The Predictive Accuracy of Anogenital Distance and Genital Tubercle Angle for First-Trimester Fetal Sex Determination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
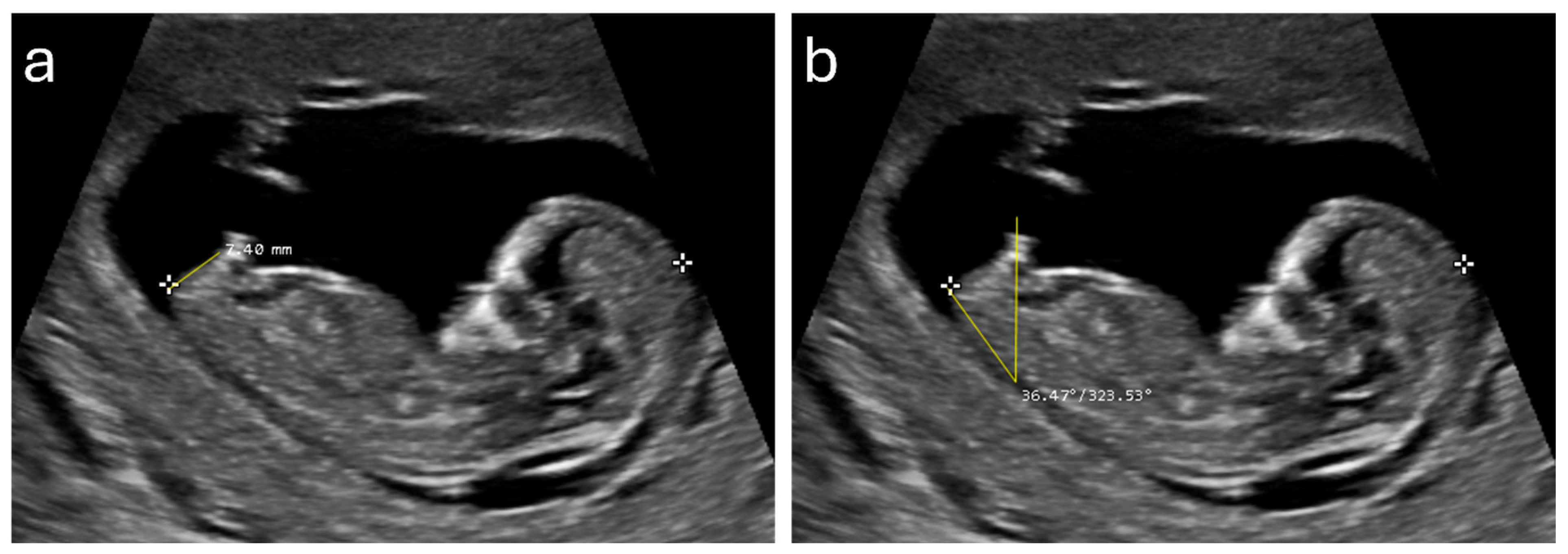
2.3. Ultrasound Measurements
2.4. Statistical Method
3. Results
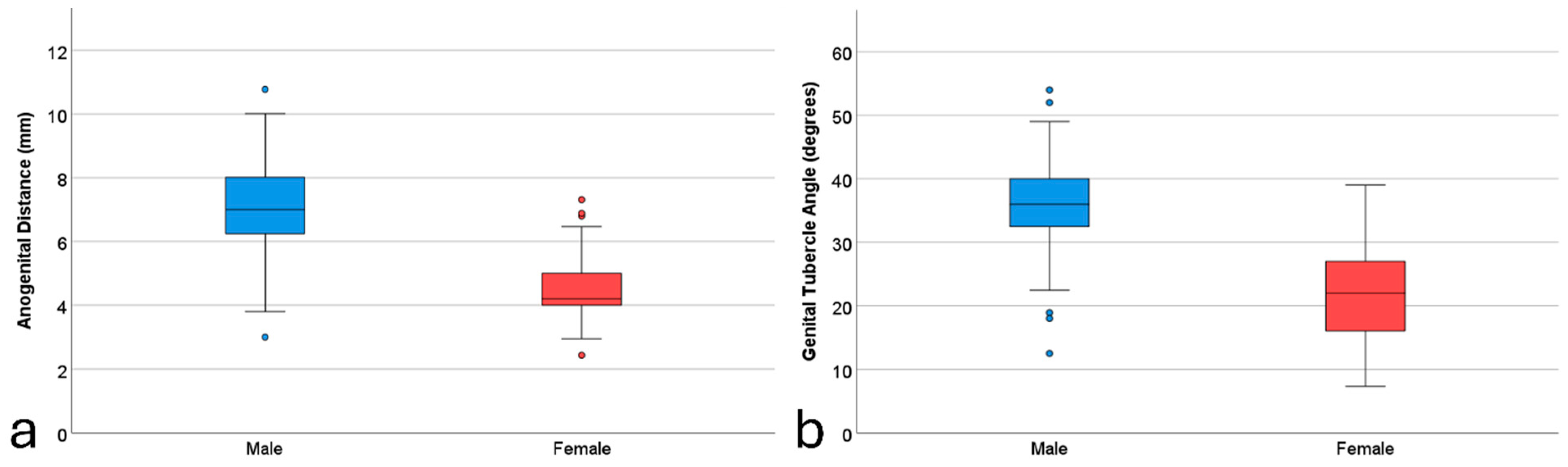
3.1. Descriptive Statistics
3.2. Gender Prediction and Accuracy
3.3. Optimal Cutoff Points
3.4. Measurement’s Reproducibility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Sousa, M.J.R.; Ribeiro, R.; Syngelaki, A.; Nicolaides, K.H. First Trimester Combined Screening in Patients with Systemic Lupus Erythematosus: Impact of Pre-Analytical Variables on Risk Assessment. Clin. Rheumatol. 2019, 38, 1251–1255. [Google Scholar] [CrossRef] [PubMed]
- Mujezinovic, F.; Alfirevic, Z. Procedure-Related Complications of Amniocentesis and Chorionic Villous Sampling: A Systematic Review. Obstet. Gynecol. 2007, 110, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Marrapodi, M.M.; Capristo, C.; Conte, A.; Molitierno, R.; Morlando, M.; Fordellone, M.; Campitiello, M.R.; Torella, M. Racial and Ethnic Disparities in Non-Invasive Prenatal Testing Adherence: A Retrospective Cohort Study. Minerva Obstet. Gynecol. 2024, online ahead of print. [Google Scholar]
- La Verde, M.; De Falco, L.; Torella, A.; Savarese, G.; Savarese, P.; Ruggiero, R.; Conte, A.; Fico, V.; Torella, M.; Fico, A. Performance of Cell-Free DNA Sequencing-Based Non-Invasive Prenatal Testing: Experience on 36,456 Singleton and Multiple Pregnancies. BMC Med. Genom. 2021, 14, 93. [Google Scholar] [CrossRef] [PubMed]
- Alberry, M.S.; Aziz, E.; Ahmed, S.R.; Abdel-fattah, S. Non Invasive Prenatal Testing (NIPT) for Common Aneuploidies and Beyond. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 258, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Alabbad, M.; Al-Badr, S.; Ismail, H.; Alghiryafi, L. Diagnostic Accuracy and Complications Rate of CT-Guided Core Needle Lung Biopsy of Solid and Part-Solid Lesion: A Single-Institution Experience. Saudi J. Radiol. 2023, 2, 1–14. [Google Scholar] [CrossRef]
- Dubois, M.-L.; Winters, P.D.; Rodrigue, M.-A.; Gekas, J. Patient Attitudes and Preferences about Expanded Noninvasive Prenatal Testing. Front. Genet. 2023, 14, 976051. [Google Scholar] [CrossRef] [PubMed]
- Stocker, J.; Evens, L. Fetal Sex Determination by Ultrasound. Obstet. Gynecol. 1977, 50, 462–466. [Google Scholar] [PubMed]
- Scholly, T.A.; Sutphen, J.H.; Hitchcock, D.A.; Mackey, S.C.; Langstaff, L.M. Sonographic Determination of Fetal Gender. Am. J. Roentgenol. 1980, 135, 1161–1165. [Google Scholar] [CrossRef]
- Weldner, B.M. Accuracy of Fetal Sex Determination by Ultrasound. Acta Obstet. Gynecol. Scand. 1981, 60, 333–334. [Google Scholar] [CrossRef] [PubMed]
- Shalev, E.; Weiner, E.; Zuckerman, H. Ultrasound Determination of Fetal Sex. Am. J. Obstet. Gynecol. 1981, 141, 582–583. [Google Scholar] [CrossRef] [PubMed]
- Schotten, A.; Giese, C. The “Female Echo”: Prenatal Determination of the Female Fetus by Ultrasound. Am. J. Obstet. Gynecol. 1980, 138, 463–464. [Google Scholar] [CrossRef]
- Natsuyama, E. Sonographic Determination of Fetal Sex from Twelve Weeks of Gestation. Am. J. Obstet. Gynecol. 1984, 149, 748–757. [Google Scholar] [CrossRef]
- Efrat, Z.; Akinfenwa, O.O.; Nicolaides, K.H. First-Trimester Determination of Fetal Gender by Ultrasound. Ultrasound Obstet. Gynecol. 1999, 13, 305–307. [Google Scholar] [CrossRef]
- Chelli, D.; Methni, A.; Dimassi, K.; Boudaya, F.; Sfar, E.; Zouaoui, B.; Chelli, H.; Chennoufi, M.B. Fetal Sex Assignment by First Trimester Ultrasound: A Tunisian Experience. Prenat. Diagn. 2009, 29, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Gharekhanloo, F. The Ultrasound Identification of Fetal Gender at the Gestational Age of 11–12 Weeks. J. Fam. Med. Prim. Care 2018, 7, 210–212. [Google Scholar] [CrossRef]
- Najdi, N.; Safi, F.; Hashemi-Dizaji, S.; Sahraian, G.; Jand, Y. First Trimester Determination of Fetal Gender by Ultrasonographic Measurement of Anogenital Distance: A Cross-Sectional Study. Int. J. Reprod. Biomed. 2019, 17, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Gilboa, Y.; Kivilevitch, Z.; Oren, M.; Cohen, Y.P.; Katorza, E.; Achiron, R. Anogenital Distance in Male and Female Fetuses at 20 to 35weeks of Gestation: Centile Charts and Reference Ranges. Prenat. Diagn. 2014, 34, 946–951. [Google Scholar] [CrossRef]
- Aydin, E.; Holt, R.; Chaplin, D.; Hawkes, R.; Allison, C.; Hackett, G.; Austin, T.; Tsompanidis, A.; Gabis, L.; Ziv, S.I.; et al. Fetal Anogenital Distance Using Ultrasound. Prenat. Diagn. 2019, 39, 527–535. [Google Scholar] [CrossRef]
- Alfuraih, A.M.; Alotaiby, S.A.; Alsaadi, M.J.; Bukhari, H.A.; Aldhebaib, A.M.; Mohtasib, R.S. Predictive Value and Reference Ranges of Anogenital Distance for Determining Fetal Gender in the First Trimester. Saudi Med. J. 2021, 42, 1057. [Google Scholar] [CrossRef]
- Elanwar, A.M.; Shazly, S.A.; Attia, N.A.I.; Heraiz, A.I. Anogenital Distance for Detection of Fetal Sex in First Trimester of Pregnancy. Egypt. J. Hosp. Med. 2023, 90, 3035–3040. [Google Scholar] [CrossRef]
- Edris, T.I.; Mohamed, M.A.; Mohamady, L.A.; El-Noury, M.A. Anogenital Distance for Detection of Fetal Sex in First Trimester. Egypt. J. Hosp. Med. 2024, 94, 885–888. [Google Scholar]
- Sipahi, M.; Tokgöz, V.Y.; Alanya Tosun, Ş. An Appropriate Way to Predict Fetal Gender at First Trimester: Anogenital Distance. J. Matern. Fetal Neonatal Med. 2019, 32, 2012–2016. [Google Scholar] [CrossRef] [PubMed]
- Arfi, A.; Cohen, J.; Canlorbe, G.; Bendifallah, S.; Thomassin-Naggara, I.; Darai, E.; Benachi, A.; Arfi, J.S. First-Trimester Determination of Fetal Gender by Ultrasound: Measurement of the Ano-Genital Distance. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 203, 177–181. [Google Scholar] [CrossRef]
- Cao, W.; Ding, X.; Dong, Z.; Tang, H. Reference Values for and Correlation Analysis of the Anogenital Distance of Chinese Han Full-Term Singleton Neonates. Front. Pediatr. 2022, 10, 905421. [Google Scholar] [CrossRef]
- Nanda, P.M.; Yadav, J.; Dayal, D.; Kumar, R.; Kumar, P.; Kumar, J.; Kaur, H.; Sikka, P. Estimation of Reference Values for External Genitalia Parameters in North Indian Preterm and Term Female Newborns. Indian J. Pediatr. 2023, 91, 548–555. [Google Scholar] [CrossRef]
- Gilboa, Y.; Perlman, S.; Kivilevitch, Z.; Messing, B.; Achiron, R. Prenatal Anogenital Distance Is Shorter in Fetuses with Hypospadias. J. Ultrasound Med. 2017, 36, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Stoykov, S. MicroDICOM Viewer; Version 2024-2; MicroDicom Ltd.: Sofia, Bugaria, 2023. [Google Scholar]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Papadopoulou, E.; Vafeiadi, M.; Agramunt, S.; Basagaña, X.; Mathianaki, K.; Karakosta, P.; Spanaki, A.; Koutis, A.; Chatzi, L.; Vrijheid, M.; et al. Anogenital Distances in Newborns and Children from Spain and Greece: Predictors, Tracking and Reliability. Paediatr. Perinat. Epidemiol. 2013, 27, 89–99. [Google Scholar] [CrossRef]
- Sathyanarayana, S.; Beard, L.; Zhou, C.; Grady, R. Measurement and Correlates of Ano-Genital Distance in Healthy, Newborn Infants. Int. J. Androl. 2010, 33, 317. [Google Scholar] [CrossRef]
- Faupel-Badger, J.M.; Wang, Y.; Karumanchi, S.A.; Stanczyk, F.; Pollak, M.; McElrath, T.; Hoover, R.N.; Troisi, R. Associations of Pregnancy Characteristics with Maternal and Cord Steroid Hormones, Angiogenic Factors, and Insulin-like Growth Factor Axis. Cancer Causes Control 2011, 22, 1587. [Google Scholar] [CrossRef] [PubMed]
- Potischman, N.; Troisi, R.; Thadhani, R.; Hoover, R.N.; Dodd, K.; Davis, W.W.; Sluss, P.M.; Hsieh, C.C.; Ballard-Barbash, R. Pregnancy Hormone Concentrations across Ethnic Groups: Implications for Later Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Odeh, M.; Ophir, E.; Bornstein, J. Hypospadias Mimicking Female Genitalia on Early Second Trimester Sonographic Examination. J. Clin. Ultrasound 2008, 36, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, E.Z.; Blazer, S.; Blumenfeld, Z.; Bronshtein, M. Fetal Transient Clitoromegaly and Transient Hypertrophy of the Labia Minora in Early and Mid Pregnancy. J. Ultrasound Med. 2012, 31, 409–415. [Google Scholar] [CrossRef]

| Variable | Male (n = 188) | Female (n = 124) | Overall (n = 312) | |||
|---|---|---|---|---|---|---|
| Mean (SD) | Min–Max | Mean (SD) | Min–Max | Mean (SD) | Min–Max | |
| GA (days) | 87.87 (5.62) | 77–97 | 86.24 (5.78) | 77–97 | 87.22 (5.73) | 77–97 |
| Maternal age (years) | 30.40 (5.19) | 16–44 | 30.17 (4.79) | 19–43 | 30.31 (5.03) | 16–44 |
| CRL (mm) | 61.25 (10.65) | 38.0–85.0 | 58.59 (10.50) | 45.0–83.9 | 60.19 (10.65) | 38.0–85.0 |
| AGD (mm) | 7.16 (1.40) | 3.0–10.77 | 4.42 (1.05) | 2.43–9.0 | 6.07 (1.85) | 2.43–10.77 |
| GTA (degree) | 35.90 (6.20) | 8.13–54.0 | 21.57 (7.29) | 7.32–39.0 | 30.21 (9.67) | 7.32–54.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfuraih, A.M.; Almajem, B.M.; Alsolai, A.A. The Predictive Accuracy of Anogenital Distance and Genital Tubercle Angle for First-Trimester Fetal Sex Determination. Diagnostics 2024, 14, 1811. https://doi.org/10.3390/diagnostics14161811
Alfuraih AM, Almajem BM, Alsolai AA. The Predictive Accuracy of Anogenital Distance and Genital Tubercle Angle for First-Trimester Fetal Sex Determination. Diagnostics. 2024; 14(16):1811. https://doi.org/10.3390/diagnostics14161811
Chicago/Turabian StyleAlfuraih, Abdulrahman M., Bashaier Mansour Almajem, and Amal Abdullah Alsolai. 2024. "The Predictive Accuracy of Anogenital Distance and Genital Tubercle Angle for First-Trimester Fetal Sex Determination" Diagnostics 14, no. 16: 1811. https://doi.org/10.3390/diagnostics14161811
APA StyleAlfuraih, A. M., Almajem, B. M., & Alsolai, A. A. (2024). The Predictive Accuracy of Anogenital Distance and Genital Tubercle Angle for First-Trimester Fetal Sex Determination. Diagnostics, 14(16), 1811. https://doi.org/10.3390/diagnostics14161811






