Breast Collagen Organization: Variance by Patient Age and Breast Quadrant
Abstract
:1. Introduction
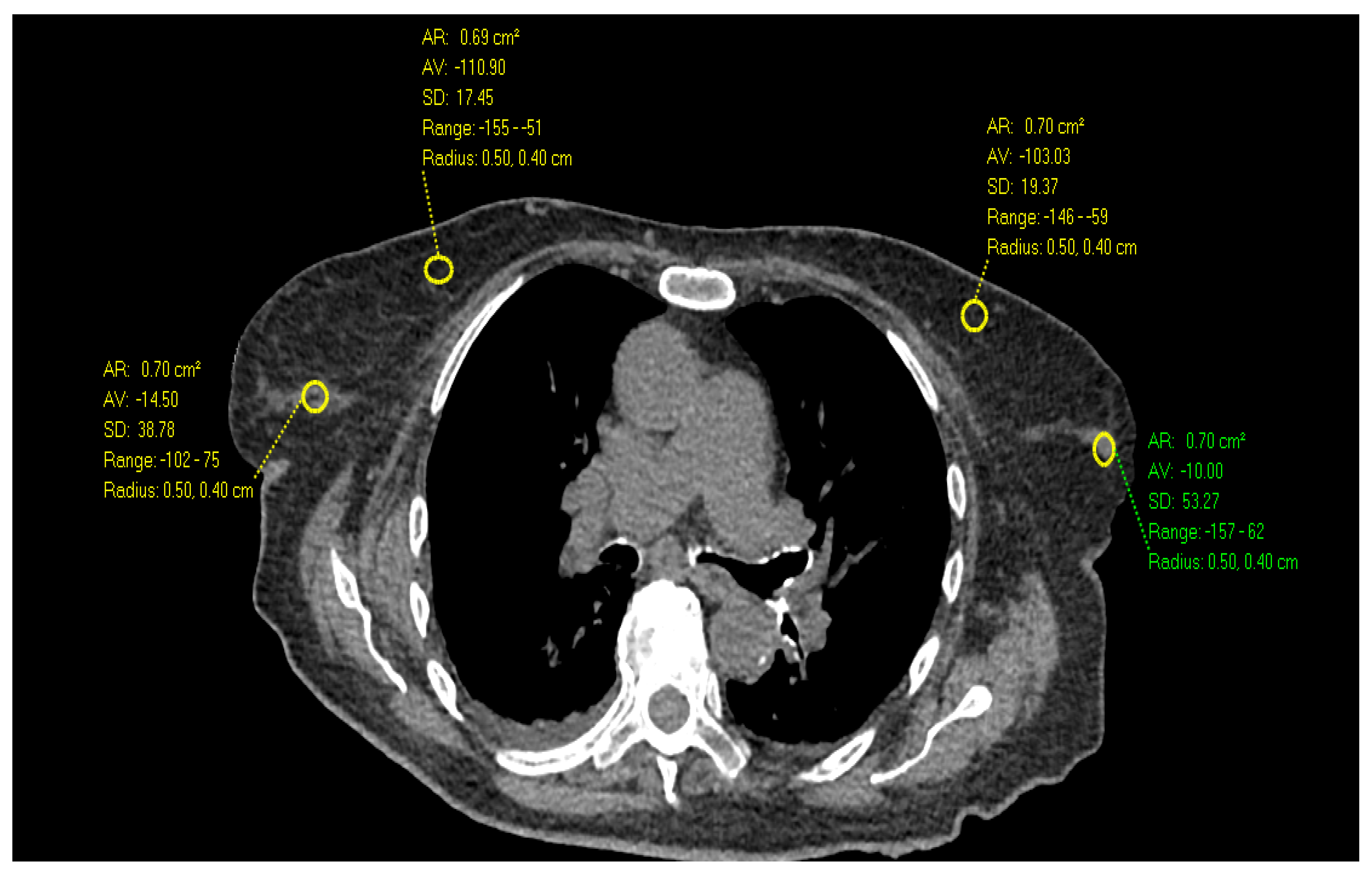
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Cancer Society. Breast Cancer Facts & Figures 2022–2024; American Cancer Society, Inc.: Atlanta, GA, USA, 2022. [Google Scholar]
- Bleyer, A.; Welch, H.G. Effect of three decades of screening mammography on breast-cancer incidence. N. Engl. J. Med. 2012, 367, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Pruthi, S.; Heisey, R.E.; Bevers, T.B. Chemoprevention for Breast Cancer. Ann. Surg. Oncol. 2015, 22, 3230–3235. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Bourguet-Kondracki, M.L.; Hussain, F.; Rauf, A.; Ibrahim, M.; Khalid, M.; Hussain, H.; Hussain, J.; Ali, I.; Khalil, A.A.; et al. The potential role of dietary plant ingredients against mammary cancer: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2580–2605. [Google Scholar] [CrossRef] [PubMed]
- Tarchi, S.M.; Pernia Marin, M.; Hossain, M.M.; Salvatore, M. Breast stiffness, a risk factor for cancer and the role of radiology for diagnosis. J. Transl. Med. 2023, 21, 582. [Google Scholar] [CrossRef] [PubMed]
- Checka, C.M.; Chun, J.E.; Schnabel, F.R.; Lee, J.; Toth, H. The relationship of mammographic density and age: Implications for breast cancer screening. AJR Am. J. Roentgenol. 2012, 198, W292–W295. [Google Scholar] [CrossRef] [PubMed]
- Flugelman, A.A.; Burton, A.; Keinan-Boker, L.; Stein, N.; Kutner, D.; Shemesh, L.; Boyd, N. Correlation between cumulative mammographic density and age-specific incidence of breast cancer: A biethnic study in Israel. Int. J. Cancer 2022, 150, 1968–1977. [Google Scholar] [CrossRef] [PubMed]
- Schreer, I. Dense breast tissue as an important risk factor for breast cancer and implications for early detection. Breast Care 2009, 4, 89–92. [Google Scholar] [CrossRef]
- Nazari, S.S.; Mukherjee, P. An overview of mammographic density and its association with breast cancer. Breast Cancer 2018, 25, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Alowami, S.; Troup, S.; Al-Haddad, S.; Kirkpatrick, I.; Watson, P.H. Mammographic density is related to stroma and stromal proteoglycan expression. Breast Cancer Res. 2003, 5, R129–R135. [Google Scholar] [CrossRef]
- Li, X.; Jin, Y.; Xue, J. Unveiling Collagen’s Role in Breast Cancer: Insights into Expression Patterns, Functions and Clinical Implications. Int. J. Gen. Med. 2024, 17, 1773–1787. [Google Scholar] [CrossRef]
- Jiang, X.; Shen, H.; Shang, X.; Fang, J.; Lu, Y.; Lu, Y.; Zheng, J.; Fu, P. Recent Advances in the Aging Microenvironment of Breast Cancer. Cancers 2022, 14, 4990. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Inman, D.R.; Eliceiri, K.W.; Knittel, J.G.; Yan, L.; Rueden, C.T.; White, J.G.; Keely, P.J. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008, 6, 11. [Google Scholar] [CrossRef]
- Northey, J.J.; Hayward, M.K.; Yui, Y.; Stashko, C.; Kai, F.; Mouw, J.K.; Thakar, D.; Lakins, J.N.; Ironside, A.J.; Samson, S.; et al. Mechanosensitive hormone signaling promotes mammary progenitor expansion and breast cancer risk. Cell Stem Cell 2024, 31, 106–126.e13. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Provenzano, P.P. Aligned forces: Origins and mechanisms of cancer dissemination guided by extracellular matrix architecture. Curr. Opin. Cell Biol. 2021, 72, 63–71. [Google Scholar] [CrossRef]
- Bahcecioglu, G.; Yue, X.; Howe, E.; Guldner, I.; Stack, M.S.; Nakshatri, H.; Zhang, S.; Zorlutuna, P. Aged Breast Extracellular Matrix Drives Mammary Epithelial Cells to an Invasive and Cancer-Like Phenotype. Adv. Sci. 2021, 8, e2100128. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, C.C.; Plymate, S.R.; Reed, M.J. Aging-related alterations in the extracellular matrix modulate the microenvironment and influence tumor progression. Int. J. Cancer 2010, 127, 2739–2748. [Google Scholar] [CrossRef]
- Schietke, R.; Warnecke, C.; Wacker, I.; Schödel, J.; Mole, D.R.; Campean, V.; Amann, K.; Goppelt-Struebe, M.; Behrens, J.; Eckardt, K.U.; et al. The lysyl oxidases LOX and LOXL2 are necessary and sufficient to repress E-cadherin in hypoxia: Insights into cellular transformation processes mediated by HIF-1. J. Biol. Chem. 2010, 285, 6658–6669. [Google Scholar] [CrossRef]
- Liburkin-Dan, T.; Toledano, S.; Neufeld, G. Lysyl Oxidase Family Enzymes and Their Role in Tumor Progression. Int. J. Mol. Sci. 2022, 23, 6249. [Google Scholar] [CrossRef]
- Taylor, M.A.; Amin, J.D.; Kirschmann, D.A.; Schiemann, W.P. Lysyl oxidase contributes to mechanotransduction-mediated regulation of transforming growth factor-β signaling in breast cancer cells. Neoplasia 2011, 13, 406–418. [Google Scholar] [CrossRef]
- Rummel, S.; Hueman, M.T.; Costantino, N.; Shriver, C.D.; Ellsworth, R.E. Tumour location within the breast: Does tumour site have prognostic ability? Ecancermedicalscience 2015, 9, 552. [Google Scholar] [CrossRef]
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The role of collagen in cancer: From bench to bedside. J. Transl. Med. 2019, 17, 309. [Google Scholar] [CrossRef] [PubMed]
- Leung, L.; Niculescu-Duvaz, D.; Smithen, D.; Lopes, F.; Callens, C.; McLeary, R.; Saturno, G.; Davies, L.; Aljarah, M.; Brown, M.; et al. Anti-metastatic Inhibitors of Lysyl Oxidase (LOX): Design and Structure-Activity Relationships. J. Med. Chem. 2019, 62, 5863–5884. [Google Scholar] [CrossRef] [PubMed]
- Lampi, M.C.; Reinhart-King, C.A. Targeting extracellular matrix stiffness to attenuate disease: From molecular mechanisms to clinical trials. Sci. Transl. Med. 2018, 10, eaao0475. [Google Scholar] [CrossRef]
- Vangangelt, K.M.H.; Kramer, C.J.H.; Bastiaannet, E.; Putter, H.; Cohen, D.; van Pelt, G.W.; Rakha, E.A.; Green, A.R.; Tollenaar, R.A.E.M.; Mesker, W.E. The intra-tumoural stroma in patients with breast cancer increases with age. Breast Cancer Res. Treat. 2020, 179, 37–45. [Google Scholar] [CrossRef]
- Desperito, E.; Schwartz, L.; Capaccione, K.M.; Collins, B.T.; Jamabawalikar, S.; Peng, B.; Patrizio, R.; Salvatore, M.M. Chest CT for Breast Cancer Diagnosis. Life 2022, 12, 1699. [Google Scholar] [CrossRef] [PubMed]



| Outer Upper | Outer Lower | Inner Upper | Inner Lower | |
|---|---|---|---|---|
| 3rd decade | 40% | 20% | 20% | 20% |
| 4th decade | 50% | 30% | 20% | 0% |
| 5th decade | 40% | 30% | 30% | 0% |
| 6th decade | 45% | 45% | 15% | 10% |
| 7th decade | 90% | 10% | 0% | 0% |
| 8th decade | 80% | 20% | 0% | 0% |
| 9th decade | 40% | 40% | 10% | 10% |
| Total average | 55% | 27% | 14% | 6% |
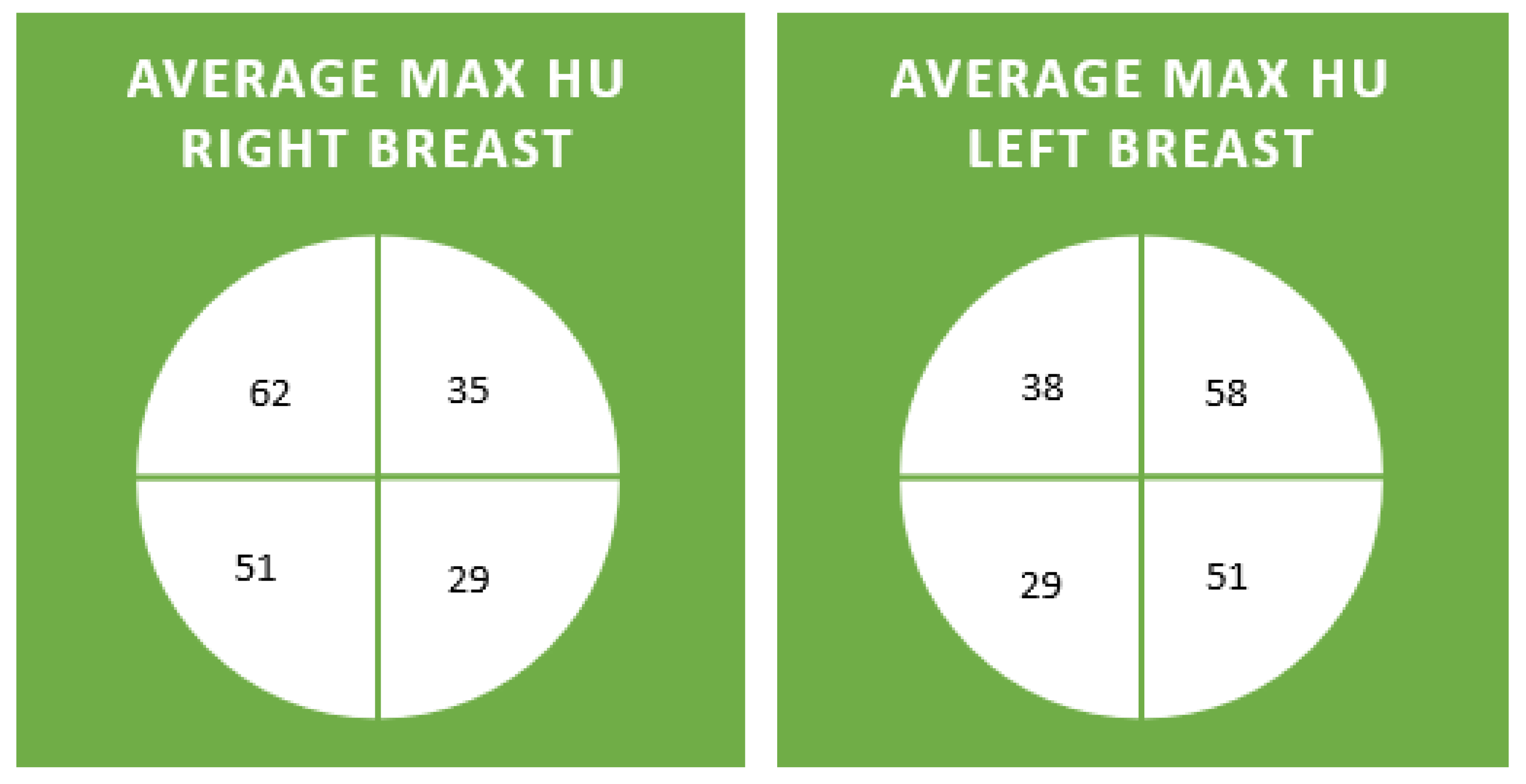
| 3rd Decade | 4th Decade | 5th Decade | 6th Decade | 7th Decade | 8th Decade | 9th Decade | |
|---|---|---|---|---|---|---|---|
| RUO Max | 54 (35–74) | 68 (35–111) | 62 (26–99) | 59 (29–97) | 71 (36–204) | 58 (31–101) | 63 (36–106) |
| LUO Max | 44 (13–71) | 62 (44–79) | 58 (16–86) | 47 (27–84) | 78 (19–195) | 57 (36–85) | 52 (41–63) |
| RLO Max | 39 (21–64) | 57 (21–92) | 57 (34–94) | 60 (29–112) | 45 (22–76) | 47 (18–104) | 53 (43–60) |
| LLO Max | 40 (−17–62) | 62 (18–108) | 51 (4–78) | 40 (23–86) | 46 (7–72) | 44 (4–97) | 59 (35–73) |
| RUI Max | 40 (9–69) | 43 (1–91) | 33 (1–123) | 38 (−19–86) | 30 (-5–59) | 30 (−1–79) | 34 (7–95) |
| LUI Max | 45 (26–81) | 42 (18–70) | 34 (4–80) | 41 (11–84) | 32 (1–84) | 34 (12–80) | 35 (21–58) |
| RLI Max | 33 (5–47) | 36 (−15–81) | 21 (−10–58) | 27 (−14–71) | 18 (−22–46) | 32 (9–86) | 33 (12–60) |
| LLI Max | 24 (−10–101) | 46 (3–75) | 29 (4–55) | 32 (8–81) | 10 (−33–46) | 22 (−9–76) | 31 (4–63) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asiimwe, A.C.; Marin, M.P.; Salvatore, M. Breast Collagen Organization: Variance by Patient Age and Breast Quadrant. Diagnostics 2024, 14, 1748. https://doi.org/10.3390/diagnostics14161748
Asiimwe AC, Marin MP, Salvatore M. Breast Collagen Organization: Variance by Patient Age and Breast Quadrant. Diagnostics. 2024; 14(16):1748. https://doi.org/10.3390/diagnostics14161748
Chicago/Turabian StyleAsiimwe, Arnold Caleb, Monica Pernia Marin, and Mary Salvatore. 2024. "Breast Collagen Organization: Variance by Patient Age and Breast Quadrant" Diagnostics 14, no. 16: 1748. https://doi.org/10.3390/diagnostics14161748




