Myocarditis, Myositis, and Myasthenia Gravis Overlap Syndrome Associated with Immune Checkpoint Inhibitors: A Systematic Review
Abstract
1. Introduction
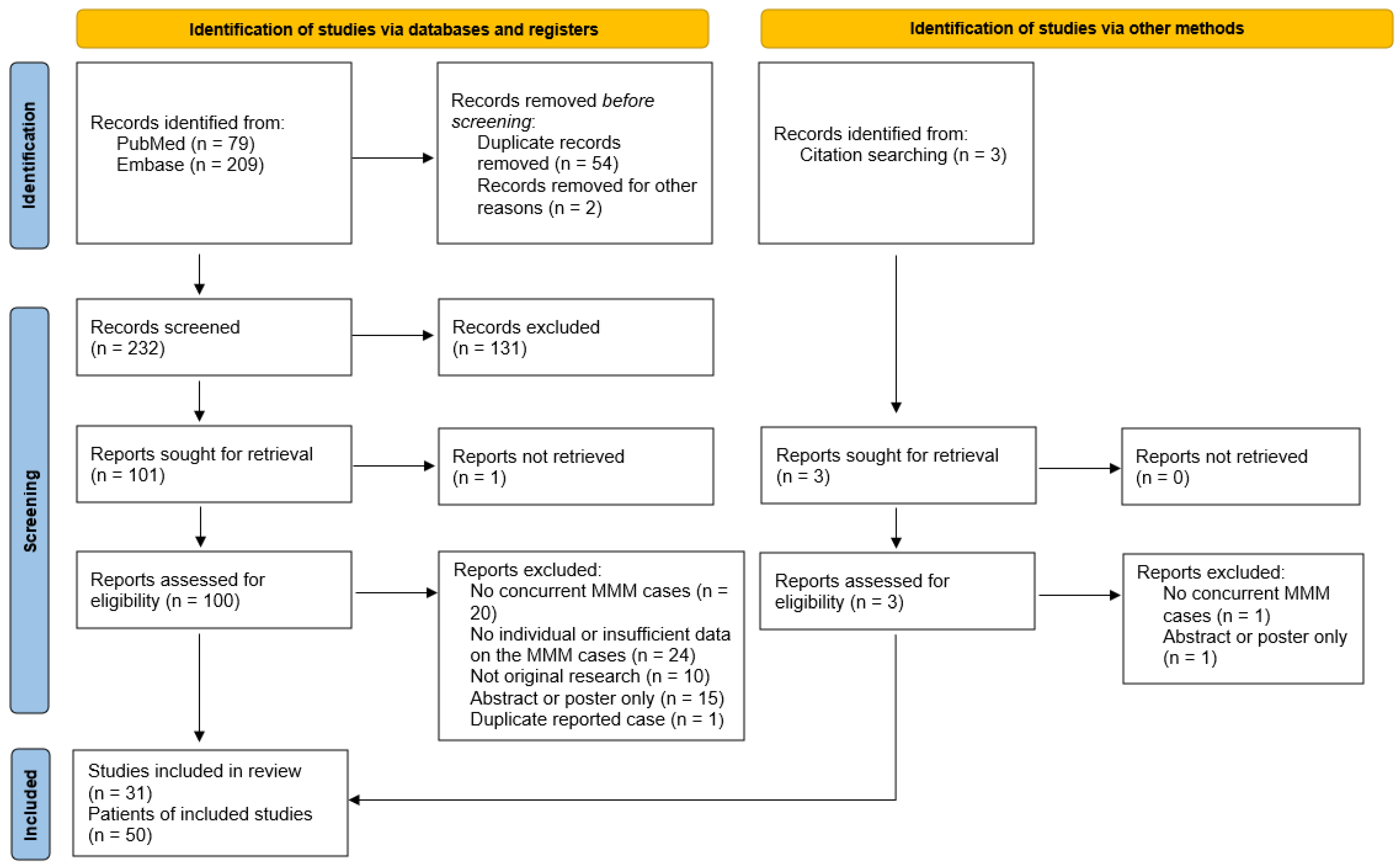
2. Materials and Methods
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, D.B.; Chandra, S.; Sosman, J.A. Immune Checkpoint Inhibitor Toxicity in 2018. JAMA 2018, 320, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Galdiero, M.R.; Marone, G.; Criscuolo, G.; Triassi, M.; Bonaduce, D.; Marone, G.; Tocchetti, C.G. Cardiotoxicity of immune checkpoint inhibitors. ESMO Open 2017, 2, e000247. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Available online: https://www.fda.gov/ (accessed on 28 September 2023).
- El Majzoub, I.; Qdaisat, A.; Thein, K.Z.; Win, M.A.; Han, M.M.; Jacobson, K.; Chaftari, P.S.; Prejean, M.; Reyes-Gibby, C.; Yeung, S.J. Adverse Effects of Immune Checkpoint Therapy in Cancer Patients Visiting the Emergency Department of a Comprehensive Cancer Center. Ann. Emerg. Med. 2019, 73, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Jomaa, M.K.; Ferrarotto, R.; Yeung, S.J.; Hanna, E.Y.; Reyes-Gibby, C.C. Serious immune-related adverse events in patients with head and neck cancer after checkpoint blockade: Systematic review. Head Neck 2019, 41, 4036–4050. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.R.; Florido, R.; Lipson, E.J.; Naidoo, J.; Ardehali, R.; Tocchetti, C.G.; Lyon, A.R.; Padera, R.F.; Johnson, D.B.; Moslehi, J. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc. Res. 2019, 115, 854–868. [Google Scholar] [CrossRef] [PubMed]
- Laenens, D.; Yu, Y.; Santens, B.; Jacobs, J.; Beuselinck, B.; Bechter, O.; Wauters, E.; Staessen, J.; Janssens, S.; Van Aelst, L. Incidence of Cardiovascular Events in Patients Treated with Immune Checkpoint Inhibitors. J. Clin. Oncol. 2022, 40, 3430–3438. [Google Scholar] [CrossRef] [PubMed]
- Raschi, E.; Rossi, S.; De Giglio, A.; Fusaroli, M.; Burgazzi, F.; Rinaldi, R.; Potena, L. Cardiovascular Toxicity of Immune Checkpoint Inhibitors: A Guide for Clinicians. Drug Saf. 2023, 46, 819–833. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Marone, G.; Mercurio, V.; Galdiero, M.R.; Bonaduce, D.; Tocchetti, C.G. Immune Checkpoint Inhibitors and Cardiac Toxicity: An Emerging Issue. Curr. Med. Chem. 2018, 25, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.Z.; Aqeel, S.B.; Lingamaneni, P.; Pichardo, R.C.; Jawed, A.; Khalid, S.; Banskota, S.U.; Fu, P.; Mangla, A. Association of Immune Checkpoint Inhibitors with Neurologic Adverse Events: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e227722. [Google Scholar] [CrossRef] [PubMed]
- Haugh, A.M.; Probasco, J.C.; Johnson, D.B. Neurologic complications of immune checkpoint inhibitors. Expert Opin. Drug Saf. 2020, 19, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Marini, A.; Bernardini, A.; Gigli, G.L.; Valente, M.; Muniz-Castrillo, S.; Honnorat, J.; Vogrig, A. Neurologic Adverse Events of Immune Checkpoint Inhibitors: A Systematic Review. Neurology 2021, 96, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Psimaras, D.; Velasco, R.; Birzu, C.; Tamburin, S.; Lustberg, M.; Bruna, J.; Argyriou, A.A. Immune checkpoint inhibitors-induced neuromuscular toxicity: From pathogenesis to treatment. J. Peripher. Nerv. Syst. 2019, 24 (Suppl. S2), S74–S85. [Google Scholar] [CrossRef] [PubMed]
- Fazel, M.; Jedlowski, P.M. Severe Myositis, Myocarditis, and Myasthenia Gravis with Elevated Anti-Striated Muscle Antibody following Single Dose of Ipilimumab-Nivolumab Therapy in a Patient with Metastatic Melanoma. Case Rep. Immunol. 2019, 2019, 2539493. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.S.; Fradley, M.G.; Cohen, J.V.; Nohria, A.; Reynolds, K.L.; Heinzerling, L.M.; Sullivan, R.J.; Damrongwatanasuk, R.; Chen, C.L.; Gupta, D.; et al. Myocarditis in Patients Treated with Immune Checkpoint Inhibitors. J. Am. Coll. Cardiol. 2018, 71, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, J.J.; Salem, J.E.; Sosman, J.A.; Lebrun-Vignes, B.; Johnson, D.B. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 2018, 391, 933. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; Lopez-Fernandez, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef] [PubMed]
- Anquetil, C.; Salem, J.E.; Lebrun-Vignes, B.; Johnson, D.B.; Mammen, A.L.; Stenzel, W.; Leonard-Louis, S.; Benveniste, O.; Moslehi, J.J.; Allenbach, Y. Immune Checkpoint Inhibitor-Associated Myositis: Expanding the Spectrum of Cardiac Complications of the Immunotherapy Revolution. Circulation 2018, 138, 743–745. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Talamo, L.; Dillon, P.; Gentzler, R.D.; Millard, T.; Salerno, M.; Slingluff, C.L., Jr.; Gaughan, E.M. Severe combined cardiac and neuromuscular toxicity from immune checkpoint blockade: An institutional case series. Cardiooncology 2020, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Cham, J.; Ng, D.; Nicholson, L. Durvalumab-induced myocarditis, myositis, and myasthenia gravis: A case report. J. Med. Case Rep. 2021, 15, 278. [Google Scholar] [CrossRef] [PubMed]
- Fazal, M.; Prentice, D.A.; Kho, L.K.; Fysh, E. Nivolumab-associated myositis myocarditis and myasthenia and anti-striated muscle antibodies. Intern. Med. J. 2020, 50, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, N.; Etchegaray, M.; Henry, J.; Lelenwa, L.; Zhao, B.; Segura, A.; Buja, L.M. The Terrible Triad of Checkpoint Inhibition: A Case Report of Myasthenia Gravis, Myocarditis, and Myositis Induced by Cemiplimab in a Patient with Metastatic Cutaneous Squamous Cell Carcinoma. Case Rep. Immunol. 2020, 2020, 5126717. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.H.; Grubb, W.; Sawlani, K.; Gibson, M.K.; Hoimes, C.J.; Rogers, L.R.; Lavertu, P.; Yao, M. Immune checkpoint-mediated myositis and myasthenia gravis: A case report and review of evaluation and management. Am. J. Otolaryngol. 2018, 39, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Fukushima, S.; Miyashita, A.; Aoi, J.; Jinnin, M.; Kosaka, T.; Ando, Y.; Matsukawa, M.; Inoue, H.; Kiyotani, K.; et al. Myasthenic crisis and polymyositis induced by one dose of nivolumab. Cancer Sci. 2016, 107, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Lipe, D.N.; Galvis-Carvajal, E.; Rajha, E.; Wechsler, A.H.; Gaeta, S. Immune checkpoint inhibitor-associated myasthenia gravis, myositis, and myocarditis overlap syndrome. Am. J. Emerg. Med. 2021, 46, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T.; Kiniwa, Y.; Sato, R.; Sano, T.; Nakamura, K.; Mikoshiba, Y.; Ohashi, N.; Sekijima, Y.; Okuyama, R. Presence of antibodies to striated muscle and acetylcholine receptor in association with occurrence of myasthenia gravis with myositis and myocarditis in a patient with melanoma treated with an anti-programmed death 1 antibody. Eur. J. Cancer 2019, 106, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Ishikawa, N.; Konoeda, F.; Seki, N.; Fukushima, S.; Takahashi, K.; Uhara, H.; Hasegawa, Y.; Inomata, S.; Otani, Y.; et al. Nivolumab-related myasthenia gravis with myositis and myocarditis in Japan. Neurology 2017, 89, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Todo, M.; Kaneko, G.; Shirotake, S.; Shimada, Y.; Nakano, S.; Okabe, T.; Ishikawa, S.; Oyama, M.; Nishimoto, K. Pembrolizumab-induced myasthenia gravis with myositis and presumable myocarditis in a patient with bladder cancer. IJU Case Rep. 2020, 3, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Xing, Q.; Zhang, Z.W.; Lin, Q.H.; Shen, L.H.; Wang, P.M.; Zhang, S.; Fan, M.; Zhu, B. Myositis-myasthenia gravis overlap syndrome complicated with myasthenia crisis and myocarditis associated with anti-programmed cell death-1 (sintilimab) therapy for lung adenocarcinoma. Ann. Transl. Med. 2020, 8, 250. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Chapter 7: Systematic reviews of etiology and risk. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: Eagan, MN, USA, 2020. [Google Scholar]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies. JBI Database Syst. Rev. Implement. Rep. 2020, 18, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Ahdi, H.S.; Abdulmujeeb, S.; Nabrinsky, E. Multiple Autoimmune Complications After a Single Dose of Pembrolizumab. Cureus 2023, 15, e35871. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Li, D.; Yang, P.; Xu, K.; Wang, Y.; Li, Q.; Liu, J.; Du, W.; Zhang, F.; Feng, R. Camrelizumab-Related Myocarditis and Myositis With Myasthenia Gravis: A Case Report and Literature Review. Front. Oncol. 2021, 11, 778185. [Google Scholar] [CrossRef] [PubMed]
- Bawek, S.J.; Ton, R.; McGovern-Poore, M.; Khoncarly, B.; Narvel, R. Nivolumab-Induced Myasthenia Gravis Concomitant With Myocarditis, Myositis, and Hepatitis. Cureus 2021, 13, e18040. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, K.; Buhlaiga, N.; Thebault, P.; Lapointe, R.; Johnson, N.A.; Miller, W.H., Jr. Alemtuzumab for Immune-Related Myocarditis Due to PD-1 Therapy. N. Engl. J. Med. 2019, 380, 2375–2376. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, E.; Bonasoni, M.P.; D’Aleo, M.; Tamagnini, I.; Tudini, M.; Fais, P.; Pelotti, S. Pembrolizumab-Induced Fatal Myasthenia, Myocarditis, and Myositis in a Patient with Metastatic Melanoma: Autopsy, Histological, and Immunohistochemical Findings-A Case Report and Literature Review. Int. J. Mol. Sci. 2023, 24, 10919. [Google Scholar] [CrossRef] [PubMed]
- Golec, S.; Mitrani, L.; Yoon, J.; Dangayach, N.; Sahni, G.D. Triple-M (Myocarditis-Myositis-Myasthenia Gravis) Syndrome in a Patient Receiving Nivolumab: A Cardio-Oncologic Emergency. J. Am. Coll. Cardiol. 2023, 81, 3459. [Google Scholar] [CrossRef]
- Hyun, J.W.; Kim, K.H.; Kim, S.H.; Kim, H.J. Severe neuromuscular immune-related adverse events of immune checkpoint inhibitors at national cancer center in Korea. J. Cancer Res. Clin. Oncol. 2023, 149, 5583–5589. [Google Scholar] [CrossRef] [PubMed]
- Konstantina, T.; Konstantinos, R.; Anastasios, K.; Anastasia, M.; Eleni, L.; Ioannis, S.; Sofia, A.; Dimitris, M. Fatal adverse events in two thymoma patients treated with anti-PD-1 immune check point inhibitor and literature review. Lung Cancer 2019, 135, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Guan, W.; Li, B.; Deng, H.; Chen, Y.; Yang, Y.; Qiu, G.; Xie, X.; Zhou, C. A case report and literature review on respiratory failure with immune checkpoint inhibitors: A life-threatening adverse event. Immunopharmacol. Immunotoxicol. 2023, 45, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Luecke, E.; Ganzert, C.; Vielhaber, S.; Haybaeck, J.; Jechorek, D.; Mawrin, C.; Schreiber, J. Immune Checkpoint Inhibitor-induced Fatal Myositis in a Patient With Squamous Cell Carcinoma and a History of Thymoma. Clin. Lung Cancer 2020, 21, e246–e249. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.B.; Tang, W.; Zeng, Q.; Duan, W.; Li, S.; Yang, X.; Bi, F. Case Report: The Neuromusclar Triad of Immune Checkpoint Inhibitors: A Case Report of Myositis, Myocarditis, and Myasthenia Gravis Overlap Following Toripalimab Treatment. Front. Cardiovasc. Med. 2021, 8, 714460. [Google Scholar] [CrossRef] [PubMed]
- Marco, C.; Simo, M.; Alemany, M.; Casasnovas, C.; Dominguez, R.; Vilarino, N.; Calvo, M.; Martin-Liberal, J.; Brenes, J.; Sabater-Riera, J.; et al. Myasthenia Gravis Induced by Immune Checkpoint Inhibitors: An Emerging Neurotoxicity in Neuro-Oncology Practice: Case Series. J. Clin. Med. 2022, 12, 130. [Google Scholar] [CrossRef] [PubMed]
- Masood, A.; Mootoo, A.; Maghsoudlou, P.; D’Cruz, D.; Srikandarajah, K.; Harries, M.; Hart, N.; Papa, S.; Spicer, J. The threat of triple M and autoimmune overlap syndromes with immune checkpoint inhibitors—A series of case reports. Autoimmun. Rev. 2023, 22, 103269. [Google Scholar] [CrossRef]
- Nakagomi, Y.; Tajiri, K.; Shimada, S.; Li, S.; Inoue, K.; Murakata, Y.; Murata, M.; Sakai, S.; Sato, K.; Ieda, M. Immune Checkpoint Inhibitor-Related Myositis Overlapping With Myocarditis: An Institutional Case Series and a Systematic Review of Literature. Front. Pharmacol. 2022, 13, 884776. [Google Scholar] [CrossRef] [PubMed]
- Saishu, Y.; Yoshida, T.; Seino, Y.; Nomura, T. Nivolumab-related myasthenia gravis with myositis requiring prolonged mechanical ventilation: A case report. J. Med. Case Rep. 2022, 16, 61. [Google Scholar] [CrossRef] [PubMed]
- Soman, B.; Dias, M.C.; Rizvi, A.; Kardos, A. Fatal Triad of Immune Checkpoint Inhibitor Therapy. JACC Cardio Oncol. 2022, 4, S11–S12. [Google Scholar] [CrossRef]
- Wai Siu, D.H.; O’Neill, R.S.; Harris, C.A.; Wang, J.; Ardolino, L.; Downton, T.; Tong, M.; Hong, J.H.; Chin, V.; Clingan, P.R.; et al. Immune checkpoint inhibitor-induced myocarditis, myositis, myasthenia gravis and transaminitis: A case series and review. Immunotherapy 2022, 14, 511–520. [Google Scholar] [CrossRef]
- Wang, S.; Peng, D.; Zhu, H.; Min, W.; Xue, M.; Wu, R.; Shao, Y.; Pan, L.; Zhu, M. Acetylcholine receptor binding antibody-associated myasthenia gravis, myocarditis, and rhabdomyolysis induced by tislelizumab in a patient with colon cancer: A case report and literature review. Front. Oncol. 2022, 12, 1053370. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-C.; Chen, M.-H. Triad of myasthenia gravis, myositis, and myocarditis after nivolumab administration in a patient with cholangiocarcinoma. J. Cancer Res. Pract. 2022, 9, 153–155. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, L.; Wang, D.; Hui, B.; Li, X.; Zhou, Y.; Chen, X.; Gu, Y. Anti-PD-1 and regorafenib induce severe multisystem adverse events in microsatellite stability metastatic colorectal cancer: A case report. Immunotherapy 2021, 13, 1317–1323. [Google Scholar] [CrossRef]
- Yang, Z.X.; Chen, X.; Tang, S.Q.; Zhang, Q. Sintilimab-Induced Myocarditis Overlapping Myositis in a Patient With Metastatic Thymoma: A Case Report. Front. Cardiovasc. Med. 2021, 8, 797009. [Google Scholar] [CrossRef]
- Yin, B.; Xiao, J.; Wang, X.; Li, X.; Guan, Y.; Chen, J.; Han, P.; Li, K.; Wang, J. Myocarditis and myositis/myasthenia gravis overlap syndrome induced by immune checkpoint inhibitor followed by esophageal hiatal hernia: A case report and review of the literature. Front. Med. 2022, 9, 950801. [Google Scholar] [CrossRef]
- Aggarwal, N.; Bianchini, D.; Parkar, R.; Turner, J. Immunotherapy-Induced Overlap Syndrome: Myositis, Myasthenia Gravis, and Myocarditis-A Case Series. Case Rep. Med. 2024, 2024, 5399073. [Google Scholar] [CrossRef]
- Basnet, A.; Sharma, N.R.; Gautam, S.; Lamichhane, S.; Kansakar, S.; Tiwari, K.; Pokhrel, M.; Singh, S. Immune checkpoint inhibitor-induced myasthenia gravis, myocarditis, and myositis: A case report. Clin. Case Rep. 2024, 12, e8968. [Google Scholar] [CrossRef] [PubMed]
- Byer, S.H.; Stewart, C.; Mansour, S.; Grewal, U.S. Novel use of abatacept and ruxolitinib as salvage therapy in steroid-refractory immune checkpoint blockade-induced myocarditis with myasthenia and myositis overlap syndrome. Eur. J. Cancer 2024, 202, 114027. [Google Scholar] [CrossRef] [PubMed]
- Cooksley, T.; Weaver, J.; McNamara, M.; Lorigan, P. Immune checkpoint inhibitor-related myasthenia gravis, myositis and myocarditis: A triad but not at the same time? QJM 2024, 117, 373–374. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, J.A.; Hanmandlu, A.; Wegner, R.; Botdorf, J.; Tummala, S.; Iliescu, C.A.; Nates, J.L.; Reddy, D.R. Management of respiratory failure in immune checkpoint inhibitors-induced overlap syndrome: A case series and review of the literature. BMC Anesthesiol. 2023, 23, 310. [Google Scholar] [CrossRef] [PubMed]
- Emile, J.; Cauquil, C.; Carpentier, D.; Routier, E.; Robert, C. Fatal myasthenia gravis (MG) associated with myositis and myocarditis in a patient with pre-existing MG treated by adjuvant nivolumab for a stage III melanoma. Eur. J. Cancer 2024, 205, 114098. [Google Scholar] [CrossRef] [PubMed]
- Otto, F.; Seiberl, M.; Bieler, L.; Moser, T.; Kleindienst, W.; Wallner-Essl, W.; Koelblinger, P.; Wipfler, P.; Harrer, A. Beyond T cell toxicity—Intrathecal chemokine CXCL13 indicating B cell involvement in immune-related adverse events following checkpoint inhibition: A two-case series and literature review. Eur. J. Neurol. 2024, 31, e16279. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Chen, S.; Gui, Q.; Zhang, T.; Li, Y.; Du, Z.; Lv, Y.; Du, X.; Hu, Y.; Liu, Z. Prognosis of immune checkpoint inhibitor-induced myasthenia gravis: A single center experience and systematic review. Front. Neurol. 2024, 15, 1372861. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Hontsu, S.; Hiraoka, J.; Yamanaka, A.; Fujioka, N.; Shimada, D.; Okuda, Y.; Sugie, K.; Muro, S. A Rare Case of Overlapping Durvalumab-induced Myositis, Takotsubo-like Morphological Changes Caused by Myocarditis, and Myasthenia Gravis. Intern. Med. 2024, 3028-23. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Young, K. Exploring Pembrolizumab-Induced Myocarditis, Myositis, and Myasthenia Gravis: A Comprehensive Literature Review and Case Presentation on Bladder Cancer. Cureus 2023, 15, e49867. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, R.; Baba, K.; Furuta, R.; Maesaka, H.; Hirosawa, H.; Bando, T.; Oshima, A.; Onoda, H.; Nukui, T.; Dougu, N.; et al. A Case of Liver Cancer with Overlapping Myasthenia Gravis, Myocarditis, Seronegative Autoimmune Autonomic Ganglionopathy, and Myositis Symptoms Induced by Atezolizumab: A Case Report. Intern. Med. 2024, 63, 2193–2198. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.M.; Dodd, K.; Knight, T.; Chaudhri, M.; Khera, R.; Lilleker, J.B.; Roberts, M.; Lorigan, P.; Cooksley, T. Improved outcomes with early immunosuppression in patients with immune-checkpoint inhibitor induced myasthenia gravis, myocarditis and myositis: A case series. Support. Care Cancer 2023, 31, 518. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, S.; Price, H.; Drews, R.; Bouffard, M.A.; Young, L.H.; Narayanaswami, P. Novel uses of complement inhibitors in myasthenia gravis-Two case reports. Muscle Nerve 2024, 69, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Haanen, J.; Obeid, M.; Spain, L.; Carbonnel, F.; Wang, Y.; Robert, C.; Lyon, A.R.; Wick, W.; Kostine, M.; Peters, S.; et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1217–1238. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.B.; Jalal, S.I.; Durm, G.A. Physician awareness of immune-related adverse events from checkpoint inhibitors. J. Clin. Oncol. 2022, 40, 6571. [Google Scholar] [CrossRef]
- Koyyala, V.P.B.; Chandra, S.; Goel, V.; Pasricha, S.; Gupta, M.; Muppalla, B.S.; Vanapala, K.; Gupta, S.K.; Gupta, D.; Sen, S.; et al. 76P Need for awareness about immune-related adverse events (iRAEs) among community physicians in India. Ann. Oncol. 2021, 32, S1405. [Google Scholar] [CrossRef]
- Wattana, M.K.; Lindsay, A.; Davenport, M.; Pettit, N.R.; Menendez, J.R.; Li, Z.; Lipe, D.N.; Qdaisat, A.; Bischof, J.J. Current gaps in emergency medicine core content education for oncologic emergencies: A targeted needs assessment. AEM Educ. Train. 2024, 8, e10987. [Google Scholar] [CrossRef] [PubMed]
- Gilon, D.; Iakobishvili, Z.; Leibowitz, D. The Diagnosis and Management of Immune Checkpoint Inhibitor Cardiovascular Toxicity: Myocarditis and Beyond. Vaccines 2022, 10, 304. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Gibby, C.C.; Qdaisat, A.; Ferrarotto, R.; Fadol, A.; Bischof, J.J.; Coyne, C.J.; Lipe, D.N.; Hanna, E.Y.; Shete, S.; Abe, J.I.; et al. Cardiovascular events after cancer immunotherapy as oncologic emergencies: Analyses of 610 head and neck cancer patients treated with immune checkpoint inhibitors. Head Neck 2024, 46, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Qdaisat, A.; Reyes-Gibby, C.C.; Abe, J.-I.; Palaskas, N.; Shete, S.S.; Yeung, S.-C. Cardiovascular Events as Oncologic Emergencies in Patients on Immune- Checkpoint Inhibitor Therapy. J. Emerg. Med. 2024, 66, e52–e53. [Google Scholar] [CrossRef]
- Herrmann, J.; Lenihan, D.; Armenian, S.; Barac, A.; Blaes, A.; Cardinale, D.; Carver, J.; Dent, S.; Ky, B.; Lyon, A.R.; et al. Defining cardiovascular toxicities of cancer therapies: An International Cardio-Oncology Society (IC-OS) consensus statement. Eur. Heart J. 2022, 43, 280–299. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chen, Z.; Song, W.; Xu, Y.; Zhao, Z.; Sun, Y.; Wang, Y.; Geng, X.; Zhao, J.; Zhang, X.; et al. Cardiovascular adverse events associated with immune checkpoint inhibitors: A retrospective multicenter cohort study. Cancer Med. 2024, 13, e7233. [Google Scholar] [CrossRef] [PubMed]
- Vasbinder, A.; Ismail, A.; Salem, J.E.; Hayek, S.S. Role of Biomarkers in the Management of Immune-Checkpoint Inhibitor-Related Myocarditis. Curr. Cardiol. Rep. 2023, 25, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Sundarrajan, C.; Bhai, S.; Dimachkie, M.M. Immune checkpoint inhibitor-related myositis: From pathophysiology to treatment. Clin. Exp. Rheumatol. 2023, 41, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Chen, Y.P.; Lin, W.C.; Su, W.C.; Sun, Y.T. Immune Checkpoint Inhibitor-Induced Myasthenia Gravis. Front. Neurol. 2020, 11, 634. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B.; Benesova, K.; Hassel, J.C.; Wick, W.; Jordan, K. How we identify and treat neuromuscular toxicity induced by immune checkpoint inhibitors. ESMO Open 2021, 6, 100317. [Google Scholar] [CrossRef] [PubMed]
- Zubair, A.S.; Roy, B.; Baehring, J.M.; Nowak, R.J. Myasthenia Gravis in the Setting of Immune Checkpoint Inhibitor Therapy: Practical Considerations and Opinion-Based Approach to Acute Management. Cureus 2022, 14, e30638. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Manouchehri, A.; Haugh, A.M.; Quach, H.T.; Balko, J.M.; Lebrun-Vignes, B.; Mammen, A.; Moslehi, J.J.; Salem, J.E. Neurologic toxicity associated with immune checkpoint inhibitors: A pharmacovigilance study. J. Immunother. Cancer 2019, 7, 134. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.J.; Qdaisat, A.; Chaftari, P.; Lipe, D.; Merlin, J.; Rajha, E.; Wechsler, A.; Sandoval, M.; Viets, J.; Al-Breiki, A.; et al. Diagnosis and management of immune-related adverse effects of immune checkpoint therapy in the emergency department. J. Am. Coll. Emerg. Physicians Open 2020, 1, 1637–1659. [Google Scholar] [CrossRef] [PubMed]
- Verheijden, R.J.; van Eijs, M.J.M.; May, A.M.; van Wijk, F.; Suijkerbuijk, K.P.M. Immunosuppression for immune-related adverse events during checkpoint inhibition: An intricate balance. NPJ Precis. Oncol. 2023, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.; Katel, A.; Massarelli, E.; Villaflor, V.M.; Sun, V.; Salgia, R. Immune Checkpoint Inhibitor-Induced Myocarditis with Myositis/Myasthenia Gravis Overlap Syndrome: A Systematic Review of Cases. Oncologist 2021, 26, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | n (%) |
|---|---|
| Total | 50 |
| Age, median (IQR), years | 70 (65–75) |
| Sex | |
| Female | 16 (32) |
| Male | 34 (68) |
| Cancer type | |
| Melanoma | 14 (28) |
| Lung cancer | 10 (20) |
| Renal cancer | 7 (14) |
| Thymoma | 6 (12) |
| Bladder cancer | 3 (6) |
| Colorectal cancer | 2 (4) |
| Esophageal cancer | 2 (4) |
| Cholangiocarcinoma | 2 (4) |
| Head and neck cancer | 1 (2) |
| Breast cancer | 1 (2) |
| Prostate cancer | 1 (2) |
| Sarcoma | 1 (2) |
| Days from ICI to presentation, median (range) | 21 (15–28) |
| Immune checkpoint inhibitor type 1 | |
| Pembrolizumab | 20 (40) |
| Nivolumab | 15 (30) |
| Ipilimumab | 8 (16) |
| Sintilimab | 5 (10) |
| Durvalumab | 4 (8) |
| Camrelizumab | 1 (2) |
| Avelumab | 1 (2) |
| Cemiplimab | 1 (2) |
| Tremelimumab | 1 (2) |
| Spartalizumab | 1 (2) |
| Tislelizumab | 1 (2) |
| Toripalimab | 1 (2) |
| Reported in-hospital mortality | |
| No | 31 (62) |
| Yes | 19 (38) |
| Sign/Symptom | n (%) 1 |
|---|---|
| Ptosis | 29 (58) |
| Dyspnea | 24 (48) |
| Diplopia | 21 (42) |
| Myalgia | 18 (36) |
| Muscle weakness | 14 (28) |
| Dysphagia | 13 (26) |
| Fatigue | 12 (24) |
| Dysphonia | 6 (12) |
| Chest pain or tightness | 4 (8) |
| Palpitations | 4 (8) |
| Dizziness | 4 (8) |
| Dysarthria | 4 (8) |
| Extraocular muscle deficit | 3 (6) |
| Neck weakness | 3 (6) |
| Presyncope | 3 (6) |
| Head droop | 2 (4) |
| Blurred vision | 2 (4) |
| Paresis | 2 (4) |
| Rash | 2 (4) |
| Gait problems | 1 (2) |
| Facial droop | 1 (2) |
| Arthralgia | 1 (2) |
| Malaise | 1 (2) |
| Fever | 1 (2) |
| Other visual problems | 6 (12) |
| Others | 8 (16) |
| Treatment | n (%) 1 |
|---|---|
| Corticosteroids | 49 (98.0) |
| Intravenous immunoglobulin | 26 (52.0) |
| Plasma exchange | 18 (36.0) |
| Pyridostigmine | 10 (20.0) |
| Mycophenolate mofetil | 9 (18.0) |
| Rituximab | 7 (14.0) |
| Cyclophosphamide | 4 (8.0) |
| Infliximab | 4 (8.0) |
| Anti-thymocyte globulin | 3 (6.0) |
| Plasmapheresis | 2 (4.0) |
| Physostigmine | 1 (2.0) |
| Alemtuzumab | 1 (2.0) |
| Author | Patient Sequence | Age, Years | Treatment Strategy 1 | Clinical Response 1 |
|---|---|---|---|---|
| Shirai et al. [30] | 1 | 83 | 3 days of methylprednisolone 1000 mg/day, then tapered prednisolone (1 mg/kg/day to 30 mg/day) and 4 cycles of plasma exchange | ECG and blood tests improved shortly after therapy initiation. Wide QRS and AV block improved in 3 days. CK and troponin-T levels decreased. Ptosis, ophthalmoplegia, and neck weakness improved after 6 weeks. |
| Esfahani et al. [41] | 1 | 71 | 3 days of methylprednisolone 1 g/day, then 200 mg/day. Mycophenolate mofetil 1 g twice/day. Plasmapheresis daily for 5 days. Rituximab IV 375 mg/m² weekly. Alemtuzumab 30 mg on day 18. Weaned off rituximab, glucocorticoids, and mycophenolate mofetil over 4 weeks. | Initial response with improved biochemical variables by day 7. Developed cardiac arrhythmias on day 18. Resolution of myocarditis and myositis by day 28. Weaned off all treatments by day 50. |
| Fazel et al. [17] | 1 | 78 | Methylprednisolone IV: 75 mg (day 1), 125 mg (days 2–3), 1000 mg (days 4–6), 150 mg (day 7), 75 mg (day 8). IVIG 2 mg/kg (days 5–6). Plasmapheresis 1 cycle (day 7). | Biomarkers decreased. Muscle weakness slightly improved. Bulbar symptoms worsened, leading to discharge to hospice. |
| Konstantina et al. [45] | 1 | 30 | Prednisolone 2 mg/kg, pyridostigmine, IVIG (400 mg/kg for 5 days), rituximab 375/m² weekly | Developed eyelid drop, diplopia, respiratory failure, liver transaminases increase. Intubated, ICU 30 days. Improved with rituximab, weaned from ventilation. Developed septic shock, died on day 64. |
| Todo et al. [32] | 1 | 63 | Prednisolone 1 mg/kg (60 mg/day), tapered over 321 days | Biomarkers gradually decreased, and symptoms improved. |
| Arora et al. [23] | 1 | 70 | IV steroids 1 mg/kg initially, then ATG and increase in steroids to 1 g methylprednisolone. MMF and cyclophosphamide on day 5, plasmapheresis on day 6 | Progressive cardiac abnormalities, cardiac arrest, intubation for respiratory failure, died after unsuccessful resuscitation. |
| 2 | 79 | IV steroids 1 mg/kg, increased to 1 g/day, ATG and MMF on day 1, cyclophosphamide on day 3, IVIG for MG | Cardiac biomarkers decreased, a permanent pacemaker was placed, and there was no improvement in generalized weakness or ophthalmoplegia. Complications including GI bleed and PE, transitioned to hospice. | |
| 3 | 61 | IV steroids 2 mg/kg, pyridostigmine, MMF added for progressive troponin increase | Troponin decreased with MMF. After discharge, she returned to the hospital with chest pressure, developed SIADH hypercapnic respiratory failure, and transitioned to comfort measures. | |
| 4 | 67 | IV steroids 2 mg/kg, MMF, increased to 1 g methylprednisolone, ATG, plasmapheresis | Developed hypercapnic respiratory failure, troponin rose while CK and transaminases decreased, transitioned to comfort measures. | |
| 5 | 70 | IV steroids, plasmapheresis, and initial steroids were increased to methylprednisolone 1 g per day. Infliximab | The patient required intubation for respiratory failure. Developed upper GI bleeding from immune-related gastritis, and Infliximab was given. Deteriorated with dysphagia, dyspnea, required intubation, GI bleeding, persistent respiratory failure, transitioned to comfort measures. | |
| 6 | 89 | IV steroids 1 mg/kg. | Multiple episodes of nonsustained ventricular tachycardia and high-degree AV block transitioned to comfort measures. | |
| Fazal et al. [25] | 1 | 82 | Started on IV immunoglobulin 0.4 g/kg/day for 5 days. Upon rapid deterioration, high-dose IV methylprednisolone 1 g was started. Dual antiplatelets were given for troponin rise. He was then transitioned to oral prednisolone, and pyridostigmine was initiated. | Showed initial improvement and was extubated within 48 h. Ptosis and dysarthria continued. Reintubated due to respiratory failure. Experienced GI bleeding, fevers, hemodynamic deterioration, and death. |
| Jejakumar et al. [26] | 1 | 86 | IV methylprednisolone 1 g, plasma exchange for 5 days, continued high-dose methylprednisolone, IV immunoglobulin | Intubated on arrival, rising troponin levels, worsening kidney function, required renal replacement therapy. Died from hyperkalemia and severe metabolic acidosis despite resuscitation efforts. |
| Luecke et al. [47] | 1 | 67 | High-dose systemic glucocorticoids, 5 cycles of plasmapheresis, pyridostigmine | Showed no clinical improvement despite immunosuppressive treatment. Required intubation and mechanical ventilation, died 18 days after ICU admission. |
| Xing et al. [33] | 1 | 66 | Methylprednisolone (MP) 2 mg/kg/day and IV immunoglobulin 400 mg/kg/day for 5 days, temporary pacemaker for 2 days. Adjusted to MP 500 mg/day for 5 days, then tapered, and pyridostigmine bromide 120 mg twice daily. | Intubated after NIPPV. Peripheral limb and eye-opening symptoms improved, serum CPK normalized, anti-AChR-Ab decreased. Respiratory muscle weakness persisted. After two PLEX courses, anti-AChR-Ab normalized, breathing improved. Now on pyridostigmine, mechanical ventilation 12 h/day, and rehab |
| Bawek et al. [40] | 1 | 68 | Pyridostigmine 60 mg TID, IVIG. Due to continued deterioration, on day 8, 1000 mg methylprednisolone (mPSL) IV daily for 3 days was started, followed by prednisone taper | Initial symptom improvement with mPSL, but developed intractable diarrhea (IVIG-related), increased oxygen requirement, multiple organ failure, and possible heparin-induced thrombocytopenia. Transferred to hospice care. |
| Cham et al. [24] | 1 | 72 | On admission corticosteroid use was considered, but the patient declined because of existing central serous retinopathy. Due to his declining respiratory status, he was transferred to the ICU and intubated on day 9. At that point, high-dose corticosteroids at 1 mg/kg/day and plasmapheresis were started on hospital day 10, completing 5 rounds. | Developed progressive axial weakness and respiratory decline, intubated on day 9, tracheostomy and PEG placement required. Transferred to long-term care facility on day 36 due to ventilation dependence. |
| Lipe et al. [29] | 1 | 49 | Steroids, PLEX, IVIG | Alive at discharge. |
| 2 | 67 | Steroids, IVIG, Cellcept | Alive at discharge. | |
| 3 | 70 | Steroids, infliximab, PLEX | Death. | |
| 4 | 81 | Steroids, infliximab, rituximab, PLEX | Alive at discharge. | |
| 5 | 75 | Steroids, PLEX | Alive at discharge. | |
| 6 | 66 | Steroids, Infliximab, Rituximab, PLEX | Alive at discharge. | |
| 7 | 74 | Steroids, infliximab, PLEX, IVIG | Death. | |
| Luo et al. [48] | 1 | 47 | IV immunoglobulin (0.4 g/kg/day for 5 days), followed by pulse methylprednisolone (500 mg, then 250 mg/day for 5 days), then oral prednisolone (60 mg/day for 4 weeks, tapering to 50 mg/day) | Developed type II respiratory failure, intubated, and mechanically ventilated. Third-degree AV block treated with a pacemaker. Improved limb strength after 69 days, but still had difficulty weaning from the ventilator. Transferred for rehab; off mechanical ventilation and on noninvasive ventilation after 1 month. |
| Yang, Xu et al. [57] | 1 | 66 | High-dose IV steroids (500 mg/day for 3 days, 250 mg/day for 3 days, 120 mg/day for 3 days, then tapered), IV immunoglobulin (25 g/day for 5 days). Additional treatments included coenzyme Q10, trimetazidine, recombinant human brain natriuretic peptide, diuretics, cetirizine, calamine lotion, magnesium isoglycyrrhizinate, nadroparin calcium, insulin, cefoperazone sulbactam, and albumin infusion | Symptoms resolved, and examination gradually normalized. |
| Yang, Chen et al. [58] | 1 | 33 | Methylprednisolone (2 mg/kg/day), human immunoglobulin (20 g/day for 5 days), and pyridostigmine (180 mg/day). Oral prednisone tapered over 6 months. | Symptoms significantly improved within days; LV normalized and QRS complexes returned to normal. |
| Bai et al. [39] | 1 | 69 | Methylprednisolone sodium succinate (120 mg/day for 5 days, reduced to 80 mg/day) on the 6th day. Due to deterioration, the steroids were restarted at 120 mg with tapering. Oral pyridostigmine bromide (30 mg qid, tapered). Immunoglobulin injections for 1 week. | Initially developed lower extremity weakness and respiratory failure. Transferred to the ICU and intubated. Diagnosed with ventilator-associated pneumonia. Weaned off the ventilator after 2 weeks. Gradual reduction in glucocorticoid dosage, improved myocardial biomarkers, and muscle strength. Treated with oral prednisone (15 mg daily, tapered) and pyridostigmine bromide (30 mg three times a day) during recovery. |
| Hyun et al. [44] | 1 | 55 | IV methylprednisolone 1 g/day for 3 days | death |
| 2 | 64 | IV methylprednisolone1 g/d for 5 d, plus IVIG at 2 g/kg | Discharged alive without significant disability, | |
| Nakagomi et al. [51] | 1 | 77 | Plasma exchange, IVIG (400 mg/kg/day for 5 days) and increasing oral prednisone. Steroid pulse therapy with IV methylprednisolone (1 g/day for 3 days), followed by a second and third pulse due to continuous increasing troponin levels. | MG and myositis symptoms improved with declining CK levels after initial therapy. However, myocarditis worsened. Troponin levels, although initially decreased, kept re-elevating, thus needing several pulses of MP. The patient was discharged alive. |
| 2 | 73 | Pulse therapy with IV methylprednisolone (1 g/day for 3 days), followed by oral prednisone. Two additional steroid pulses were required due to persistent troponin elevation. | Rapid recovery from myositis and MG with reduced CK and improved eyelid ptosis, but increased myocarditis activity (elevated troponin and persistent chest discomfort). Discharged on day 36. | |
| Saishu et al. [52] | 1 | 55 | Initially treated with IV immunoglobulin and prednisolone (20 mg/day) before definitive diagnosis and based on presenting symptoms only. Continued with immunoglobulin, corticosteroids (methylprednisolone and prednisolone), and plasma exchange (five times). | Improved muscle weakness, ptosis, ocular motility disorder, and CK levels. Despite initial ICU admission and intubation due to respiratory failure, symptoms gradually improved, and the patient could walk with a cane after rehabilitation. |
| Soman et al. [53] | 1 | 73 | Prednisolone, immunoglobulin infusion, physostigmine. | Developed complete heart block; required isoproterenol. Pacemaker implantation failed. Desaturated, needed advanced noninvasive ventilation. Raised right hemidiaphragm due to phrenic nerve palsy. Died on day 7 of admission. |
| Wai Siu et al. [54] | 1 | 73 | Prednisone Methylprednisolone PLEX IVIG Mycophenolate | Resolution of toxicity. |
| 2 | 74 | Prednisone Methylprednisolone Mycophenolate | Resolution of toxicity. | |
| 3 | 73 | Methylprednisolone Prednisone IVIG | Resolution of toxicity. | |
| Wang et al. [55] | 1 | 65 | Methylprednisolone (1 g/day for 3 days, then taper) and IVIG (0.4 g/kg/day for 3 days). | Myocardial enzymes decreased gradually; biomarkers normalized over 20 days; discharged with intermittent ventilator support. |
| Wu et al. [56] | 1 | 48 | Pyridostigmine, IV methylprednisolone (1 mg/kg/day, tapered every 3 days), normal saline hydration for 5 days. Continued with Pyridostigmine 60 mg 1 tablet/day and dexamethasone 4 mg 2 tablet per day after discharge | Symptoms gradually improved; a 1-month follow-up showed normal eye movement and reduced diplopia. |
| Yin et al. [59] | 1 | 71 | Methylprednisolone (500 mg/day for 5 days, then taper) and IVIG (0.4 g/kg/day for 5 days). Tacrolimus (3 mg/day) added due to weakness and soreness bilateral extremities. | Significant clinical improvement: biomarker levels declined; discharged. |
| Ahdi et al. [38] | 1 | 58 | IVIG (667 mg/kg/day for 3 days, total 2 g/kg) and pyridostigmine. | Symptoms and liver function improved; discharged on oral prednisone. |
| Giovannini et al. [42] | 1 | 65 | Intravenous methylprednisolone (60 mg/day) and oral pyridostigmine (60 mg, three times daily). Anticoagulant therapy initiated. | On day 2, developed dyspnea, atrial fibrillation, and severe hypoxemia. Despite noninvasive ventilation, dialysis, and resuscitation, the patient died from ventricular tachycardia and fibrillation. |
| Golec et al. [43] | 1 | 74 | Methylprednisolone (1 mg/kg, escalated to 1000 mg/day), plasma exchange (PLEX), mycophenolate mofetil. PLEX was initially held but later resumed. | Developed tamponade (treated with pericardiocentesis), complete heart block (treated with pacemaker), worsening myasthenia, intubation, and 6 additional PLEX sessions. He transitioned to comfort care and died. |
| Lin X. et al. [46] | 1 | 51 | Methylprednisolone (500 mg/day, reduced to 250 mg/day), IVIG (5 g/day), pyridostigmine, and low-flow oxygen. | Symptoms of overlap syndrome improved; respiratory weakness and biomarkers (cTnI and CK) normalized. Discharged on day 18. |
| Marco et al. [49] | 1 | 77 | Corticosteroids, PLEX, mechanical ventilation | Death at 60 days follow-up. |
| 2 | 78 | Corticosteroids, immunoglobulins, plasma exchange, Rituximab, mechanical ventilation | Death at 133 days follow-up. | |
| 3 | 70 | Corticosteroids, immunoglobulins, Ciclofosfamide, mechanical ventilation | Death at 53 days follow up. | |
| 4 | 85 | Corticosteroids, immunoglobulins | Death at 30 days follow up. | |
| Masood et al. [50] | 1 | 75 | On day 1, the patient received 500 mg IV methylprednisolone, followed by 1 g IV methylprednisolone (3 doses), IVIG (2 g/kg over 5 days), and Cyclophosphamide (500 mg IV) on day 10. On day 26, 1 g rituximab (with a repeat dose in 2 weeks) was given. The patient continued with IVIG (0.4 g/kg/day for 5 days) and monthly IVIG for 3 months. | Developed RBBB, complete heart block, asystole, and cardiac arrest; required a permanent pacemaker. Progressive muscle weakness, dysphagia, and respiratory failure. Tracheal decannulation on day 133. Post-discharge remained on prednisolone with normal muscle strength but progressed melanoma with cutaneous metastases. |
| 2 | 77 | Three pulses of 1 g IV methylprednisolone, five days of IVIg (2 g/kg). On day 6 the patient received 500 mg IV cyclophosphamide (plus 5 cycles every 2 weeks), IV rituximab (days 7 and 21), and monthly IVIG for 5 months. | Intubated and received a pacemaker and tracheostomy on day 9. CK improved; tracheostomy decannulated on day 119. Discharged home and maintained on Prednisolone (5 mg daily). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lipe, D.N.; Qdaisat, A.; Krishnamani, P.P.; Nguyen, T.D.; Chaftari, P.; El Messiri, N.; Srinivasan, A.; Galvis-Carvajal, E.; Reyes-Gibby, C.C.; Wattana, M.K. Myocarditis, Myositis, and Myasthenia Gravis Overlap Syndrome Associated with Immune Checkpoint Inhibitors: A Systematic Review. Diagnostics 2024, 14, 1794. https://doi.org/10.3390/diagnostics14161794
Lipe DN, Qdaisat A, Krishnamani PP, Nguyen TD, Chaftari P, El Messiri N, Srinivasan A, Galvis-Carvajal E, Reyes-Gibby CC, Wattana MK. Myocarditis, Myositis, and Myasthenia Gravis Overlap Syndrome Associated with Immune Checkpoint Inhibitors: A Systematic Review. Diagnostics. 2024; 14(16):1794. https://doi.org/10.3390/diagnostics14161794
Chicago/Turabian StyleLipe, Demis N., Aiham Qdaisat, Pavitra P. Krishnamani, Trung D. Nguyen, Patrick Chaftari, Nour El Messiri, Aswin Srinivasan, Elkin Galvis-Carvajal, Cielito C. Reyes-Gibby, and Monica K. Wattana. 2024. "Myocarditis, Myositis, and Myasthenia Gravis Overlap Syndrome Associated with Immune Checkpoint Inhibitors: A Systematic Review" Diagnostics 14, no. 16: 1794. https://doi.org/10.3390/diagnostics14161794
APA StyleLipe, D. N., Qdaisat, A., Krishnamani, P. P., Nguyen, T. D., Chaftari, P., El Messiri, N., Srinivasan, A., Galvis-Carvajal, E., Reyes-Gibby, C. C., & Wattana, M. K. (2024). Myocarditis, Myositis, and Myasthenia Gravis Overlap Syndrome Associated with Immune Checkpoint Inhibitors: A Systematic Review. Diagnostics, 14(16), 1794. https://doi.org/10.3390/diagnostics14161794







