Club Cell Secretory Protein-16 (CC16) as a Prognostic Biomarker for COVID-19 and H1N1 Viral Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Plasma Specimens and Ethics Approval
2.2. Enzyme-Linked Immunosorbent Assay (ELISA)
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. CC16 Levels in COVID-19 and H1N1 Patients
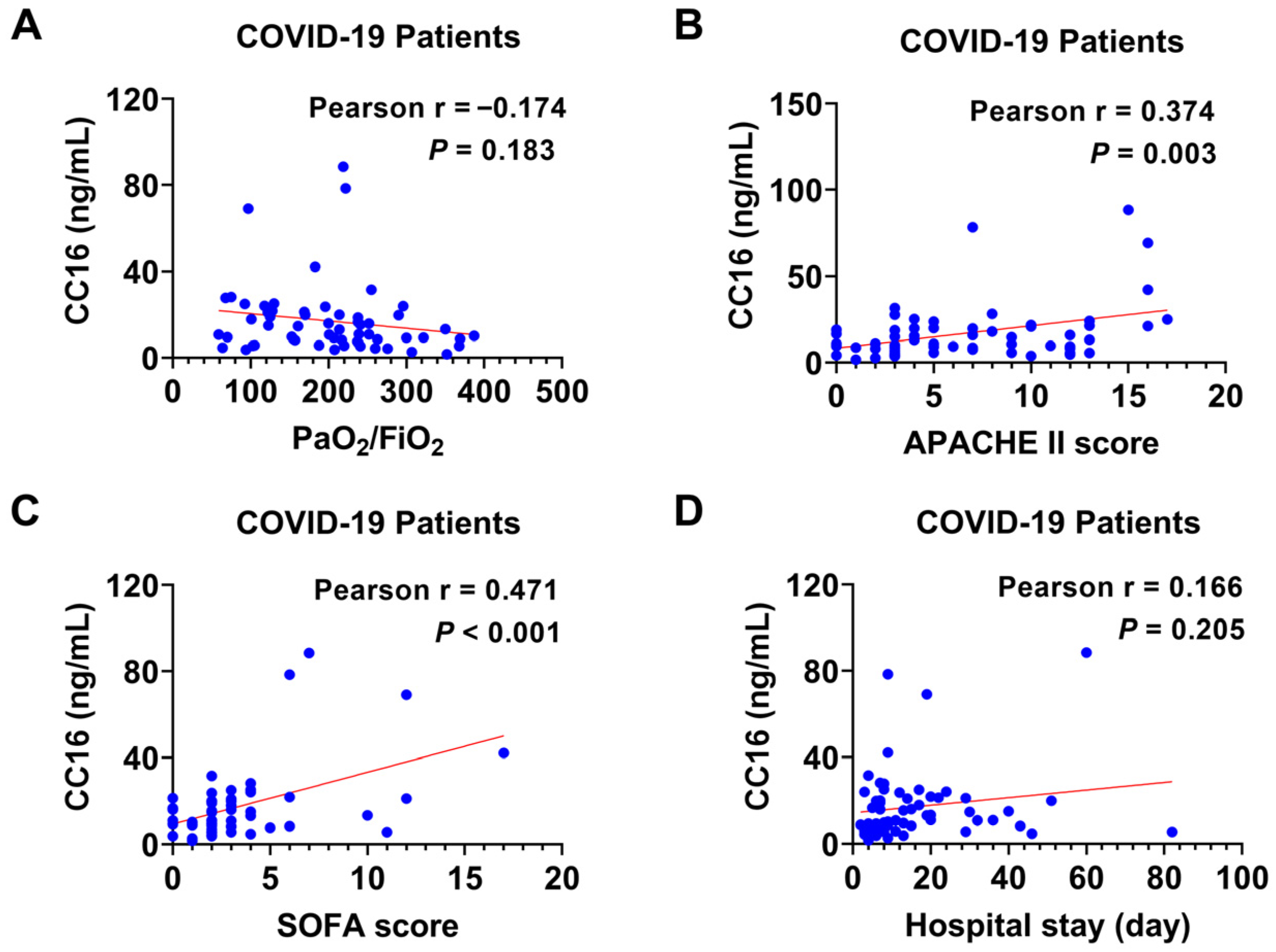
3.3. The Relation of Plasma CC16 Level to the Severity of COVID-19
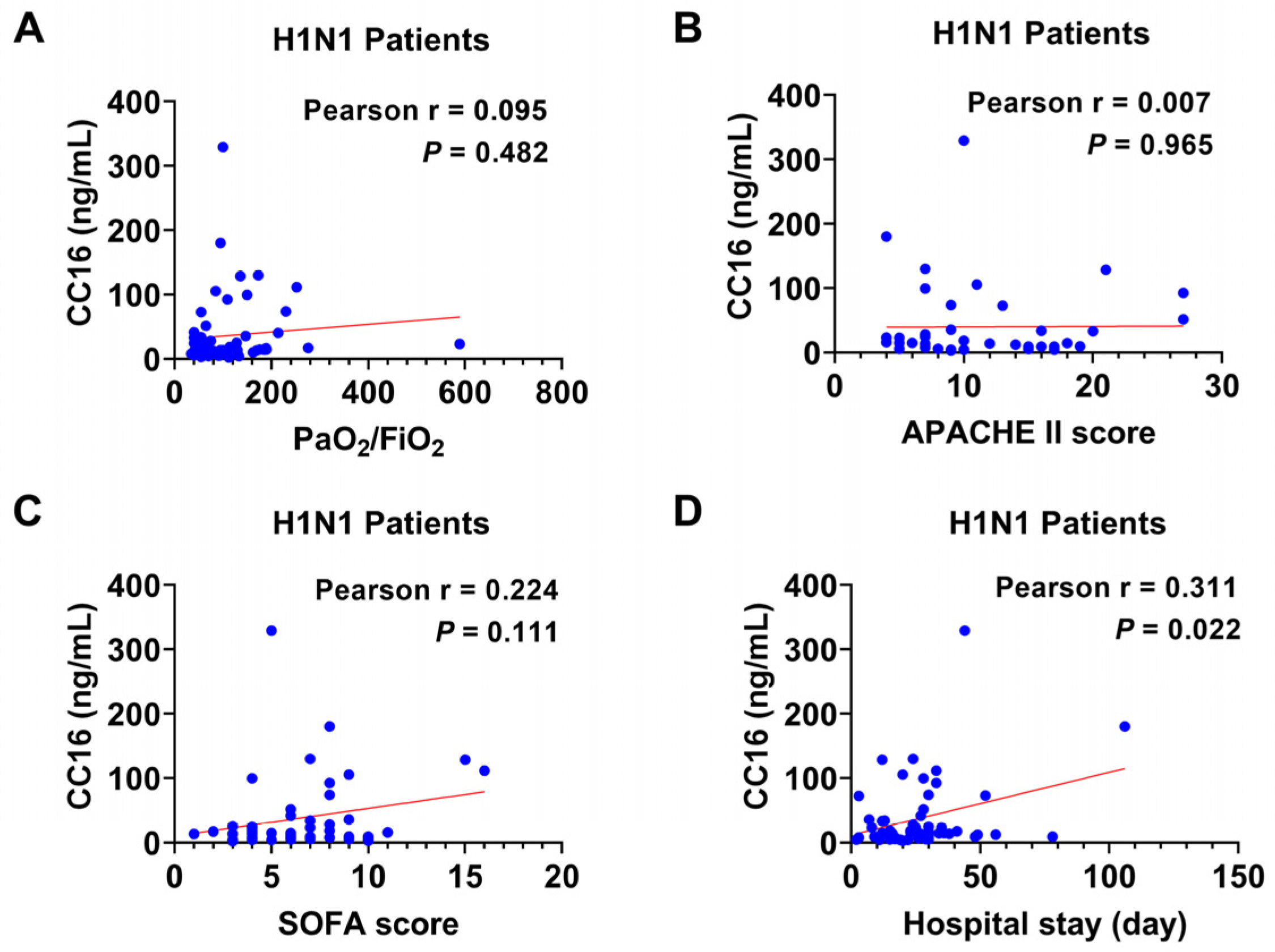
3.4. The Relation of Plasma CC16 Level to the Severity of H1N1 Infection
3.5. The Correlation between Plasma CC16 and the Disease Severity in Combined Groups
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Berhanu, G.; Desalegn, C.; Kandi, V. Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2): An Update. Cureus 2020, 12, e7423. [Google Scholar] [CrossRef]
- Grasselli, G.; Tonetti, T.; Protti, A.; Langer, T.; Girardis, M.; Bellani, G.; Laffey, J.; Carrafiello, G.; Carsana, L.; Rizzuto, C.; et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: A multicentre prospective observational study. Lancet Respir. Med. 2020, 8, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Birkenfeld, A.L.; Schulze, M.B. Global pandemics interconnected - obesity, impaired metabolic health and COVID-19. Nat. Rev. Endocrinol. 2021, 17, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Nishiga, M.; Wang, D.W.; Han, Y.; Lewis, D.B.; Wu, J.C. COVID-19 and cardiovascular disease: From basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020, 17, 543–558. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, Y.; Zheng, H.; Liu, L. Risk Factors for Long COVID in Older Adults. Biomedicines 2023, 11, 3002. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers 2019, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Bivona, G.; Agnello, L.; Ciaccio, M. Biomarkers for Prognosis and Treatment Response in COVID-19 Patients. Ann. Lab. Med. 2021, 41, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Taubenberger, J.K.; Kash, J.C. Influenza virus evolution, host adaptation, and pandemic formation. Cell Host Microbe 2010, 7, 440–451. [Google Scholar] [CrossRef]
- Flerlage, T.; Boyd, D.F.; Meliopoulos, V.; Thomas, P.G.; Schultz-Cherry, S. Influenza virus and SARS-CoV-2: Pathogenesis and host responses in the respiratory tract. Nat. Rev. Microbiol. 2021, 19, 425–441. [Google Scholar] [CrossRef]
- Roberts, N.J., Jr.; Krilov, L.R. The Continued Threat of Influenza A Viruses. Viruses 2022, 14, 883. [Google Scholar] [CrossRef] [PubMed]
- Nuwarda, R.F.; Alharbi, A.A.; Kayser, V. An Overview of Influenza Viruses and Vaccines. Vaccines 2021, 9, 1032. [Google Scholar] [CrossRef] [PubMed]
- Denney, L.; Ho, L.P. The role of respiratory epithelium in host defence against influenza virus infection. Biomed. J. 2018, 41, 218–233. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Thomas, P.G. Influenza virus-related critical illness: Pathophysiology and epidemiology. Crit. Care 2019, 23, 258. [Google Scholar] [CrossRef] [PubMed]
- Potey, P.M.; Rossi, A.G.; Lucas, C.D.; Dorward, D.A. Neutrophils in the initiation and resolution of acute pulmonary inflammation: Understanding biological function and therapeutic potential. J. Pathol. 2019, 247, 672–685. [Google Scholar] [CrossRef]
- Ragaller, M.; Richter, T. Acute lung injury and acute respiratory distress syndrome. Emerg. Trauma. Shock. 2010, 3, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Almuntashiri, S.; Han, Y.; Youngblood, H.A.; Chase, A.; Zhu, Y.; Wang, X.; Linder, D.F.; Siddiqui, B.; Sikora, A.; Liu, Y.; et al. Identification of circulating microvesicle-encapsulated miR-223 as a potential novel biomarker for ARDS. Physiol. Rep. 2022, 10, e15494. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.W.; Almuntashiri, S.; Chase, A.; Alhumaid, A.; Somanath, P.R.; Sikora, A.; Zhang, D. Plasma matrix metalloproteinase-3 predicts mortality in acute respiratory distress syndrome: A biomarker analysis of a randomized controlled trial. Respir. Res. 2023, 24, 166. [Google Scholar] [CrossRef] [PubMed]
- Almuntashiri, S.; Jones, T.W.; Wang, X.; Sikora, A.; Zhang, D. Plasma TIMP-1 as a sex-specific biomarker for acute lung injury. Biol. Sex. Differ. 2022, 13, 70. [Google Scholar] [CrossRef] [PubMed]
- Almuntashiri, S.; Chase, A.; Sikora, A.; Zhang, D. Validation of Prognostic Club Cell Secretory Protein (CC16) Cut-point in an Independent ALTA Cohort. Biomark. Insights 2023, 18, 11772719231156308. [Google Scholar] [CrossRef]
- Chase, A.; Almuntashiri, S.; Sikora, A.; Zhang, D. Club Cell Secretory Protein-Derived Acute Respiratory Distress Syndrome Phenotypes Predict 90-Day Mortality: A Reanalysis of the Fluids and Catheter Treatment Trial. Crit. Care Explor. 2022, 4, e0711. [Google Scholar] [CrossRef]
- Almuntashiri, S.; Zhu, Y.; Han, Y.; Wang, X.; Somanath, P.R.; Zhang, D. Club Cell Secreted Protein CC16: Potential Applications in Prognosis and Therapy for Pulmonary Diseases. J. Clin. Med. 2020, 9, 4039. [Google Scholar] [CrossRef]
- Kropski, J.A.; Fremont, R.; Calfee, C.S.; Ware, L.B. Clara cell protein (CC16), a marker of lung epithelial injury, is decreased in plasma and pulmonary edema fluid from patients with acute lung injury. Chest 2009, 135, 1440–1447. [Google Scholar] [CrossRef]
- Martinu, T.; Todd, J.L.; Gelman, A.E.; Guerra, S.; Palmer, S.M. Club Cell Secretory Protein in Lung Disease: Emerging Concepts and Potential Therapeutics. Annu. Rev. Med. 2023, 74, 427–441. [Google Scholar] [CrossRef]
- Iannuzo, N.; Dy, A.B.C.; Guerra, S.; Langlais, P.R.; Ledford, J.G. The Impact of CC16 on Pulmonary Epithelial-Driven Host Responses during Mycoplasma pneumoniae Infection in Mouse Tracheal Epithelial Cells. Cells 2023, 12, 1984. [Google Scholar] [CrossRef]
- Almuntashiri, S.; Han, Y.; Zhu, Y.; Dutta, S.; Niazi, S.; Wang, X.; Siddiqui, B.; Zhang, D. CC16 Regulates Inflammation, ROS Generation and Apoptosis in Bronchial Epithelial Cells during Klebsiella pneumoniae Infection. Int. J. Mol. Sci. 2021, 22, 11459. [Google Scholar] [CrossRef]
- Broeckaert, F.; Bernard, A. Clara cell secretory protein (CC16): Characteristics and perspectives as lung peripheral biomarker. Clin. Exp. Allergy 2000, 30, 469–475. [Google Scholar] [CrossRef]
- Han, Y.; Zhu, Y.; Almuntashiri, S.; Wang, X.; Somanath, P.R.; Owen, C.A.; Zhang, D. Extracellular vesicle-encapsulated CC16 as novel nanotherapeutics for treatment of acute lung injury. Mol. Ther. 2023, 31, 1346–1364. [Google Scholar] [CrossRef]
- Rojas-Quintero, J.; Wang, X.; Tipper, J.; Burkett, P.R.; Zuniga, J.; Ashtekar, A.R.; Polverino, F.; Rout, A.; Yambayev, I.; Hernandez, C.; et al. Matrix metalloproteinase-9 deficiency protects mice from severe influenza A viral infection. JCI Insight 2018, 3, e99022. [Google Scholar] [CrossRef]
- Choreno-Parra, J.A.; Jimenez-Alvarez, L.A.; Cruz-Lagunas, A.; Rodriguez-Reyna, T.S.; Ramirez-Martinez, G.; Sandoval-Vega, M.; Hernandez-Garcia, D.L.; Choreno-Parra, E.M.; Balderas-Martinez, Y.I.; Martinez-Sanchez, M.E.; et al. Clinical and Immunological Factors That Distinguish COVID-19 From Pandemic Influenza A(H1N1). Front. Immunol. 2021, 12, 593595. [Google Scholar] [CrossRef]
- Park, J.; Pabon, M.; Choi, A.M.K.; Siempos, I.I.; Fredenburgh, L.E.; Baron, R.M.; Jeon, K.; Chung, C.R.; Yang, J.H.; Park, C.M.; et al. Plasma surfactant protein-D as a diagnostic biomarker for acute respiratory distress syndrome: Validation in US and Korean cohorts. BMC Pulm. Med. 2017, 17, 204. [Google Scholar] [CrossRef]
- Greene, K.E.; Wright, J.R.; Steinberg, K.P.; Ruzinski, J.T.; Caldwell, E.; Wong, W.B.; Hull, W.; Whitsett, J.A.; Akino, T.; Kuroki, Y.; et al. Serial changes in surfactant-associated proteins in lung and serum before and after onset of ARDS. Am. J. Respir. Crit. Care Med. 1999, 160, 1843–1850. [Google Scholar] [CrossRef]
- Jabaudon, M.; Blondonnet, R.; Ware, L.B. Biomarkers in acute respiratory distress syndrome. Curr. Opin. Crit. Care 2021, 27, 46–54. [Google Scholar] [CrossRef]
- Celli, B.R.; Owen, C.A. The club cell and its protein, CC16: Time to shine. Lancet Respir. Med. 2013, 1, 757–759. [Google Scholar] [CrossRef]
- Rohmann, N.; Sturmer, P.; Geisler, C.; Schlicht, K.; Hartmann, K.; Turk, K.; Hollstein, T.; Tran, F.; Rosenstiel, P.; Franke, A.; et al. Brief Research Report: Serum clara cell 16 kDa protein levels are increased in patients hospitalized for severe SARS-CoV-2 or sepsis infection. Front. Immunol. 2022, 13, 1037115. [Google Scholar] [CrossRef]
- Tiezzi, M.; Morra, S.; Seminerio, J.; Van Muylem, A.; Godefroid, A.; Law-Weng-Sam, N.; Van Praet, A.; Corbiere, V.; Orte Cano, C.; Karimi, S.; et al. SP-D and CC-16 Pneumoproteins’ Kinetics and Their Predictive Role During SARS-CoV-2 Infection. Front. Med. 2021, 8, 761299. [Google Scholar] [CrossRef]
- Short, K.R.; Kroeze, E.; Fouchier, R.A.M.; Kuiken, T. Pathogenesis of influenza-induced acute respiratory distress syndrome. Lancet Infect. Dis. 2014, 14, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhang, W.; Wang, L.; Tian, F. Diagnostic and prognostic values of Club cell protein 16 (CC16) in critical care patients with acute respiratory distress syndrome. J. Clin. Lab. Anal. 2018, 32. [Google Scholar] [CrossRef] [PubMed]
- Gibot, S.; Bene, M.C.; Noel, R.; Massin, F.; Guy, J.; Cravoisy, A.; Barraud, D.; De Carvalho Bittencourt, M.; Quenot, J.P.; Bollaert, P.E.; et al. Combination biomarkers to diagnose sepsis in the critically ill patient. Am. J. Respir. Crit. Care Med. 2012, 186, 65–71. [Google Scholar] [CrossRef]


| All (n = 121) | COVID-19 (n = 60) | H1N1 (n = 61) | p Value 1 | |
|---|---|---|---|---|
| Age in years | 49.09 (12.15) | 50.30 (12.96) | 47.90 (11.27) | 0.28 |
| Female gender n (%) | 60 (49.6) | 30 (50) | 30 (49.2) | 0.92 |
| PaO2/FiO2 | 130 (79–219.5) | 207.5 (124.25–252) | 92 (60–141.5) 2 | <0.001 |
| >300 n (%) | 8 (6.84) | 7 (11.67) | 1 (1.75) | 0.10 |
| 201–300 n (%) | 29 (24.79) | 25 (41.67) | 4 (7.02) | <0.001 |
| 101–200 n (%) | 38 (32.48) | 20 (33.33) | 18 (31.58) | 0.98 |
| ≤100 n (%) | 42 (35.90) | 8 (13.33) | 34 (59.65) | <0.001 |
| APACHE II | 7 (4–12) | 5 (3–10.75) | 10 (7–16) 3 | <0.001 |
| SOFA | 4 (2–7) | 2 (2–4) | 6 (4–8) 4 | <0.001 |
| Hospital stay in days | 17 (8–29.25) | 9 (6–20) | 25.5 (15–33) 5 | <0.001 |
| Survivors n (%) | 96 (79.34) | 47 (78.33) | 49 (80.33) | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moore, S.; Gopichandran, K.; Sevier, E.; Gamare, S.; Almuntashiri, S.; Ramírez, G.; Regino, N.; Jiménez-Alvarez, L.; Cruz-Lagunas, A.; Rodriguez-Reyna, T.S.; et al. Club Cell Secretory Protein-16 (CC16) as a Prognostic Biomarker for COVID-19 and H1N1 Viral Infections. Diagnostics 2024, 14, 1720. https://doi.org/10.3390/diagnostics14161720
Moore S, Gopichandran K, Sevier E, Gamare S, Almuntashiri S, Ramírez G, Regino N, Jiménez-Alvarez L, Cruz-Lagunas A, Rodriguez-Reyna TS, et al. Club Cell Secretory Protein-16 (CC16) as a Prognostic Biomarker for COVID-19 and H1N1 Viral Infections. Diagnostics. 2024; 14(16):1720. https://doi.org/10.3390/diagnostics14161720
Chicago/Turabian StyleMoore, Shane, Keerthana Gopichandran, Elizabeth Sevier, Siddhika Gamare, Sultan Almuntashiri, Gustavo Ramírez, Nora Regino, Luis Jiménez-Alvarez, Alfredo Cruz-Lagunas, Tatiana S. Rodriguez-Reyna, and et al. 2024. "Club Cell Secretory Protein-16 (CC16) as a Prognostic Biomarker for COVID-19 and H1N1 Viral Infections" Diagnostics 14, no. 16: 1720. https://doi.org/10.3390/diagnostics14161720
APA StyleMoore, S., Gopichandran, K., Sevier, E., Gamare, S., Almuntashiri, S., Ramírez, G., Regino, N., Jiménez-Alvarez, L., Cruz-Lagunas, A., Rodriguez-Reyna, T. S., Zuñiga, J., Owen, C. A., Wang, X., & Zhang, D. (2024). Club Cell Secretory Protein-16 (CC16) as a Prognostic Biomarker for COVID-19 and H1N1 Viral Infections. Diagnostics, 14(16), 1720. https://doi.org/10.3390/diagnostics14161720







