Abstract
Mucopolysaccharidoses (MPS) comprise a group of 12 metabolic disorders where defects in specific enzyme activities lead to the accumulation of glycosaminoglycans (GAGs) within lysosomes. This classification expands to 13 when considering MPS IIIE. This type of MPS, associated with pathogenic variants in the ARSG gene, has thus far been described only in the context of animal models. However, pathogenic variants in this gene also occur in humans, but are linked to a different disorder, Usher syndrome (USH) type IV, which is sparking increasing debate. This paper gathers, discusses, and summarizes arguments both for and against classifying dysfunctions of arylsulfatase G (due to pathogenic variants in the ARSG gene) in humans as another subtype of MPS, called MPS IIIE. Specific difficulties in diagnostics and the classification of some inherited metabolic diseases are also highlighted and discussed.
1. Introduction
Mucopolysaccharidoses (MPS) are a heterogeneous group of genetically determined metabolic disorders, belonging to lysosomal storage diseases (LSD) with complex pathomechanisms [1]. The primary cause of the disease is a disturbance in the activity of lysosomal enzymes responsible for the hydrolysis of glycosaminoglycans (GAGs)-long, linear polysaccharides that are a multifunctional group of compounds due to their polyanionic nature. Incompletely degraded GAGs accumulate in lysosomes, thereby disrupting normal cell functions and subsequently causing tissue-level abnormalities and organ dysfunctions [1,2,3]. There are currently 12 ‘classical’ (conventional, i.e., those in which GAG accumulation arises from a severe decrease in an activity of an enzyme directly involved in GAG degradation; not counting MPS-plus syndrome (MPSPS), where GAG storage is caused by another mechanism) types and subtypes of MPS, where the classification is based on both the type(s) of accumulated GAG(s) and the enzyme affected by the defect. In addition, depending on the absence/presence of symptoms of the nervous system disorders, MPS can be subdivided into non-neuronopathic types (MPS IVA and IVB, VI, VII and X) and neuronopathic types (MPS I, II, all subtypes of MPS III and VII) [4].
Recently, several papers have mentioned the 13th type of MPS, MPS IIIE [5,6,7,8,9]. Until recently, MPS IIIE was described as a subtype found only in animal models [10]. The gene variant causing this MPS has been detected in humans relatively recently and has been described as Usher disease (USH) type IV [11,12,13]. The aim of this study is to discuss the arguments both for and against the recognition of MPS IIIE in humans.
2. Mucopolysaccharidosis
As mentioned earlier, MPS is a heterogeneous group of diseases in which symptoms can vary not only between types but also within subtypes [6] (see Table 1 for details). Nevertheless, some symptoms, like coarse facial features, dysostosis multiplex, hepatosplenomegaly, cardiovascular disorders, cognitive impairment (in neuronopathic forms), recurrent respiratory infections, and diarrhea, occur in the majority of patients, though with various severities [14,15].

Table 1.
Characteristics of classical/conventional types of MPS a.
Disorders that develop in the course of MPS, particularly those affecting the osteoarticular and central nervous systems, are irreversible after reaching a specific threshold, thus, early diagnosis and treatment are extremely important [16,17]. However, the diagnosis of MPS is difficult for a number of reasons. MPS is a multisystemic, rare disease with a progressive nature [3,15]. In practice, this means that in a child who appears healthy at birth, the first symptoms appear over time and may not be obvious. As the disease progresses, the abnormalities become more and more apparent, at the same time that further disorders appear which may be regarded as symptoms of another disease [16,18]. An additional difficulty is the heterogeneity of symptoms among patients and the rarity of MPS. This results in a delayed diagnosis at best (often preceded by a misdiagnosis of another disease), while at worst, a lack of diagnosis [19]. The diagnosis of MPS is often based on the clinical picture, and sometime a patient’s history (possibility of MPS in relatives). When MPS is suspected, the first test performed is usually the assessment of the urinary GAG level determination. The next step is assessing the activities of lysosomal enzymes, only the result of which allows a diagnosis of MPS to be made. Molecular tests are then performed to identify gene variants to assess the progression of the disease and plan appropriate treatment [19,20].
Sanfilippo disease is a type III of MPS in which only one GAG, heparan sulfate (HS), accumulates. Because the HS degradation pathway involves several enzymes, subtypes of Sanfilippo disease are distinguished, where the current classification includes four of them (subtypes A–D) [21]. Patients with MPS III, like those with other MPS types, show various symptoms, i.e., facial dysmorphia, hepatosplenomegaly, osteoarticular abnormalities, etc., but the somatic disturbances are relatively mild, relative to those with other MPS types. In contrast, the central nervous system (CNS) disorders are especially severe [19].
In the course of MPS III, children initially develop normally until around 2–3 years of age, then the first symptoms begin to appear. The slowing down or stopping of normal development and a gradual loss of acquired skills are observed [9,22]. Features of facial dysmorphia begin to become apparent. Symptoms such as frequent ear and upper respiratory tract infections or diarrhea are often underestimated. Around 3–4 years of age, cognitive functions deteriorate significantly, and sleep disorders and behavioral problems appear (including aggressive-like behavior, impulsivity, anxiety disorders, and/or autistic disorders) [23,24]. Skeletal abnormalities become more severe and more apparent. In the teenage years, a child’s quiescence, cognitive decline, and dementia are observed. Patients lose the ability to function and move independently. In milder forms, the symptoms of the disease usually appear later and are not as pronounced as in severe forms [19].
Animal studies have shown the existence of another enzyme involved in HS degradation, arylsulfatase G (ARSG) [10,25]. The results of experiments with Arsg-knockout mice showed a similarity to mouse models of the other four types of MPS III. The Arsg dysfunction and the deficiency of the corresponding enzyme led to HS accumulation in lysosomes [10]. HS storage was evident in both CNS and somatic organs, like the liver and kidney [10,25,26]. Secondary accumulations of complex lipids (glycolipids, gangliosides, lipofuscins, cholesterol) and subunit c of mitochondrial ATP synthase (SCMAS) were also observed. Some behavioral abnormalities, typical for animal models of the other 4 MPS III subtypes, were also indicated [10]. Since a pathogenic variant in the ARSG gene in a homozygous configuration has also been identified in humans [11,12,13], while not causing GAG accumulation, the question arises as to whether the classification of MPS should be expanded to include another subtype?
3. MPS IIIE in Humans—Whether It Is or Not?
The correct diagnosis of a disorder, even when effective treatment options are not available, is always important, at least to provide the best possible management and care to patients. From the perspective of parents/guardians and the patient, knowing and understanding the cause of the disorder also allows for a kind of psychological comfort. By knowing the cause, they gain an insight into the illness, which reduces feelings of helplessness, enables them to get used to the situation and gives them a sense of control. From a medical point of view, a correct diagnosis, even in the absence of drugs to cure the patient, allows optimal care to be planned. By knowing the disease, it is possible to more or less anticipate its course and introduce preventive measures for possible complications. It is also possible to plan and implement symptomatic treatment, seek experimental therapy programs, and, above all, prevent the use of treatment methods that may be not ineffective or even harmful. Therefore, the discussion regarding the possible occurrence of MPS IIIE in humans is an important issue requiring attention.
3.1. Why Yes?
3.1.1. ARSG Activity
In 2010, Abitbol et al. published a paper describing adult American Staffordshire terriers (ASTs) as a canine model of neuronal ceroid lipofuscinoses (NCLs), a lysosomal storage disease with neurodegeneration and neurological disorders, caused by the accumulation of autofluorescent lipofuscin and fatty lipopigment [27,28]). Symptoms of the disease begin to be seen in the juvenile period, very rarely only in adulthood. At the time of publication of that paper [27], the cause of inherited forms of CLN4 was not known. It was observed that ASTs sometimes develop symptoms similar to those characteristic of CLN4, i.e., locomotor ataxia, absence of visual impairment, marked cerebellar atrophy, and accumulation of a specific lipopigment in Purkinje cells and thalamic neurons (revealed abnormal lysosomes filled with inclusions). In addition, the disorders appeared late and developed slowly [27]. Studies have shown the presence of variants in the ARSG gene, leading to a decrease in the activity (by as much as 75%) of the N-sulfoglucosamine-3-O-sulfatase (arylsulfatase G), an enzyme encoded by this gene. Variants in this gene have been proposed as a possible cause of NCLs with onset in adulthood [27]. However, the exact function of the enzyme was first described in 2012 by Kowalewski et al. [10]. In order to determine the function of the enzyme, the researchers created the knock-down (KO) mouse mutant in the Arsg gene. The results of that study showed that ARSG is one of the lysosomal hydrolases involved in GAG degradation [25]. Loss of activity of this enzyme led to HS accumulation in mice due to the inability to remove 3-O-sulfated N-sulfoglucosamine residues of heparan sulfate (a simplified diagram of HS degrading enzymes, including ARSG activity, is shown in Figure 1) [10]. Not only did the KO mouse model of ARSG deficiency show features similar to MPS III (described in the next section), but also the accumulation of autofluorescent material, which was observed in ARSG-deficient dogs [25,27], was observed. In view of the results described above, a link between ARSG variants and the pathogenesis of MPS was proposed and it was suggested that the previously described canine model of NCLs may also be an animal model of MPS III [10]. It is now indeed considered that the variant in the ARSG gene, previously described in the canine model, is associated with the pathogenesis of MPS, at least in animals (specifically, in dogs and mice) [29,30].
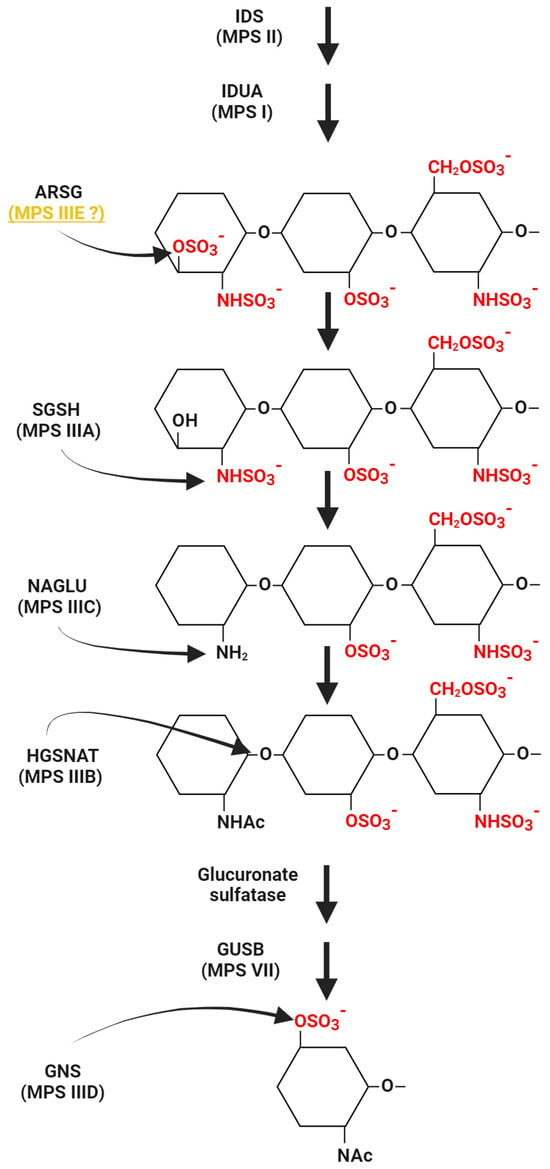
Figure 1.
The pathway of HS degradation with a special emphasis on enzymes for which dysfunctions cause specific subtypes of MPS III. Reactions catalyzed by these enzymes are indicated, while other steps and enzymes for which deficiencies are responsible for other MPS types are shown in a simplified form. Whether deficiency of arylsulfatase G (ARSG) causes MPS IIIE in humans or not is a disputable issue; thus, this MPS subtype is followed by a question mark. Abbreviations: IDS, iduronate-2-sulfatase; IDUA, α-L-iduronidase; ARSG, arylsulfatase G; SGSH, Heparan-N-sulfatase; NAGLU, α-N-acetylglucosaminidase; HGSNAT, heparan α-glucosaminide-N-acetyltransferase; GUSB, β-glucuronidase; GNS, N-acetylglucosamine-6-sulfatase. This scheme was created using BioRender.com (license no. YA2720J3QO).
3.1.2. Mild/Attenuated Type of MPS
As mentioned earlier, in 2012, Kowalewski et al. published a paper describing the phenotype of the constructed Arsg-knockout mouse line (ARSG KO) [10]. This model showed similarities to other mouse models of MPS III in terms of both biochemical and behavioral abnormalities. HS accumulation was observed in the liver and brain, the organs typically affected in MPS III, but without features of organomegaly. Accumulation of GAG was also observed in organs [10,31]. In addition, significant enlargement of lysosomes was observed in the cells of some brain areas. A secondary accumulation of gangliosides and other lipids has also been observed, as also occurs in the course of other MPS types [10,32]. As the mice aged, difficulties in acquiring new skills have been observed [25]. Stenosis (SCMAS) has been documented in MPS III and has also been confirmed in the ARSG KO mice [25,33,34]. A common feature of several lysosomal storage disorders, including MPS, is retinal degeneration, which was the earliest observed disorder in the ARSG KO mice studied [26,35,36]. In short, mice with the dysfunctional Arsg gene (in the homozygous state) show some characteristics of LSD, and especially MPS III.
The aforementioned ARSG KO model has features characteristic of MPS; however, compared to other MPS III models, they appear to be relatively mild [25,26]. Nevertheless, it is not a very unusual situation, as, for example, MPS I is subdivided into clinical subtypes (from extremely severe, through moderate, to mild; called also Hurler, Hurler/Scheie, and Scheie disease, respectively) not because of the different molecular basis, but because of differences in the severity of the symptoms [15]. Similarly, MPS II, often very severe in its course, can develop into the attenuated type, although the variant in both cases involves the same gene [37]. The severity of the symptoms, in both cases (MPS I and II), depends on the HS concentration and the ratio between different kinds of GAGs [38]. Although the subdivision of MPS III into subtypes is due to differences in molecular basis (mutant gene and deficient gene product–an enzyme), it is not obvious why subtypes IIIA and IIIB are known to be more severe in their courses than subtypes IIIC and IIID [39]. It also happens that people with the theoretically more severe MPS III subtypes (A and B) remain undiagnosed for years because they have developed mild symptoms (with minor cognitive impairment or even non-neuronopathic). An interesting series of 12 patients with MPS III was described, with adult-onset phenotypes and mild cognitive impairment or even non-neuronopathic phenotypes [40]. In six of these patients, the main symptom (in addition to decreased enzyme activity and elevated urinary GAG levels) was retinal dystrophy (RD)/RD with visual impairment [40]. In four more patients, RD developed after diagnosis. It should be mentioned that the oldest patients were 50–70 years old (7 patients) and three were over 40 years old, while the life expectancy of patients with classic MPS III is about 20–30 years [19,40].
De Falco et al. described the case of a patient who was diagnosed with Usher’s disease (USH) at around 30 years of age due to retinitis pigmentosa (RP) and sensorineural hearing loss (SNHL) [41]. The patient’s condition deteriorated over the next 20 years. At the age of 53 years, the patient was re-diagnosed. No behavioral, cognitive or memory disorders were detected. Physical examination did not reveal features of facial dysmorphia or organomegaly [41]. On neurological examination, no specific abnormalities were detected except for minimal cerebellar features, including mild telekinetic tremor (left > right) and dysdiadochokinesia [41]. Attention could be drawn to the short stature of the male patient (150 cm). Exome sequencing did not reveal variants conditioning USH, but variants in the SGSH gene, causing MPS IIIA, were detected. GAG measurement and enzyme activity tests showed elevated HS levels and no detectable SGSH activity. Thus, the final diagnosis of MPS IIIA was made [41]. This case indicates that Sanfilippo disease may present with atypical symptoms, but two crucial features should be evident, i.e., HS storage and deficiency in one of enzymes responsible for GAG degradation.
The above statement is supported by the facts that attenuated forms of MPS may run their course without the characteristic dysmorphic facial features, and patients’ life expectancies may not differ from those of healthy individuals [40,42]. The main symptoms may not necessarily be retardation/stunting, cognitive impairment, or hepatosplenomegaly, but they can include visual impairment, skeletal problems without inflammatory features, and hearing and cardiac problems [42]. Moreover, urinary GAG levels may be normal [42]. Therefore, the ARSG KO mouse model may indeed be a model of MPS III, whose phenotype is benign.
3.2. Why Not?
3.2.1. The Animal Model and Humans
Doubts about the validity of the MPS diagnosis in the cases of variants in the ARSG gene may already arise at the animal model level. Admittedly, studies in the mouse model demonstrated a number of disorders converging (and key) to those observed in animal models of other MPS III subtypes, i.e., high levels of HS in lysosomes, secondary lipid accumulation, liver involvement, or CNS abnormalities [10,25,26]. However, some differences emerged. First of all, it is important to note that the mouse model described by Kowalewski et al. is of the knockout type, i.e., it is completely devoid of ARSG activity, thus a severe phenotype and a rapid course could be expected [10,43,44,45,46]. Kruszweski et al. pointed to a relatively late onset of symptoms compared to mouse models of other MPS III types, and the authors of the model themselves indicated a milder phenotype compared to other MPS III subtypes [25,26]. The mice did not show symptoms of the disease until the age of 12 months, despite HS storage in CNS and peripheral organs [10]. The major neurological finding of ARSG KO was ataxia with massive Purkinje cell degradation; however, the earliest sign was retinal degradation occurring between 1–6 months of age in mice, even before the onset of neurological abnormalities, while changes in the brain structure were rather limited to the cerebellum [26]. Retinal degradation also occurs earlier in mouse models of MPS III, but the earliest signs of neurological disorders are primarily motor and behavioral abnormalities, such as hyperactivity or aggression [43,44,45,46]. Although behavioral and cognitive abnormalities were observed in the ARSG KO mice, they appear significantly later and are not as severe as in other MPS III mouse models [43,44,45,46]. Moreover, motor dysfunction was not observed in the ARSG KO mouse model [25]. Pathological changes in brain structures are much more extensive (hippocampus, cortex, cerebellum, spinal cord, microglia, astroglia) in other MPS III subtypes than in the Arsg mouse mutants [44,45,46,47].
In addition to HS accumulation, dermatan sulfate (DS) accumulation has also been observed in the mouse Args knockout model [25]. It is worth noting that in MPS II, both HS and DS accumulate [38]. Clinically, MPS II is divided into severe and attenuated forms [37], which could account for the relatively milder course, compared to MPS III [25]. Furthermore, the aforementioned accumulation of SCMAS proteins as well as retinal degradation are observed in MPS II [34,35]. In fact, ARSG KO mice resemble, to some extent, a mild form of MPS II, with a defined difference in molecular basis.
Arsg-knockout mice were created to study the action of ARSG [10]. By design, this model was not intended to reflect human disease, as at the time of its development, MPS IIIE has not been reported in humans [25,26]. In fact, mouse models of MPS III A–D largely correspond to the disorders observed in patients. The differences are quite minor (with some obvious limitations, like the impossibility of studying speech disorders in animals) and relate to the speed of onset of symptoms rather than the subtype of MPS III [43,44,45,46]. Although the mouse model of ARSG deficiency does indeed show disorders similar to those observed in mouse models of the other types of MPS, in humans it causes a completely different disorder, defined as USH type IV [13].
USH is a recessively-inherited disease with SNHL and RP, with or without vestibular dysfunction [48]. Based on the age of onset of symptoms, their severity and the speed with which the disease progresses, there are three main types of USH, namely I, II, and III [13]. In 2018, Katheb et al. described variants in the ARSG gene (a homozygous missense variant) as a cause of USH, describing it as an atypical USH phenotype [11]. No vestibular system involvement was observed in any of the subjects, SNHL and RP (the characteristic phenotype of ring-shaped retinal atrophy along the arcades) with late, for USH, onset were observed in all five patients. Investigations showed no neurological abnormalities, and magnetic resonance imaging showed no brain abnormalities, similar to abdominal ultrasound, which also showed no changes. Three patients showed skeletal changes (osteoporosis), but it should be noted that the patients were 50–72 years old at the time of the study [11]. It was tested whether the variant detected in the patients’ ARSG gene (D45Y) affects the activity of ARSG and other lysosomal enzymes. The results showed a decrease in ARSG activity without affecting other lysosomal hydrolases. At the same time, GAG levels remained at the upper limit of the norm [11]. It was acknowledged that the described patients’ phenotypically did not fit into the classical USH variants, but also did not show features typical of MPS. It was suggested that updating the USH classification be considered [11]. Since then, a total of 15 variants in the ARSG gene have been described, leading to the development of the disorder now recognized as USH type IV [11,12,13,49,50,51].
The diagnosis of USH type IV, rather than Sanfilippo disease, in the above described patients, was based on the fact that decreased ARSG activity did not correlate with other features typical of MPS, like lysosomal abnormalities, somatic abnormalities (like hepatosplenomegaly or osteoarticular disorders), and especially elevated GAG levels (which remained around the upper limit of normal values in these patients) [11,12,13]. Although hearing abnormalities are typical of many types of MPS, including MPS III, and visual abnormalities (corneal clouding, glaucoma, retinopathy, or optic nerve abnormalities) are characteristic of neuronopathic types of MPS [52,53], no CNS abnormalities typical of the neuronopathic types of MPS, including MPS III, were found in the described patients [11,12,13,45,46,47]. In 2021, Kowalewski et al. confirmed that the ARSG dysfunction is connected to USH, particularly untypical USH type IV [54]. Interestingly, it was suggested that other, as yet unidentified, 3-O-sulfatase(s) of HS may exist, as ARSG could not catalyze the reaction using a substrate which contains a free amino group [54]. Indeed, this might explain the discrepancy between the lack of ARSG function and normal GAG levels in patients diagnosed for USH type IV, as the auxiliary function of another, fully functional enzyme might compensate for ARSG deficiency. Nevertheless, the discovery of such sulfatase(s) is necessary to verify this hypothesis positively.
Interestingly, a similar situation applied to the classification of variants in the ARSK gene. There is a mouse model, where HS and DS accumulation occurs due to dysfunction of one of the lysosomal hydrolases, arylsulfatase K (ARSK) [19]. In 2020, Trabszo et al. published a paper on the ARSK-deficient mouse model, in which elevated levels of HS and DS were noted in the liver, spleen, and brain, with a concomitant decrease in ARSK enzymatic activity [8]. Although behavioral changes in ARSK-deficient mice were observed, they were considerably milder than those in models of other types of MPS. Additionally, features indicative of CNS abnormalities were absent. The effects of ARSK deficiency on the skeletal system (whose abnormalities are typical in MPS) were also not observed. The authors of that study proposed distinguishing MPS IIB, as a subtype of MPS [8]. However, MPS II (Hunter syndrome) is one of the neuronopathic types of MPS, in which iduronate 2-sulfatase (IDS) function is impaired, resulting in deposition in HS and DS in lysosomes [38]. Abnormalities characteristic for MPS II include skeletal abnormalities, organomegaly, facial dysmorphia, short stature, inguinal and umbilical hernias, hearing impairment/loss, and neurological abnormalities with functional impairment and corneal opacity [38]. Sometimes, however, patients with a benign phenotype do not develop CNS abnormalities [38]. One year later, Verheyen et al. published a paper in which a variant leading to ARSK deficiency was described in humans [55]. However, the observed disorders differed from those described in the mouse model devoid of the Arsk function. The patients developed osteoarticular disorders without behavioral abnormalities. Symptoms typical of MPS were observed, i.e., short stature, recurrent ear infections, sleep problems, mild visual system abnormalities, and facial dysmorphic features. MPS IV was initially suspected, but subsequent investigations (assessment of enzymatic activity) ruled out this possibility. Although urinary GAG levels were normal, liquid chromatography/mass spectrometry (LC-MS/MS) showed a significantly increased level of DS [55]. Therefore, a diagnosis of MPS type X was proposed [55]. Over time, additional patients with similar disorders and a similar diagnostic process began to be reported [56,57]. Skeletal abnormalities, no behavioral incapacity, or CNS abnormalities with typical symptoms suggestive of MPS were found. Initial assessment of urinary GAG levels showed no abnormalities, but repeated testing suggested MPS IV. Evaluation of enzymatic activity together with genetic testing and GAG examination with LC-MS/MS confirmed ARSK deficiency with accumulation of DS [56,57]. Like Verheyen et al., both Rustad et al. and Sun et al. classified the described cases as MPS X [50,51,52].
Animal models of human diseases are certainly much more advanced than cellular models, on which it is not possible to show/consider the bigger picture of how the organism as a whole works. However, an animal model is still only a model. Indeed, there are many examples of studies where potential therapeutics with promising results in animal studies have not worked in humans. The same principle applies to disorders observed in the course of a disease. Not all symptoms seen in humans can be transferred to animal models and, as is evident in some MPS types, this also works the other way round.
3.2.2. MPS-like Symptoms
Mucopolysaccharidosis-plus (MPSPS) is a disease in which variants in the VPS33A gene result in cellular accumulation of DS and HS (high urinary concentrations of both GAGs) while the activities of all the enzymes responsible for GAG degradation remain unaltered [58,59]. The clinical picture is remarkably similar to that seen in patients with ‘classic’ MPS, including skeletal abnormalities such as excessive joint stiffness or dysostosis multiplex, facial dysmorphic features, and cardiovascular abnormalities. In addition, as in the neuronopathic types of MPS, CNS abnormalities (psychomotor retardation and developmental delay) appear, which may be associated with HS accumulation [19,22,58]. Nevertheless, the variant in the gene that causes MPSPS represents a distinct disease entity within LSD [60]. No classification as benign/atypical MPS I or II has been proposed, as the molecular mechanisms of this disease are significantly different from those of ‘classic’ MPS disorders.
3.2.3. One Gene, Different Diseases
GM1 gangliosidosis and MPS IVB are LSDs in which multiple disorders are caused by variants in the GLB1 gene which encodes acid β-galactosidase [16]. As a result, keratan sulphate (KS) and ganglioside GM1 accumulate in cells [16,61]. Both GM1 gangliosidosis and MPS IVB present with skeletal abnormalities, facial dysmorphic features, hepatosplenomegaly, visual disturbances, and frequent diarrhea (symptoms typical of MPS) [62,63]. However, GM1 gangliosidosis is a sphingolipidosis, a neurodegenerative disease in which the accumulation of primarily GM1 and GA1 gangliosides occurs in the nervous system [62], while MPS IVB is characterized by predominant KS accumulation in bones and cartilage with little or no CNS involvement (if present, CNS dysfunctions are relatively mild and develop secondarily to the main disorders) [19]. Although these two diseases are remarkably similar, not only in terms of genetic background, but also the stored materials and the observed disorders, MPS IVB is still classified as a mucopolysaccharidosis rather than another subtype of gangliosidosis. Conversely, no reclassification of GM1 gangliosidosis as another MPS type/subtype has been proposed so far. This raises the question of why a variant in the ARSG gene, which does not even give LSD-typical symptoms and is already classified as a specific disease entity, USH type IV, should be renamed as MPS IIIE?
RP is the most common type of inherited retinal degeneration with diverse genetic backgrounds [64]. At first, RP usually manifests with night blindness, followed by visual field loss, and eventually leads to total blindness [64]. RP can be a disease in itself or a component of other conditions such as USH, mitochondrial disorders, or inborn defects of metabolism, like MPS [64,65,66]. Haer-Wigman et al. described six patients with a variant in the HGSNAT gene, a defect of which leads to impaired heparan-α-glucosaminide-N-acetyltransferase (HGSANT) activity, HS accumulation, and a diagnosis of MPS IIIC [64]. In order to exclude the possibility of a benign type of MPS IIIC, patients underwent physical examinations and biochemical tests, which are standard procedures in the diagnosis of MPS. Physical examination did not reveal abnormalities typical of LSD/MPS. One patient was found to have mild hearing loss at 59 years of age, and none of the six patients showed CNS disorders or behavioral abnormalities. Examination of the HGSANT activity showed its significant decrease, compared to the reference value for healthy subjects, but the patients’ scores remained significantly higher than those of individuals diagnosed with MPS [64]. The final diagnosis of all six patients was non-syndromic RP [64]. In 2020, Schiff et al. conducted a study in a group of 20 patients with a variant in the HGSNAT gene who were diagnosed with RP [67]. Biallelic variants of the gene were detected in 17 patients, but none showed features typical of MPS IIIC. Enzyme testing from leukocytes showed a significant but mild reduction in the activity [67]. It is noteworthy that four of the identified variants were previously described as occurring in MPS IIIC [67].
Despite these ostensibly paradoxical cases, the fact that the same variant can lead to radically different phenotypes is not surprising. It is important to remember that when investigating the effect of single gene variants, one must take into account the broader genetic and environmental context. The ‘final result’ is influenced not only by the main variant, directly determining the development of the disease, but also by the genetic background, epigenetic factors, gene-gene interactions, and environmental factors [68]. All of these can affect differential penetrance and expression, and exacerbate/mitigate primary disorders [68]. RP resulting from variants in genes that normally lead to LSD also involves genes that condition neuronal ceroid lipofuscinoses (NCLs) [13]. Pathogenic variants in the CLN3, CLN5, and CLN7 genes usually lead to accumulation of autofluorescent lipofuscin and fatty lipopigment in lysosomes of neuronal cells. This results in progressive neurological deterioration with dementia, epilepsy, loss of vision, motor disturbances, and early death [69]. However, several research groups described variants in the above-mentioned genes associated with the diagnosis of isolated retina degeneration [70,71,72,73]. A similar situation could apply to the ARSG gene variants described in USH type IV, but these are variants that lead to loss of function/activity and yet do not cause the typical LSD/MPS symptoms. Therefore, it is difficult to assume that other variants will be associated with a wider spectrum of symptoms or that symptoms will develop later [13]. It should be emphasized that the diagnosis of atypical/mild forms of MPS/NCLs was not proposed in the described cases. In contrast, the diagnosis was a completely different disease entity, RP.
3.2.4. Diagnostics
The first step in the diagnosis of MPS is to suspect the presence of this disorder based on the patient’s clinical features and information obtained from the patient’s history [19]. Clinical features that may suggest a diagnostic workup for MPS include facial dysmorphia (thickened features, prominent forehead and lips), coarse hair, dental abnormalities, thoracolumbar kyphosis, joint stiffness, short stature, and concomitant CNS disorders (diagnosis for neuronopathic types of MPS) (details presented in Table 2) [18,19]. A family history of MPS should be of particular interest, but also frequent respiratory tract and ear infections, recurrent diarrhea, or problems with sight or hearing, or a history of hepato- or splenomegaly is also considered [15,18].

Table 2.
Symptoms that may indicate a need for diagnosis in the direction of MPS.
The next step is to perform a test to assess urinary GAG levels, but it is important to remember that the results of such a test are not the basis of confirmi7ng the diagnosis of MPS (if elevated GAG levels are found) or rejecting the diagnosis (if GAG levels remain normal) [3,74]. GAG levels may change with age, additionally they may not be elevated in individuals with a mild disease phenotype, and it is also the case that results may be false positives. Only the assessment of enzymatic activity, together with the clinical picture and history, allows the diagnosis of MPS to be made [75]. Additional investigations are helpful during diagnosis, including radiographs to evaluate joints and bones (especially hip joints and metacarpal bones), brain magnetic resonance imaging, or various biochemical tests [19,74]. Molecular analysis to detect pathogenic gene variant(s) can finally confirm the disease, while is usually performed after initial diagnosis. However, this also facilitates assessing the possible course of the disease and planning appropriate treatment [3,15,19]. A simplified diagnostic scheme for MPS is shown in Figure 2.
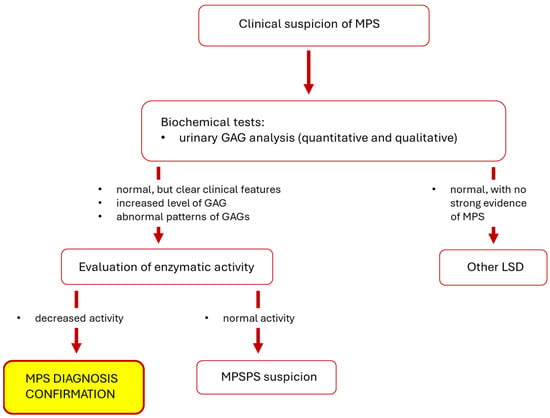
Figure 2.
Simplified diagnostic scheme for MPS. Abbreviations: GAG, glycosaminoglycan; LSD, lysosomal storage disease; MPS, mucopolysaccharidosis; MPSPS, mucopolysaccharidosis-plus syndrome.
Examples of MPS misdiagnosis or diagnostic difficulties were published in the literature [19]. An interesting example is the paper by Langereis et al. who described a patient in whom diagnosis was undertaken for Fabre disease [76], an LSD in which, due to a deficiency in α-galactosidase A (α-Gal A) activity, there is an accumulation of glycosphingolipids [77]. A 32-year-old man was hospitalized due to ischemic stroke. However, because of the lack of predisposing factors, the diagnosis of Fabry disease was started. However, α-Gal A activity did not reveal abnormalities, while the test revealed a decreased activity of α-L-iduronidase, an enzyme whose defect leads to MPS I [76]. Thus, diagnosis for this MPS type was initiated. Despite the low activity of the enzyme (while still higher than in MPS patients), the urinary GAG content (both quantitatively and qualitatively) remained normal. Genetic analysis confirmed the presence of variants in the IDUA gene, suggesting MPS I, Scheie phenotype (the mild form of MPS I), but the diagnosis was not confirmed [76]. Until the incident from which the man returned to full function and for which he was hospitalized, the patient remained healthy. He negated any joint mobility problems, and his family history also did not indicate a possible genetic disorder burden associated with metabolic diseases [76]. The patient’s height (180 cm) also did not indicate typical abnormalities for MPS. No organomegaly or dysmorphic features were found on examination. Ultimately, the suspicion of mild MPS type I was rejected [76].
4. Conclusions
There are arguments for and against the classification of the disease caused by pathogenic variants in the ARSG gene as MPS IIIE in humans. They are summarized in Table 3. Nevertheless, since both GAG storage and deficiency in activity of an enzyme involved in GAG degradation are required to make an MPS diagnosis, in our opinion, despite mice with Arsg dysfunction resembling those used as models in other subtypes of MPS III, classification of the corresponding disease in humans as MPS IIIE should be considered only if patients are described with ARSG variants as the only genetic defect, while developing biochemical features and symptoms characteristic of MPS. Otherwise, we suggest that there is no reason to include MPS IIIE in the list of human MPS types/subtypes.

Table 3.
Summary of arguments for and against the occurrence of MPS IIIE in humans.
Author Contributions
K.W., J.W. and K.P. prepared a draft of the manuscript. L.G., A.S. and M.Ż. reviewed and completed the text of the paper. K.W., K.P. and G.W. prepared the final version, which was approved by all the authors. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the University of Gdansk within the UGrants Program (grants no. 533-0C20-GS0D-24 to K.W. and 533-D000-GS0U-24 to K.P.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Figure 1 was created using BioRender.com (license no. YA2720J3QO).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gaffke, L.; Pierzynowska, K.; Podlacha, M.; Brokowska, J.; Węgrzyn, G. Changes in Cellular Processes Occurring in Mucopolysaccharidoses as Underestimated Pathomechanisms of These Diseases. Cell Biol. Int. 2021, 45, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chi, L. The Alterations and Roles of Glycosaminoglycans in Human Diseases. Polymers 2022, 14, 5014. [Google Scholar] [CrossRef] [PubMed]
- Kubaski, F.; de Oliveira Poswar, F.; Michelin-Tirelli, K.; Burin, M.G.; Rojas-Málaga, D.; Brusius-Facchin, A.C.; Leistner-Segal, S.; Giugliani, R. Diagnosis of Mucopolysaccharidoses. Diagnostics 2020, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska, K.; Gaffke, L.; Żabińska, M.; Wegrzyn, G.; Pierzynowska, K. Cellular Organelle-Related Transcriptomic Profile Abnormalities in Neuronopathic Types of Mucopolysaccharidosis: A Comparison with Other Neurodegenerative Diseases. Curr. Issues Mol. Biol. 2024, 46, 2678–2700. [Google Scholar] [CrossRef] [PubMed]
- Spahiu, L.; Behluli, E.; Peterlin, B.; Nefic, H.; Hadziselimovic, R.; Liehr, T.; Temaj, G. Mucopolysaccharidosis III: Molecular Basis and Treatment. Pediatr. Endocrinol. Diabetes Metab. 2021, 27, 201–208. [Google Scholar] [CrossRef]
- Rintz, E.; Banacki, M.; Ziemian, M.; Kobus, B.; Wegrzyn, G. Causes of Death in Mucopolysaccharidoses. Mol. Genet. Metab. 2024, 142, 108507. [Google Scholar] [CrossRef] [PubMed]
- Sahin, O.; Thompson, H.P.; Goodman, G.W.; Li, J.; Urayama, A. Mucopolysaccharidoses and the Blood–Brain Barrier. Fluids Barriers CNS 2022, 19, 76. [Google Scholar] [CrossRef]
- Trabszo, C.; Ramms, B.; Chopra, P.; Lüllmann-Rauch, R.; Stroobants, S.; Sproß, J.; Jeschke, A.; Schinke, T.; Boons, G.-J.; Esko, J.D.; et al. Arylsulfatase K Inactivation Causes Mucopolysaccharidosis Due to Deficient Glucuronate Desulfation of Heparan and Chondroitin Sulfate. Biochem. J. 2020, 477, 3433–3451. [Google Scholar] [CrossRef]
- Winner, L.K.; Rogers, M.-L.; Snel, M.F.; Hemsley, K.M. Biomarkers for Predicting Disease Course in Sanfilippo Syndrome: An Urgent Unmet Need in Childhood-Onset Dementia. J. Neurochem. 2023, 166, 481–496. [Google Scholar] [CrossRef]
- Kowalewski, B.; Lamanna, W.C.; Lawrence, R.; Damme, M.; Stroobants, S.; Padva, M.; Kalus, I.; Frese, M.-A.; Lübke, T.; Lüllmann-Rauch, R.; et al. Arylsulfatase G Inactivation Causes Loss of Heparan Sulfate 3-O-Sulfatase Activity and Mucopolysaccharidosis in Mice. Proc. Natl. Acad. Sci. USA 2012, 109, 10310–10315. [Google Scholar] [CrossRef]
- Khateb, S.; Kowalewski, B.; Bedoni, N.; Damme, M.; Pollack, N.; Saada, A.; Obolensky, A.; Ben-Yosef, T.; Gross, M.; Dierks, T.; et al. A Homozygous Founder Missense Variant in Arylsulfatase G Abolishes Its Enzymatic Activity Causing Atypical Usher Syndrome in Humans. Genet. Med. 2018, 20, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Peter, V.G.; Quinodoz, M.; Sadio, S.; Held, S.; Rodrigues, M.; Soares, M.; Sousa, A.B.; Coutinho Santos, L.; Damme, M.; Rivolta, C. New Clinical and Molecular Evidence Linking Mutations in ARSG to Usher Syndrome Type IV. Hum. Mutat. 2021, 42, 261–271. [Google Scholar] [CrossRef]
- Velde, H.M.; Reurink, J.; Held, S.; Li, C.H.Z.; Yzer, S.; Oostrik, J.; Weeda, J.; Haer-Wigman, L.; Yntema, H.G.; Roosing, S.; et al. Usher Syndrome Type IV: Clinically and Molecularly Confirmed by Novel ARSG Variants. Hum. Genet. 2022, 141, 1723–1738. [Google Scholar] [CrossRef] [PubMed]
- Galzerano, D.; Saba, S.; Sergani, A.A.; Vriz, O.; Alghalayini, K.; Ramzan, K.; Elmahi, I.; Cittadini, A.; Salvo, G.D.; Pergola, V. Features and Behavior of Valvular Abnormalities in Adolescent and Adult Patients in Mucopolysaccharidosis: An Echocardiographic Study. Monaldi Arch. Chest Dis. 2021, 91, 1767. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, J.; Leung, W.T.; Wang, L. A Basic Understanding of Mucopolysaccharidosis: Incidence, Clinical Features, Diagnosis, and Management. Intractable Rare Dis. Res. 2020, 9, 1–9. [Google Scholar] [CrossRef]
- Celik, B.; Tomatsu, S.C.; Tomatsu, S.; Khan, S.A. Epidemiology of Mucopolysaccharidoses Update. Diagnostics 2021, 11, 273. [Google Scholar] [CrossRef]
- Ago, Y.; Rintz, E.; Musini, K.S.; Ma, Z.; Tomatsu, S. Molecular Mechanisms in Pathophysiology of Mucopolysaccharidosis and Prospects for Innovative Therapy. Int. J. Mol. Sci. 2024, 25, 1113. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, M.; Arunkumar, N.; Kubaski, F.; Mason, R.W.; Tadao, O.; Tomatsu, S. Clinical Presentation and Diagnosis of Mucopolysaccharidoses. Mol. Genet. Metab. 2018, 125, 4–17. [Google Scholar] [CrossRef]
- Wiśniewska, K.; Wolski, J.; Gaffke, L.; Cyske, Z.; Pierzynowska, K.; Węgrzyn, G. Misdiagnosis in Mucopolysaccharidoses. J. Appl. Genet. 2022, 63, 475–495. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, N.; Vu, D.C.; Khan, S.; Kobayashi, H.; Ngoc Can, T.B.; Oguni, T.; Watanabe, J.; Tanaka, M.; Yamaguchi, S.; Taketani, T.; et al. Diagnosis of Mucopolysaccharidoses and Mucolipidosis by Assaying Multiplex Enzymes and Glycosaminoglycans. Diagnostics 2021, 11, 1347. [Google Scholar] [CrossRef]
- Mohammed, E.E.A.; Fayez, A.G.; Abdelfattah, N.M.; Fateen, E. Novel Gene-Specific Bayesian Gaussian Mixture Model to Predict the Missense Variants Pathogenicity of Sanfilippo Syndrome. Sci. Rep. 2024, 14, 12148. [Google Scholar] [CrossRef] [PubMed]
- Węgrzyn, G.; Jakóbkiewicz-Banecka, J.; Narajczyk, M.; Wiśniewski, A.; Piotrowska, E.; Gabig-Cimińska, M.; Kloska, A.; Słomińska-Wojewódzka, M.; Korzon-Burakowska, A.; Węgrzyn, A. Why Are Behaviors of Children Suffering from Various Neuronopathic Types of Mucopolysaccharidoses Different? Med. Hypotheses 2010, 75, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Ozbay, H.; Ozbek, M.; Sevinçok, D.; Tas, K.; Aksu, H.; Tosun, A. Autism Spectrum Disorder in a Child with Hunter Syndrome. Psychiatry Clin. Psychopharmacol. 2020, 30, 1. [Google Scholar] [CrossRef]
- Kalyoncu, D.; Gümüştekin, R.; Urganci, N. Is Mucopolysaccharidosis a Cause of Sleep and Speech Disorders? Report of Four Cases? JAREM 2020, 10, 204–207. [Google Scholar] [CrossRef]
- Kowalewski, B.; Lübke, T.; Kollmann, K.; Braulke, T.; Reinheckel, T.; Dierks, T.; Damme, M. Molecular Characterization of Arylsulfatase G: Expression, Processing, Glycosylation, Transport, and Activity. J. Biol. Chem. 2014, 289, 27992–28005. [Google Scholar] [CrossRef] [PubMed]
- Kruszewski, K.; Lüllmann-Rauch, R.; Dierks, T.; Bartsch, U.; Damme, M. Degeneration of Photoreceptor Cells in Arylsulfatase G-Deficient Mice. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Abitbol, M.; Thibaud, J.-L.; Olby, N.J.; Hitte, C.; Puech, J.-P.; Maurer, M.; Pilot-Storck, F.; Hédan, B.; Dréano, S.; Brahimi, S.; et al. A Canine Arylsulfatase G (ARSG) Mutation Leading to a Sulfatase Deficiency Is Associated with Neuronal Ceroid Lipofuscinosis. Proc. Natl. Acad. Sci. USA 2010, 107, 14775–14780. [Google Scholar] [CrossRef] [PubMed]
- Kido, J.; Nakamura, K.; Era, T. Role of Induced Pluripotent Stem Cells in Lysosomal Storage Diseases. Mol. Cell. Neurosci. 2020, 108, 103540. [Google Scholar] [CrossRef] [PubMed]
- Cocostîrc, V.; Paștiu, A.I.; Pusta, D.L. An Overview of Canine Inherited Neurological Disorders with Known Causal Variants. Animals 2023, 13, 3568. [Google Scholar] [CrossRef]
- Katz, M.L.; Rustad, E.; Robinson, G.O.; Whiting, R.E.H.; Student, J.T.; Coates, J.R.; Narfstrom, K. Canine Neuronal Ceroid Lipofuscinoses: Promising Models for Preclinical Testing of Therapeutic Interventions. Neurobiol. Dis. 2017, 108, 277–287. [Google Scholar] [CrossRef]
- Rintz, E.; Podlacha, M.; Cyske, Z.; Pierzynowska, K.; Węgrzyn, G.; Gaffke, L. Activities of (Poly)Phenolic Antioxidants and Other Natural Autophagy Modulators in the Treatment of Sanfilippo Disease: Remarkable Efficacy of Resveratrol in Cellular and Animal Models. Neurotherapeutics 2023, 20, 254–271. [Google Scholar] [CrossRef]
- Pierzynowska, K.; Deresz, P.; Węgrzyn, G.; Gaffke, L. Dysregulation of Genes Coding for Proteins Involved in Metabolic Processes in Mucopolysaccharidoses, Evidenced by a Transcriptomic Approach. Metab. Brain Dis. 2023, 38, 2133–2144. [Google Scholar] [CrossRef]
- Ryazantsev, S.; Yu, W.-H.; Zhao, H.-Z.; Neufeld, E.F.; Ohmi, K. Lysosomal Accumulation of SCMAS (Subunit c of Mitochondrial ATP Synthase) in Neurons of the Mouse Model of Mucopolysaccharidosis III B. Mol. Genet. Metab. 2007, 90, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Elleder, M.; Sokolová, J.; Hřebíček, M. Follow-up Study of Subunit c of Mitochondrial ATP Synthase (SCMAS) in Batten Disease and in Unrelated Lysosomal Disorders. Acta Neuropathol. 1997, 93, 379–390. [Google Scholar] [CrossRef]
- Del Longo, A.; Piozzi, E.; Schweizer, F. Ocular Features in Mucopolysaccharidosis: Diagnosis and Treatment. Ital. J. Pediatr. 2018, 44, 125. [Google Scholar] [CrossRef]
- Moro, E. Lysosomal Storage Disorders: Molecular Basis and Therapeutic Approaches. Biomolecules 2021, 11, 964. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Pantoom, S.; Petters, J.; Pandey, A.K.; Hermann, A.; Lukas, J. A Molecular Genetics View on Mucopolysaccharidosis Type II. Mutat. Res./Rev. Mutat. Res. 2021, 788, 108392. [Google Scholar] [CrossRef] [PubMed]
- Hampe, C.S.; Yund, B.D.; Orchard, P.J.; Lund, T.C.; Wesley, J.; McIvor, R.S. Differences in MPS I and MPS II Disease Manifestations. Int. J. Mol. Sci. 2021, 22, 7888. [Google Scholar] [CrossRef]
- Wiśniewska, K.; Gaffke, L.; Krzelowska, K.; Węgrzyn, G.; Pierzynowska, K. Differences in Gene Expression Patterns, Revealed by RNA-Seq Analysis, between Various Sanfilippo and Morquio Disease Subtypes. Gene 2022, 812, 146090. [Google Scholar] [CrossRef]
- Nijmeijer, S.C.M.; van den Born, L.I.; Kievit, A.J.A.; Stepien, K.M.; Langendonk, J.; Marchal, J.P.; Roosing, S.; Wijburg, F.A.; Wagenmakers, M.A.E.M. The Attenuated End of the Phenotypic Spectrum in MPS III: From Late-Onset Stable Cognitive Impairment to a Non-Neuronopathic Phenotype. Orphanet. J. Rare Dis. 2019, 14, 249. [Google Scholar] [CrossRef]
- De Falco, A.; Karali, M.; Criscuolo, C.; Testa, F.; Barillari, M.R.; Scarpato, M.; Gaudieri, V.; Cuocolo, A.; Russo, A.; Nigro, V.; et al. Late-Onset Mucopolysaccharidosis Type IIIA Mimicking Usher Syndrome. Am. J. Med. Genet. Part A 2024, 194, e63517. [Google Scholar] [CrossRef]
- Rigoldi, M.; Verrecchia, E.; Manna, R.; Mascia, M.T. Clinical Hints to Diagnosis of Attenuated Forms of Mucopolysaccharidoses. Ital. J. Pediatr. 2018, 44, 132. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.A.; King, B.M.; Thorsen, C.L.; Hassiotis, S.; Beard, H.; Trim, P.J.; Whyte, L.S.; Tamang, S.J.; Duplock, S.K.; Snel, M.F.; et al. A Novel Conditional Sgsh Knockout Mouse Model Recapitulates Phenotypic and Neuropathic Deficits of Sanfilippo Syndrome. J. Inherit. Metab. Dis. 2017, 40, 715–724. [Google Scholar] [CrossRef]
- Petrova, R.; Patil, A.R.; Trinh, V.; McElroy, K.E.; Bhakta, M.; Tien, J.; Wilson, D.S.; Warren, L.; Stratton, J.R. Disease Pathology Signatures in a Mouse Model of Mucopolysaccharidosis Type IIIB. Sci. Rep. 2023, 13, 16699. [Google Scholar] [CrossRef]
- Marcó, S.; Pujol, A.; Roca, C.; Motas, S.; Ribera, A.; Garcia, M.; Molas, M.; Villacampa, P.; Melia, C.S.; Sánchez, V.; et al. Progressive Neurologic and Somatic Disease in a Novel Mouse Model of Human Mucopolysaccharidosis Type IIIC. Dis. Models Mech. 2016, 9, 999–1013. [Google Scholar] [CrossRef] [PubMed]
- Heon-Roberts, R.; Nguyen, A.L.A.; Pshezhetsky, A.V. Molecular Bases of Neurodegeneration and Cognitive Decline, the Major Burden of Sanfilippo Disease. J. Clin. Med. 2020, 9, 344. [Google Scholar] [CrossRef]
- Bhaumik, M.; Muller, V.J.; Rozaklis, T.; Linda, J.; Dobrenis, K.; Bhattacharyya, R.; Wurzelmann, S.; Finamore, P.; Hopwood, J.J.; Walkley, S.U.; et al. A Mouse Model for Mucopolysaccharidosis Type III A (Sanfilippo Syndrome). Glycobiology 1999, 9, 1389–1396. [Google Scholar] [CrossRef]
- Nolen, R.M.; Hufnagel, R.B.; Friedman, T.B.; Turriff, A.E.; Brewer, C.C.; Zalewski, C.K.; King, K.A.; Wafa, T.; Griffith, A.J.; Brooks, B.; et al. Atypical and Ultra-Rare Usher Syndrome: A Review. Ophthalmic Genet. 2020, 41, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Abad-Morales, V.; Navarro, R.; Burés-Jelstrup, A.; Pomares, E. Identification of a Novel Homozygous ARSG Mutation as the Second Cause of Usher Syndrome Type 4. Am. J. Ophthalmol. Case Rep. 2020, 19, 100736. [Google Scholar] [CrossRef]
- Fowler, N.; El-Rashedy, M.; Chishti, E.; Vander Kooi, C.W.; Maldonado, R. Multimodal Imaging and Genetic Findings in a Case of ARSG-Related Atypical Usher Syndrome. Ophthalmic Genet. 2021, 42, 338–343. [Google Scholar] [CrossRef]
- Igelman, A.D.; Ku, C.; da Palma, M.M.; Georgiou, M.; Schiff, E.R.; Lam, B.L.; Sankila, E.-M.; Ahn, J.; Pyers, L.; Vincent, A.; et al. Expanding the Clinical Phenotype in Patients with Disease Causing Variants Associated with Atypical Usher Syndrome. Ophthalmic Genet. 2021, 42, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Ponzin, D.; Ashworth, J.L.; Fahnehjelm, K.T.; Summers, C.G.; Harmatz, P.R.; Scarpa, M. Diagnosis and Management of Ophthalmologic Features in Patients with Mucopolysaccharidosis. Br. J. Ophthalmol. 2010, 95, 613. [Google Scholar] [CrossRef]
- Wolfberg, J.; Chintalapati, K.; Tomatsu, S.; Nagao, K. Hearing Loss in Mucopolysaccharidoses: Current Knowledge and Future Directions. Diagnostics 2020, 10, 554. [Google Scholar] [CrossRef]
- Kowalewski, B.; Lange, H.; Galle, S.; Dierks, T.; Lübke, T.; Damme, M. Decoding the Consecutive Lysosomal Degradation of 3-O-Sulfate Containing Heparan Sulfate by Arylsulfatase G (ARSG). Biochem. J. 2021, 478, 3221–3237. [Google Scholar] [CrossRef]
- Verheyen, S.; Blatterer, J.; Speicher, M.R.; Bhavani, G.S.; Boons, G.-J.; Ilse, M.-B.; Andrae, D.; Sproß, J.; Vaz, F.M.; Kircher, S.G.; et al. Novel Subtype of Mucopolysaccharidosis Caused by Arylsulfatase K (ARSK) Deficiency. J. Med. Genet. 2022, 59, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Rustad, C.F.; Prescott, T.E.; Merckoll, E.; Kristensen, E.; Salvador, C.L.; Nordgarden, H.; Tveten, K. Phenotypic Expansion of ARSK-Related Mucopolysaccharidosis. Am. J. Med. Genet. Part A 2022, 188, 3369–3373. [Google Scholar] [CrossRef]
- Sun, M.; Kaminsky, C.K.; Deppe, P.; Ilse, M.-B.; Vaz, F.M.; Plecko, B.; Lübke, T.; Randolph, L.M. A Novel Homozygous Missense Variant in ARSK Causes MPS X, a New Subtype of Mucopolysaccharidosis. Genes Dis. 2024, 11, 101025. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Maksimova, N.; Otomo, T.; Kato, H.; Imai, A.; Asano, Y.; Kobayashi, K.; Nojima, S.; Nakaya, A.; Hamada, Y.; et al. Mutation in VPS33A Affects Metabolism of Glycosaminoglycans: A New Type of Mucopolysaccharidosis with Severe Systemic Symptoms. Hum. Mol. Genet. 2017, 26, 173–183. [Google Scholar] [CrossRef]
- Vasilev, F.; Sukhomyasova, A.; Otomo, T. Mucopolysaccharidosis-Plus Syndrome. Int. J. Mol. Sci. 2020, 21, 421. [Google Scholar] [CrossRef]
- Faraguna, M.C.; Musto, F.; Crescitelli, V.; Iascone, M.; Spaccini, L.; Tonduti, D.; Fedeli, T.; Kullmann, G.; Canonico, F.; Cattoni, A.; et al. Mucopolysaccharidosis-Plus Syndrome, a Rapidly Progressive Disease: Favorable Impact of a Very Prolonged Steroid Treatment on the Clinical Course in a Child. Genes 2022, 13, 442. [Google Scholar] [CrossRef]
- Caciotti, A.; Donati, M.A.; Procopio, E.; Filocamo, M.; Kleijer, W.; Wuyts, W.; Blaumeiser, B.; d’Azzo, A.; Simi, L.; Orlando, C.; et al. GM1 Gangliosidosis: Molecular Analysis of Nine Patients and Development of an RT-PCR Assay for GLB1 Gene Expression Profiling. Hum. Mutat. 2007, 28, 204. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Jin, D.-K. GLB1-Related Disorders: GM1 Gangliosidosis and Morquio B Disease. J. Genet. Med. 2021, 18, 16–23. [Google Scholar] [CrossRef]
- Kingma, S.D.K.; Ceulemans, B.; Kenis, S.; Jonckheere, A.I. Are GMI Gangliosidosis and Morquio Type B Two Different Disorders or Part of One Phenotypic Spectrum? JIMD Rep. 2021, 59, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Haer-Wigman, L.; Newman, H.; Leibu, R.; Bax, N.M.; Baris, H.N.; Rizel, L.; Banin, E.; Massarweh, A.; Roosing, S.; Lefeber, D.J.; et al. Non-Syndromic Retinitis Pigmentosa Due to Mutations in the Mucopolysaccharidosis Type IIIC Gene, Heparan-Alpha-Glucosaminide N-Acetyltransferase (HGSNAT). Hum. Mol. Genet. 2015, 24, 3742–3751. [Google Scholar] [CrossRef] [PubMed]
- Holanda, I.P.; Rim, P.H.H.; Rare Genomes Project Consortium; Guaragna, M.S.; Gil-da-Silva-Lopes, V.L.; Steiner, C.E. Syndromic Retinitis Pigmentosa: A 15-Patient Study. Genes 2024, 15, 516. [Google Scholar] [CrossRef]
- Tomatsu, S.; Pitz, S.; Hampel, U. Ophthalmological Findings in Mucopolysaccharidoses. J. Clin. Med. 2019, 8, 1467. [Google Scholar] [CrossRef]
- Schiff, E.R.; Daich Varela, M.; Robson, A.G.; Pierpoint, K.; Ba-Abbad, R.; Nutan, S.; Zein, W.M.; Ullah, E.; Huryn, L.A.; Tuupanen, S.; et al. A Genetic and Clinical Study of Individuals with Nonsyndromic Retinopathy Consequent upon Sequence Variants in HGSNAT, the Gene Associated with Sanfilippo C Mucopolysaccharidosis. Am. J. Med. Genet. C Semin. Med. Genet. 2020, 184, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Kammenga, J.E. The Background Puzzle: How Identical Mutations in the Same Gene Lead to Different Disease Symptoms. FEBS J. 2017, 284, 3362–3373. [Google Scholar] [CrossRef]
- Kohlschütter, A.; Schulz, A.; Bartsch, U.; Storch, S. Current and Emerging Treatment Strategies for Neuronal Ceroid Lipofuscinoses. CNS Drugs 2019, 33, 315–325. [Google Scholar] [CrossRef]
- Bauwens, M.; Storch, S.; Weisschuh, N.; Ceuterick-de Groote, C.; De Rycke, R.; Guillemyn, B.; De Jaegere, S.; Coppieters, F.; Van Coster, R.; Leroy, B.P.; et al. Functional Characterization of Novel MFSD8 Pathogenic Variants Anticipates Neurological Involvement in Juvenile Isolated Maculopathy. Clin. Genet. 2020, 97, 426–436. [Google Scholar] [CrossRef]
- Khan, K.N.; El-Asrag, M.E.; Ku, C.A.; Holder, G.E.; McKibbin, M.; Arno, G.; Poulter, J.A.; Carss, K.; Bommireddy, T.; Bagheri, S.; et al. Specific Alleles of CLN7/MFSD8, a Protein That Localizes to Photoreceptor Synaptic Terminals, Cause a Spectrum of Nonsyndromic Retinal Dystrophy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2906–2914. [Google Scholar] [CrossRef]
- Ku, C.A.; Hull, S.; Arno, G.; Vincent, A.; Carss, K.; Kayton, R.; Weeks, D.; Anderson, G.W.; Geraets, R.; Parker, C.; et al. Detailed Clinical Phenotype and Molecular Genetic Findings in CLN3-Associated Isolated Retinal Degeneration. JAMA Ophthalmol. 2017, 135, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Magliyah, M.S.; Geuer, S.; Alsalamah, A.K.; Lenzner, S.; Drasdo, M.; Schatz, P. Association of the Recurrent Rare Variant c.415T>C p.Phe139Leu in CLN5 With a Recessively Inherited Macular Dystrophy. JAMA Ophthalmol. 2021, 139, 1–5. [Google Scholar] [CrossRef]
- Lehman, T.J.A.; Miller, N.; Norquist, B.; Underhill, L.; Keutzer, J. Diagnosis of the Mucopolysaccharidoses. Rheumatology 2011, 50, v41–v48. [Google Scholar] [CrossRef] [PubMed]
- Chih-Kuang, C.; Shuan-Pei, L.; Shyue-Jye, L.; Tuen-Jen, W. MPS Screening Methods, the Berry Spot and Acid Turbidity Tests, Cause a High Incidence of False-Negative Results in Sanfilippo and Morquio Syndromes. J. Clin. Lab. Anal. 2002, 16, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Langereis, E.J.; van den Berg, I.E.T.; Halley, D.J.J.; Poorthuis, B.J.H.M.; Vaz, F.M.; Wokke, J.H.J.; Linthorst, G.E. Considering Fabry, but Diagnosing MPS I: Difficulties in the Diagnostic Process. JIMD Rep. 2012, 9, 117–120. [Google Scholar] [CrossRef]
- Klingelhöfer, D.; Braun, M.; Seeger-Zybok, R.K.; Quarcoo, D.; Brüggmann, D.; Groneberg, D.A. Global Research on Fabry’s Disease: Demands for a Rare Disease. Mol. Genet. Genom. Med. 2020, 8, e1163. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).