreira, J.P.; Bozec, E.; Huby, A.C.; Preud’homme, G.; Duarte, K.; Lamiral, Z.; Dalleau, K.; et al. Machine Learning-Derived Echocardiographic Phenotypes Predict Heart Failure Incidence in Asymptomatic Individuals. JACC Cardiovasc. Imaging 2022, 15, 193–208. [Google Scholar] [CrossRef]
- Coller, J.M.; Campbell, D.J.; Krum, H.; Prior, D.L. Early identification of asymptomatic subjects at increased risk of heart failure and cardiovascular events: Progress and future directions. Heart Lung Circ. 2013, 22, 171–178. [Google Scholar] [CrossRef]
- Souffront, K.; PNelson, B.; Lukas, M.; Reyes Garay, H.; Gordon, L.; Matos, T.; Hanesworth, I.; Mantel, R.; Shubeck, C.; Bernstein, C.; et al. Stage B Heart Failure Is Ubiquitous in Emergency Patients with Asymptomatic Hypertension. West. J. Emerg. Med. 2024, 25, 160–165. [Google Scholar] [CrossRef]
- Bayes-Genis, A.; Docherty, K.F.; Petrie, M.C.; Januzzi, J.L.; Mueller, C.; Anderson, L.; Bozkurt, B.; Butler, J.; Chioncel, O.; Cleland, J.G.F.; et al. Practical algorithms for early diagnosis of heart failure and heart stress using NT-proBNP: A clinical consensus statement from the Heart Failure Association of the ESC. Eur. J. Heart Fail. 2023, 25, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.M.; Morrow, D.A.; Bellavia, A.; Berg, D.D.; Bhatt, D.L.; Jarolim, P.; Leiter, L.A.; McGuire, D.K.; Raz, I.; Steg, P.G.; et al. Natriuretic peptides, body mass index and heart failure risk: Pooled analyses of SAVOR-TIMI 53, DECLARE-TIMI 58 and CAMELLIA-TIMI 61. Eur. J. Heart Fail. 2024, 26, 260–269. [Google Scholar] [CrossRef]
- Nyberg, M.; Terzic, D.; Ludvigsen, T.P.; Mark, P.D.; Michaelsen, N.B.; Abildstrøm, S.Z.; Engelmann, M.; Richards, A.M.; Goetze, J.P. A State of Natriuretic Peptide Deficiency. Endocr. Rev. 2023, 44, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.G.; Trevaskis, J.L.; Lam, D.D.; Sutton, G.M.; Koza, R.A.; Chouljenko, V.N.; Kousoulas, K.G.; Rogers, P.M.; Kesterson, R.A.; Thearle, M.; et al. Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab. 2008, 8, 468–481. [Google Scholar] [CrossRef]
- Thapa, D.; Xie, B.; Zhang, M.; Stoner, M.W.; Manning, J.R.; Huckestein, B.R.; Edmunds, L.R.; Mullett, S.J.; McTiernan, C.F.; Wendell, S.G.; et al. Adropin treatment restores cardiac glucose oxidation in pre-diabetic obese mice. J. Mol. Cell. Cardiol. 2019, 129, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Altamimi, T.R.; Gao, S.; Karwi, Q.G.; Fukushima, A.; Rawat, S.; Wagg, C.S.; Zhang, L.; Lopaschuk, G.D. Adropin regulates cardiac energy metabolism and improves cardiac function and efficiency. Metabolism 2019, 98, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.M.; Yosten, G.L.; Samson, W.K. Adropin acts in brain to inhibit water drinking: Potential interaction with the orphan G protein-coupled receptor, GPR19. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R476–R480. [Google Scholar] [CrossRef] [PubMed]
- Thapa, D.; Stoner, M.W.; Zhang, M.; Xie, B.; Manning, J.R.; Guimaraes, D.; Shiva, S.; Jurczak, M.J.; Scott, I. Adropin regulates pyruvate dehydrogenase in cardiac cells via a novel GPCR-MAPK-PDK4 signaling pathway. Redox Biol. 2018, 18, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Ying, T.; Wu, L.; Lan, T.; Wei, Z.; Hu, D.; Ke, Y.; Jiang, Q.; Fang, J. Adropin inhibits the progression of atherosclerosis in ApoE-/-/Enho-/- mice by regulating endothelial-to-mesenchymal transition. Cell Death Discov. 2023, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zeng, K.; Liu, Q.C.; Guo, Z.; Zhang, S.; Chen, X.R.; Lin, J.H.; Wen, J.P.; Zhao, C.F.; Lin, X.H.; et al. Adropin deficiency worsens HFD-induced metabolic defects. Cell Death Dis. 2017, 8, e3008. [Google Scholar] [CrossRef] [PubMed]
- Gunraj, R.E.; Yang, C.; Liu, L.; Larochelle, J.; Candelario-Jalil, E. Protective roles of adropin in neurological disease. Am. J. Physiol. Cell Physiol. 2023, 324, C674–C678. [Google Scholar] [CrossRef]
- Fujie, S.; Hasegawa, N.; Horii, N.; Uchida, M.; Sanada, K.; Hamaoka, T.; Padilla, J.; Martinez-Lemus, L.A.; Maeda, S.; Iemitsu, M. Aerobic Exercise Restores Aging-Associated Reductions in Arterial Adropin Levels and Improves Adropin-Induced Nitric Oxide-Dependent Vasorelaxation. J. Am. Heart Assoc. 2021, 10, e020641. [Google Scholar] [CrossRef]
- Altincik, A.; Sayin, O. Evaluation of the relationship between serum adropin levels and blood pressure in obese children. J. Pediatr. Endocrinol. Metab. 2015, 28, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Kolahdouz-Mohammadi, R.; Aydin, S.; Yosaee, S.; Clark, C.C.T.; Abdollahi, S. Circulating levels of adropin and overweight/obesity: A systematic review and meta-analysis of observational studies. Hormones 2022, 21, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Oruc, C.U.; Akpinar, Y.E.; Dervisoglu, E.; Amikishiyev, S.; Salmaslıoglu, A.; Gurdol, F.; Omer, B. Low concentrations of adropin are associated with endothelial dysfunction as assessed by flow-mediated dilatation in patients with metabolic syndrome. Clin. Chem. Lab. Med. 2017, 55, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Jurrissen, T.J.; Ramirez-Perez, F.I.; Cabral-Amador, F.J.; Soares, R.N.; Pettit-Mee, R.J.; Betancourt-Cortes, E.E.; McMillan, N.J.; Sharma, N.; Rocha, H.N.M.; Fujie, S.; et al. Role of adropin in arterial stiffening associated with obesity and type 2 diabetes. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H879–H891. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Cui, B.; Zhao, X.; Wu, Y.; Qin, H.; Guo, Y.; Wang, H.; Lu, M.; Zhang, S.; Shen, J.; et al. Correlation of Serum Adropin Levels with Risk Factors of Cardiovascular Disease in Hemodialysis Patients. Metab. Syndr. Relat. Disord. 2021, 19, 401–408. [Google Scholar] [CrossRef]
- Altintas Kadirhan, O.; Kucukdagli, O.T.; Gulen, B. The effectiveness of serum S100B, TRAIL, and adropin levels in predicting clinical outcome, final infarct core, and stroke subtypes of acute ischemic stroke patients. Biomedica 2022, 42 (Suppl. S1), 55–63. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Jin, F.; Wang, L.; Jiang, Y.; Wang, P.; Liu, J.; Zhao, L. Adropin—A new player in energy regulation predicts long-term prognosis of patients with acute myocardial infarction. Heliyon 2023, 9, e17803. [Google Scholar] [CrossRef] [PubMed]
- Bozic, J.; Kumric, M.; Ticinovic Kurir, T.; Males, I.; Borovac, J.A.; Martinovic, D.; Vilovic, M. Role of Adropin in Cardiometabolic Disorders: From Pathophysiological Mechanisms to Therapeutic Target. Biomedicines 2021, 9, 1407. [Google Scholar] [CrossRef]
- Berezina, T.A.; Obradovic, Z.; Boxhammer, E.; Berezin, A.A.; Lichtenauer, M.; Berezin, A.E. Adropin Predicts Chronic Kidney Disease in Type 2 Diabetes Mellitus Patients with Chronic Heart Failure. J. Clin. Med. 2023, 12, 2231. [Google Scholar] [CrossRef]
- Močnik, M.; Marčun Varda, N. Current Knowledge of Selected Cardiovascular Biomarkers in Pediatrics: Kidney Injury Molecule-1, Salusin-α and -β, Uromodulin, and Adropin. Children 2022, 9, 102. [Google Scholar] [CrossRef]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Ve-lazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.M.; Rørth, R.; Liu, J.; Kristensen, S.L.; Anand, I.S.; Claggett, B.L.; Cleland, J.G.F.; Chopra, V.K.; Desai, A.S.; Ge, J.; et al. Diabetes and pre-diabetes in patients with heart failure and preserved ejection fraction. Eur. J. Heart Fail. 2022, 24, 497–509. [Google Scholar] [CrossRef]
- Selvaraj, S.; Kim, J.; Ansari, B.A.; Zhao, L.; Cvijic, M.E.; Fronheiser, M.; Mohan-Rao Vanjarapu, J.; Kumar, A.A.; Suri, A.; Yenigalla, S.; et al. Body Composition, Natriuretic Peptides, and Adverse Outcomes in Heart Failure with Preserved and Reduced Ejection Fraction. JACC Cardiovasc. Imaging 2021, 14, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Pieske, B.; Tschöpe, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.M.; Huang, J.; Pandey, A.; Hashim, I.A.; Kerr, D.; Pop-Busui, R.; Rhee, C.M.; Shah, V.N.; Bally, L.; Bayes-Genis, A.; et al. Biomarkers for the Diagnosis of Heart Failure in People with Diabetes: A Consensus Report from Diabetes Technology Society. Prog. Cardiovasc. Dis. 2023, 79, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Sujana, C.; Salomaa, V.; Kee, F.; Costanzo, S.; Söderberg, S.; Jordan, J.; Jousilahti, P.; Neville, C.; Iacoviello, L.; Oskarsson, V.; et al. Natriuretic Peptides and Risk of Type 2 Diabetes: Results from the Biomarkers for Cardiovascular Risk Assessment in Europe (BiomarCaRE) Consortium. Diabetes Care 2021, 44, 2527–2535. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; Vaduganathan, M.; Claggett, B.L.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Baseline Characteristics of Patients with HF With Mildly Reduced and Preserved Ejection Fraction: DELIVER Trial. JACC Heart Fail. 2022, 10, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Salah, K.; Stienen, S.; Pinto, Y.M.; Eurlings, L.W.; Metra, M.; Bayes-Genis, A.; Verdiani, V.; Tijssen, J.G.P.; Kok, W.E. Prognosis and NT-proBNP in heart failure patients with preserved versus reduced ejection fraction. Heart 2019, 105, 1182–1189. [Google Scholar] [CrossRef]
- Lam, C.S.P.; Gamble, G.D.; Ling, L.H.; Sim, D.; Leong, K.T.G.; Yeo, P.S.D.; Ong, H.Y.; Jaufeerally, F.; Ng, T.P.; Cameron, V.A.; et al. Mortality associated with heart failure with preserved vs. reduced ejection fraction in a prospective international multi-ethnic cohort study. Eur. Heart J. 2018, 39, 1770–1780. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, K.N.; Gupta, D.K.; Xu, M.; Brittain, E.; Farber-Eger, E.; Arora, P.; Collins, S.; Wells, Q.S.; Wang, T.J. Unexpectedly Low Natriuretic Peptide Levels in Patients with Heart Failure. JACC Heart Fail. 2021, 9, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Gruden, G.; Landi, A.; Bruno, G. Natriuretic peptides, heart, and adipose tissue: New findings and future developments for diabetes research. Diabetes Care 2014, 37, 2899–2908. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.A.; Obradovic, Z.; Fushtey, I.M.; Berezina, T.A.; Novikov, E.V.; Schmidbauer, L.; Lichtenauer, M.; Berezin, A.E. The Impact of SGLT2 Inhibitor Dapagliflozin on Adropin Serum Levels in Men and Women with Type 2 Diabetes Mellitus and Chronic Heart Failure. Biomedicines 2023, 11, 457. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.A.; Obradovic, Z.; Novikov, E.V.; Boxhammer, E.; Lichtenauer, M.; Berezin, A.E. Interplay between Myokine Profile and Glycemic Control in Type 2 Diabetes Mellitus Patients with Heart Failure. Diagnostics 2022, 12, 2940. [Google Scholar] [CrossRef]
- Berezina, T.A.; Fushtey, I.M.; Berezin, A.A.; Pavlov, S.V.; Berezin, A.E. Predictors of Kidney Function Outcomes and Their Relation to SGLT2 Inhibitor Dapagliflozin in Patients with Type 2 Diabetes Mellitus Who Had Chronic Heart Failure. Adv. Ther. 2024, 41, 292–314. [Google Scholar] [CrossRef] [PubMed]
- Lian, W.; Gu, X.; Qin, Y.; Zheng, X. Elevated plasma levels of adropin in heart failure patients. Intern. Med. 2011, 50, 1523–1527. [Google Scholar] [CrossRef] [PubMed]
- Jasaszwili, M.; Billert, M.; Strowski, M.Z.; Nowak, K.W.; Skrzypski, M. Adropin as A Fat-Burning Hormone with Multiple Functions-Review of a Decade of Research. Molecules 2020, 25, 549. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejski, P.A.; Pruszyńska-Oszmałek, E.; Wojciechowicz, T.; Sassek, M.; Leciejewska, N.; Jasaszwili, M.; Billert, M.; Małek, E.; Szczepankiewicz, D.; Misiewicz-Mielnik, M.; et al. The Role of Peptide Hormones Discovered in the 21st Century in the Regulation of Adipose Tissue Functions. Genes 2021, 12, 756. [Google Scholar] [CrossRef]
- Wang, Q.; An, Y.; Zhang, L.; Zhang, Y.; Wang, G.; Liu, J. Regulation of Adropin by Sitagliptin monotherapy in participants with newly diagnosed type 2 Diabetes. BMC Endocr. Disord. 2022, 22, 306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, L.; Wu, X.; Li, X.; Chang, X.; Ding, X.; Wang, Q.; Jiang, T.; Wang, G.; Liu, J. Longitudinal changes in serum adropin levels and liver fat content during liraglutide treatment in newly diagnosed patients with type 2 diabetes mellitus and metabolic dysfunction-associated fatty liver disease. Acta Diabetol. 2023, 60, 971–979. [Google Scholar] [CrossRef]
- Tičinović Kurir, T.; Miličević, T.; Novak, A.; Vilović, M.; Božić, J. Adropin—Potential link in cardiovascular protection for obese male type 2 diabetes mellitus patients treated with liraglutide. Acta Clin. Croat. 2020, 59, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Beckley, N.; Nazareth, I.; Petersen, I. Effectiveness of sitagliptin compared to sulfonylureas for type 2 diabetes mellitus inadequately controlled on metformin: A systematic review and meta-analysis. BMJ Open 2017, 7, e017260. [Google Scholar] [CrossRef] [PubMed]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.L.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Furtado, R.H.M.; et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019, 393, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.B.; Gul, F.; Ram, P.; Kluger, A.Y.; Tecson, K.M.; McCullough, P.A.; Rangaswami, J. The Effects of SGLT2 Inhibitors on Cardiovascular and Renal Outcomes in Diabetic Patients: A Systematic Review and Meta-Analysis. Cardiorenal Med. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- The EMPA-KIDNEY Collaborative Group; Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Mayne, K.J.; et al. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef]
| Variables | Entire Group (n = 561) | Low-NT-proBNP Group (n = 162) | Elevated-NT-proBNP Group (n = 399) | p Value |
|---|---|---|---|---|
| Age (years) | 58 (47–70) | 62 (52–68) | 56 (44–69) | 0.044 |
| Male/female (n (%)) | 291 (51.7)/270 (48.2) | 91 (56.2)/71 (43.8) | 200 (50.1)/45 (49.8) | 0.688 |
| Diabetes duration (years) | 11.20± 6.75 | 10.40± 6.80 | 11.90± 6.33 | 0.722 |
| BMI (kg/m2) | 26.90 ± 5.20 | 26.40 ± 2.70 | 27.10 ± 3.10 | 0.440 |
| Waist circumference (cm) | 96.60 ± 3.60 | 99.20 ± 3.70 | 91.10 ± 3.90 | 0.160 |
| WHR (units) | 0.88 ± 0.10 | 0.92 ± 0.09 | 0.84 ± 0.07 | 0.240 |
| Dyslipidemia (n (%)) | 437 (77.9) | 126 (77.8) | 311 (77.0) | 0.762 |
| Hypertension (n (%)) | 415 (74.0) | 124 (76.5) | 291 (72.9) | 0.430 |
| Stable CAD (n (%)) | 173 (30.9) | 44 (27.1) | 129 (32.3) | 0.020 |
| Atrial fibrillation (n (%)) | 95 (16.9) | 21 (12.9) | 83 (20.8) | 0.012 |
| Smoking (n (%)) | 230 (41.0) | 61 (37.7) | 169 (42.4) | 0.050 |
| Abdominal obesity (n (%)) | 218 (38.9) | 62 (38.3) | 156 (39.1) | 0.780 |
| LVH (n (%)) | 509 (90.7) | 141 (87.0) | 368 (92.2) | 0.050 |
| CKD stages 1–3 (n (%)) | 151 (29.6) | 43 (26.5) | 108 (27.1) | 0.344 |
| Systolic BP (mm Hg) | 137 ± 10 | 138± 9 | 136 ± 7 | 0.780 |
| Diastolic BP (mm Hg) | 84 ± 9 | 86 ± 7 | 81 ± 8 | 0.650 |
| LVEDV (mL) | 153 (146–160) | 155 (139–161) | 156 (143–164) | 0.460 |
| LVESV (mL) | 71 (61–80) | 73 (65–86) | 69 (62–76) | 0.058 |
| LVEF (%) | 54 (51–57) | 53 (51–55) | 56 (51–59) | 0.054 |
| LVMMI (g/m2) | 139 ± 14 | 145 ± 11 | 132 ± 14 | 0.071 |
| LAVI (mL/m2) | 42 (35–50) | 41 (36–48) | 43 (38–49) | 0.064 |
| E/e′ (units) | 16 ± 5 | 15 ± 4 | 18 ± 5 | 0.448 |
| GLS (%) | −14.8 (−12.7; −15.7) | −15.3 (−12.4; −16.1) | −13.5 (−10.2; −15.7) | 0.152 |
| eGFR (mL/min/1.73 m2) | 79 ± 17 | 68 ± 15 | 88 ± 11 | 0.046 |
| HOMA-IR (units) | 7.12 ± 2.7 | 7.07± 2.5 | 7.30 ± 2.6 | 0.740 |
| Fasting glucose (mmol/L) | 6.42 ± 1.9 | 6.30 ± 1.6 | 6.52 ± 1.7 | 0.812 |
| HbA1c (%) | 6.45 ± 0.18 | 6.25 ± 0.13 | 6.50 ± 0.19 | 0.565 |
| Creatinine (µmol/L) | 100.9 ± 20.1 | 94.1 ± 21.7 | 115.2 ± 30.1 | 0.640 |
| SUA (mcmol/L) | 321 ± 115 | 302 ± 75 | 385 ± 925 | 0.042 |
| Total cholesterol (mmol/L) | 5.98 ± 1.22 | 5.96 ± 1.20 | 6.02 ± 1.25 | 0.676 |
| HDL-C (mmol/L) | 0.96 ± 0.20 | 0.99 ± 0.18 | 0.93 ± 0.20 | 0.548 |
| LDL-C (mmol/L) | 3.31± 0.24 | 3.26 ± 0.21 | 3.35± 0.24 | 0.490 |
| Triglycerides (mmol/L) | 1.38 ± 0.22 | 1.35 ± 0.18 | 1.41 ± 0.24 | 0.480 |
| hs-CRP (mg/L) | 4.39 (1.98–6.80) | 4.02 (2.10–6.22) | 4.90 (2.25–7.22) | 0.060 |
| TNF-alpha (pg/mL) | 2.70 (1.90–3.62) | 2.42 (1.69–3.15) | 2.98 (1.85–3.90) | 0.056 |
| NT-proBNP (pmol/mL) | 147 (65–231) | 87 (57–113) | 188 (143–227) | 0.001 |
| Adropin (ng/mL) | 3.50 (2.05–4.67) | 4.20 (3.70–4.80) | 2.40 (1.93–2.82) | 0.001 |
| ACE inhibitors (n (%)) | 221 (39.4) | 48 (29.6) | 173 (43.4) | 0.042 |
| Angiotensin-II receptor blockers (n (%)) | 207 (36.8) | 49 (30.2) | 158 (39.6) | 0.050 |
| Beta-blockers (n (%)) | 179 (31.9) | 51 (31.4) | 128 (32.1) | 0.782 |
| Ivabradine (n (%)) | 123 (21.9) | 35 (21.6) | 88 (22.1) | 0.862 |
| Calcium channel blockers (n (%)) | 161 (28.7) | 45 (27.7) | 116 (29.1) | 0.438 |
| Thiazide-like diuretics (n (%)) | 168 (29.9) | 42 (25.9) | 126 (31.6) | 0.124 |
| Antiplatelet agents (n (%)) | 173 (30.9) | 64 (39.5) | 109 (27.3) | 0.020 |
| Anticoagulants (n (%)) | 95 (16.9) | 21 (12.9) | 83 (20.8) | 0.012 |
| Metformin (n (%)) | 447 (79.8) | 129 (79.6) | 318 (79.7) | 0.878 |
| DPP4 inhibitors (n (%)) | 81 (14.4) | 25 (15.4) | 56 (14.0) | 0.060 |
| GLP-1 receptor agonists (n (%)) | 103 (18.4) | 30 (18.5) | 73 (18.3) | 0.886 |
| SGLT2 inhibitors (n (%)) | 492 (87.7) | 129 (79.6) | 363 (91.0) | 0.048 |
| Statins (n (%)) | 516 (91.2) | 144 (88.9) | 372 (93.2) | 0.070 |
| Variables | Dependent Variable: HFpEF | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Cox Regression | Multivariate Cox Regression | |||||||
| HR | 95% CI | p-Value | C-Index | HR | 95% CI | p-Value | C-Index | |
| Low NT-proBNP vs. elevated NT-proBNP | 0.93 | 0.81–1.05 | 0.644 | 0.33 | - | |||
| Low adropin vs. elevated adropin | 1.09 | 1.04–1.13 | 0.001 | 0.48 | 1.07 | 1.03–1.13 | 0.001 | 0.52 |
| Low NT-proBNP + low adropin vs. elevated NT-proBNP | 1.12 | 1.04–2.10 | 0.001 | 0.65 | 1.10 | 1.02–2.03 | 0.001 | 0.70 |
| Low NT-proBNP + elevated adropin vs. elevated NT-proBNP | 0.98 | 0.91–1.10 | 0.650 | 0.22 | - | |||
| Elevated NT-proBNP + low adropin | 1.17 | 1.02–1.33 | 0.001 | 0.67 | 1.14 | 1.01–1.29 | 0.001 | 0.63 |
| Elevated NT-proBNP + elevated adropin | 1.05 | 1.00–1.12 | 0.224 | 0.46 | - | |||
| AF vs. non-AF | 1.05 | 1.01–1.08 | 0.044 | 0.43 | 1.05 | 1.00–1.10 | 0.064 | 0.41 |
| LVH vs. non-LVH | 1.04 | 1.01–1.09 | 0.042 | 0.39 | 1.02 | 1.00–1.05 | 0.166 | 0.30 |
| Administration of SGLT2i | 0.91 | 0.86–0.98 | 0.044 | 0.34 | 0.94 | 0.88–0.96 | 0.041 | 0.38 |
| Predictive Models | Dependent Variable: HFpEF | |||||
|---|---|---|---|---|---|---|
| AUC | NRI | IDI | ||||
| M (95% CI) | p Value | M (95% CI) | p Value | M (95% CI) | p Value | |
| Model 1 (low adropin) | 0.802 (0.773–0.857) | - | Reference | - | Reference | - |
| Model 2 (low NT-proBNP + low adropin) | 0.783 (0.722–0.853) | 0.122 | 0.28 (0.19–0.35) | 0.213 | 0.23 (0.14–0.37) | 0.426 |
| Model 3 (elevated NT-proBNP + low adropin) | 0.815 (0.801–0.833) | 0.050 | 0.38 (0.22–0.51) | 0.044 | 0.29 (0.17–0.41) | 0.042 |
| Model 4 (administration of SGLT2 inhibitors) | 0.791 (0.760–0.862) | 0.444 | 0.14 (0.10–0.19) | 0.668 | 0.23 (0.19–0.29) | 0.668 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berezina, T.A.; Berezin, O.O.; Hoppe, U.C.; Lichtenauer, M.; Berezin, A.E. Adropin Predicts Asymptomatic Heart Failure in Patients with Type 2 Diabetes Mellitus Independent of the Levels of Natriuretic Peptides. Diagnostics 2024, 14, 1728. https://doi.org/10.3390/diagnostics14161728
Berezina TA, Berezin OO, Hoppe UC, Lichtenauer M, Berezin AE. Adropin Predicts Asymptomatic Heart Failure in Patients with Type 2 Diabetes Mellitus Independent of the Levels of Natriuretic Peptides. Diagnostics. 2024; 14(16):1728. https://doi.org/10.3390/diagnostics14161728
Chicago/Turabian StyleBerezina, Tetiana A., Oleksandr O. Berezin, Uta C. Hoppe, Michael Lichtenauer, and Alexander E. Berezin. 2024. "Adropin Predicts Asymptomatic Heart Failure in Patients with Type 2 Diabetes Mellitus Independent of the Levels of Natriuretic Peptides" Diagnostics 14, no. 16: 1728. https://doi.org/10.3390/diagnostics14161728
APA StyleBerezina, T. A., Berezin, O. O., Hoppe, U. C., Lichtenauer, M., & Berezin, A. E. (2024). Adropin Predicts Asymptomatic Heart Failure in Patients with Type 2 Diabetes Mellitus Independent of the Levels of Natriuretic Peptides. Diagnostics, 14(16), 1728. https://doi.org/10.3390/diagnostics14161728








