Clinical Applications of Cardiac Magnetic Resonance Parametric Mapping
Abstract
1. Introduction
2. Physical Principles
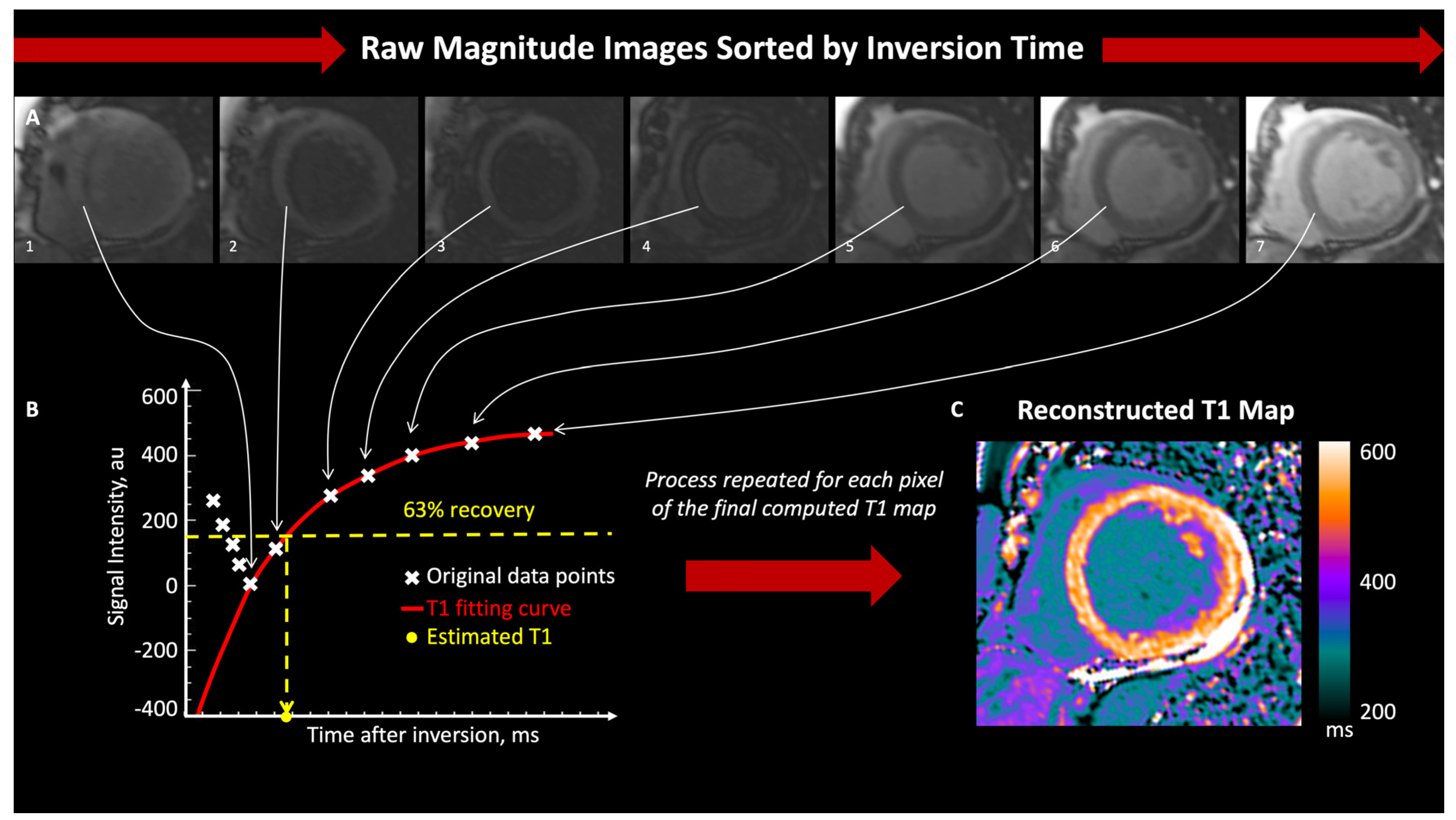
3. T1 and T2 Mapping Imaging
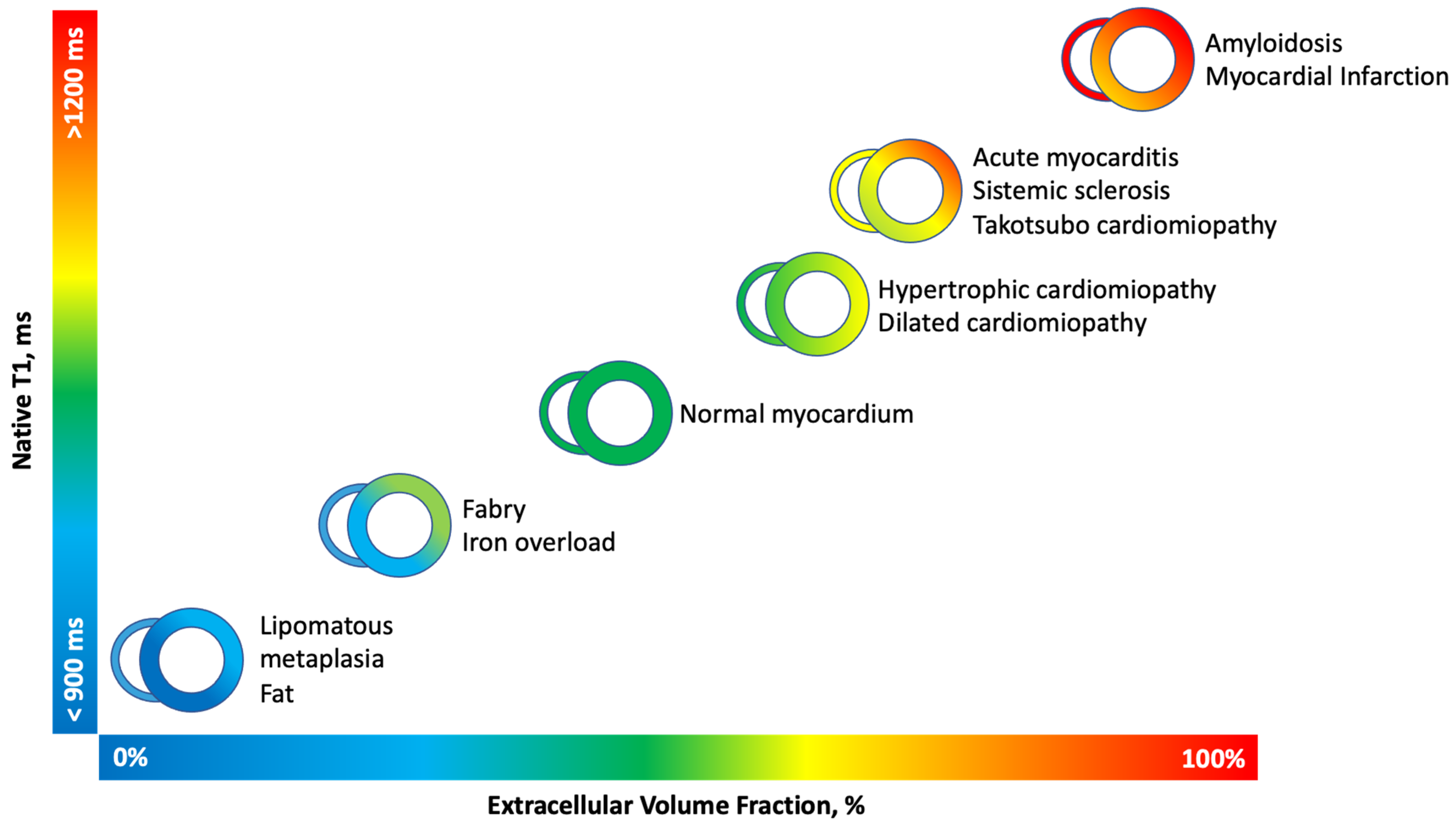
3.1. T1 Mapping
3.2. T2 Mapping
3.3. Limitations
4. Clinical Applications
4.1. Ischemic Heart Disease
4.2. Takotsubo Cardiomyopathy
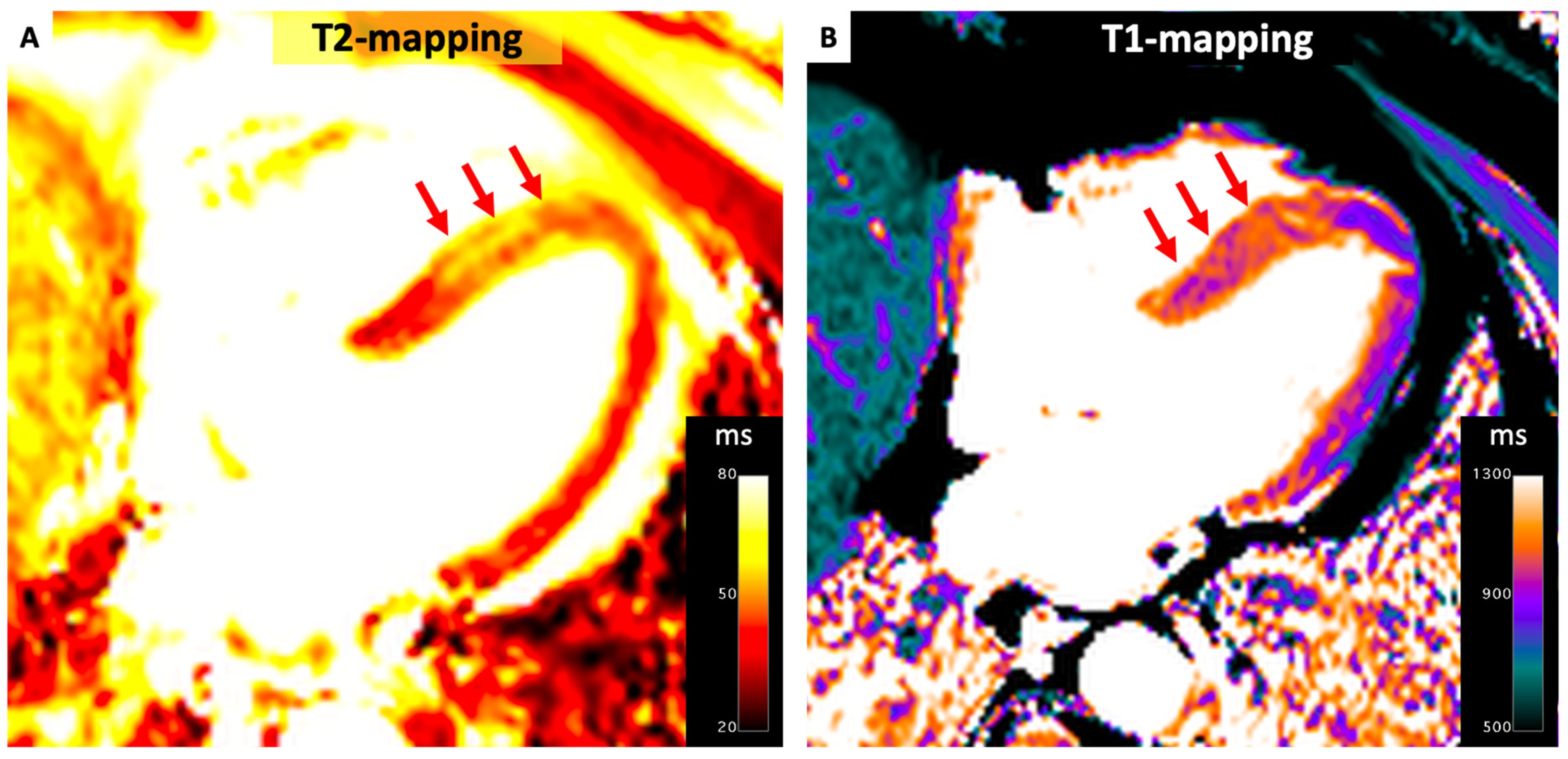
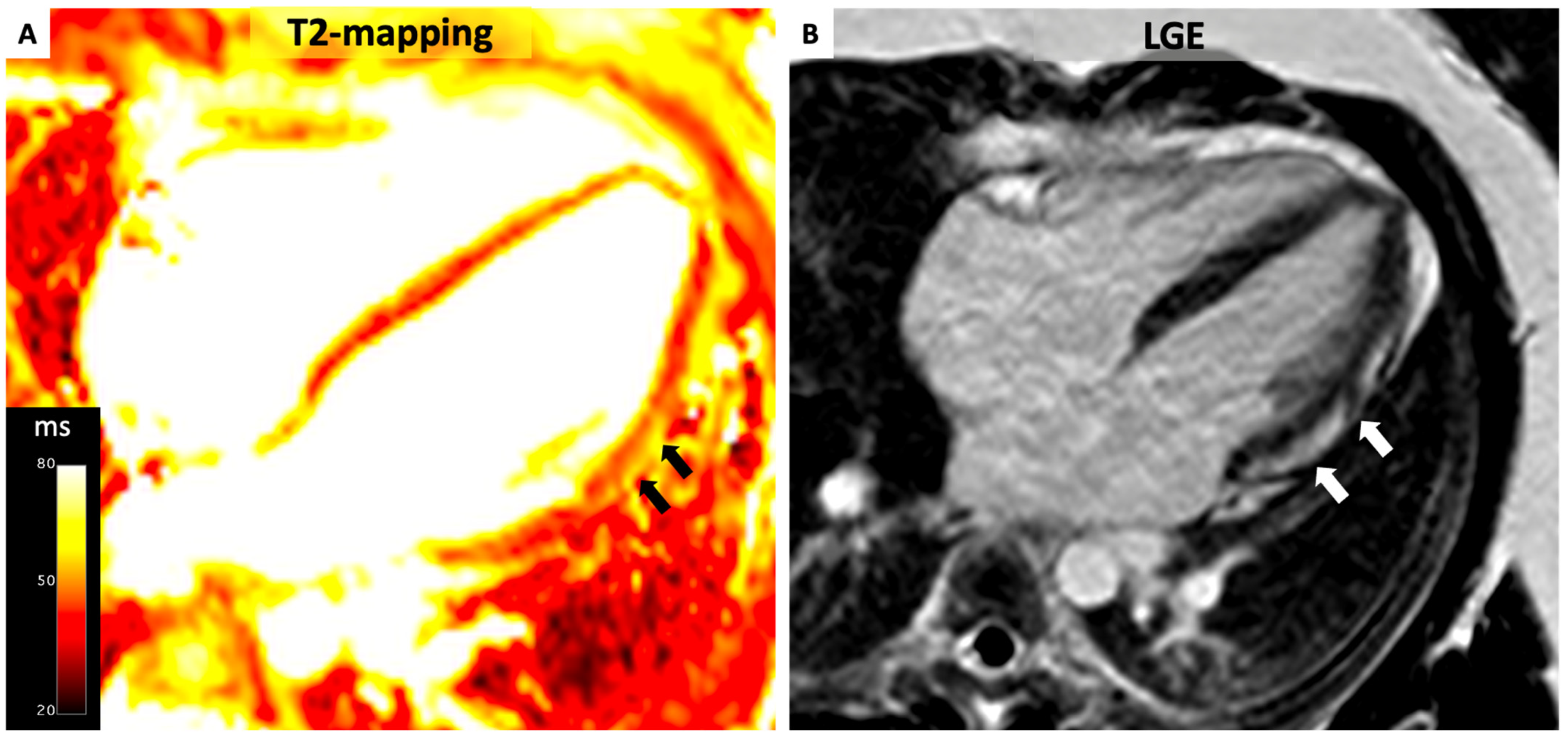
4.3. Myocarditis
- (1)
- Myocardial edema:
- -
- Regional/global increase in T2 signal intensity;
- -
- Regional/global increase in native T2.
- (2)
- Non-ischemic myocardial injury:
- -
- Regional/global increase in native T1;
- -
- Regional/global increase in ECV;
- -
- Regional LGE signal increase.
4.4. Cardiac Sarcoidosis
4.5. Autoimmune Disorders
4.6. Transplant Rejection
4.7. Non-Ischemic Dilated Cardiomyopathy
4.8. Left Ventricular Non-Compaction
4.9. Hypertrophic Cardiomyopathy
4.10. Cardiac Amyloidosis
4.11. Anderson–Fabry Disease
4.12. Iron Overload
4.13. Chemotherapy
4.14. Valvular Heart Disease
4.15. Future Applications
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Puntmann, V.O.; Carr-White, G.; Jabbour, A.; Yu, C.-Y.; Gebker, R.; Kelle, S.; Hinojar, R.; Doltra, A.; Varma, N.; Child, N.; et al. T1-Mapping and Outcome in Nonischemic Cardiomyopathy: All-Cause Mortality and Heart Failure. JACC Cardiovasc. Imaging 2016, 9, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2017, 19, 75. [Google Scholar]
- Muser, D.; Nucifora, G.; Castro, S.A.; Enriquez, A.; Chahal, C.A.A.; Magnani, S.; Kumareswaran, R.; Arkles, J.; Supple, G.; Schaller, R.; et al. Myocardial Substrate Characterization by CMR T1 Mapping in Patients with NICM and No LGE Undergoing Catheter Ablation of VT. JACC Clin. Electrophysiol. 2021, 7, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Weingärtner, S.; Meßner, N.M.; Budjan, J.; Loßnitzer, D.; Mattler, U.; Papavassiliu, T.; Zöllner, F.G.; Schad, L.R. Myocardial T1-mapping at 3T using saturation-recovery: Reference values, precision and comparison with MOLLI. J. Cardiovasc. Magn. Reson. 2016, 18, 84. [Google Scholar] [CrossRef]
- Rosmini, S.; Bulluck, H.; Captur, G.; Treibel, T.A.; Abdel-Gadir, A.; Bhuva, A.N.; Culotta, V.; Merghani, A.; Fontana, M.; Maestrini, V.; et al. Myocardial native T1 and extracellular volume with healthy ageing and gender. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Piechnik, S.K.; Ferreira, V.M.; Dall’Armellina, E.; Cochlin, L.E.; Greiser, A.; Neubauer, S.; Robson, M.D. Shortened Modified Look-Locker Inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J. Cardiovasc. Magn. Reson. 2010, 12, 69. [Google Scholar] [CrossRef]
- Chow, K.; Flewitt, J.A.; Green, J.D.; Pagano, J.J.; Friedrich, M.G.; Thompson, R.B. Saturation recovery single-shot acquisition (SASHA) for myocardial T(1) mapping. Magn. Reson. Med. 2014, 71, 2082–2095. [Google Scholar] [CrossRef]
- Burt, J.R.; Zimmerman, S.L.; Kamel, I.R.; Halushka, M.; Bluemke, D.A. Myocardial T1 mapping: Techniques and potential applications. Radiographics 2014, 34, 377–395. [Google Scholar] [CrossRef]
- Weingärtner, S.; Moeller, S.; Schmitter, S.; Auerbach, E.; Kellman, P.; Shenoy, C.; Akçakaya, M. Simultaneous multislice imaging for native myocardial T1 mapping: Improved spatial coverage in a single breath-hold. Magn. Reson. Med. 2017, 78, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Kellman, P.; Hansen, M.S. T1-mapping in the heart: Accuracy and precision. J. Cardiovasc. Magn. Reson. 2014, 16, 2. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Piechnik, S.K.; Dall’Armellina, E.; Karamitsos, T.D.; Francis, J.M.; Ntusi, N.; Holloway, C.; Choudhury, R.P.; Kardos, A.; Robson, M.D.; et al. Native T1-mapping detects the location, extent and patterns of acute myocarditis without the need for gadolinium contrast agents. J. Cardiovasc. Magn. Reson. 2014, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Karamitsos, T.D.; Piechnik, S.K.; Banypersad, S.M.; Fontana, M.; Ntusi, N.B.; Ferreira, V.M.; Whelan, C.J.; Myerson, S.G.; Robson, M.D.; Hawkins, P.N.; et al. Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovasc. Imaging 2013, 6, 488–497. [Google Scholar] [CrossRef]
- Sado, D.M.; White, S.K.; Piechnik, S.K.; Banypersad, S.M.; Treibel, T.; Captur, G.; Fontana, M.; Maestrini, V.; Flett, A.S.; Robson, M.D.; et al. Identification and assessment of Anderson-Fabry disease by cardiovascular magnetic resonance noncontrast myocardial T1 mapping. Circ. Cardiovasc. Imaging 2013, 6, 392–398. [Google Scholar] [CrossRef]
- Sado, D.M.; Maestrini, V.; Piechnik, S.K.; Banypersad, S.M.; White, S.K.; Flett, A.S.; Robson, M.D.; Neubauer, S.; Ariti, C.; Arai, A.; et al. Noncontrast myocardial T1 mapping using cardiovascular magnetic resonance for iron overload. J. Magn. Reson. Imaging 2015, 41, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.C.; Messroghli, D.R.; Kellman, P.; Piechnik, S.K.; Robson, M.D.; Ugander, M.; Gatehouse, P.D.; E Arai, A.; Friedrich, M.G.; Neubauer, S.; et al. Myocardial T1 mapping and extracellular volume quantification: A Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J. Cardiovasc. Magn. Reson. 2013, 15, 92. [Google Scholar] [CrossRef]
- Miller, C.A.; Naish, J.H.; Bishop, P.; Coutts, G.; Clark, D.; Zhao, S.; Ray, S.G.; Yonan, N.; Williams, S.G.; Flett, A.S.; et al. Comprehensive validation of cardiovascular magnetic resonance techniques for the assessment of myocardial extracellular volume. Circ. Cardiovasc. Imaging 2013, 6, 373–383. [Google Scholar] [CrossRef]
- Chen, W.; Doeblin, P.; Al-Tabatabaee, S.; Klingel, K.; Tanacli, R.; Jakob Weiß, K.; Stehning, C.; Patel, A.R.; Pieske, B.; Zou, J.; et al. Synthetic Extracellular Volume in Cardiac Magnetic Resonance Without Blood Sampling: A Reliable Tool to Replace Conventional Extracellular Volume. Circ. Cardiovasc. Imaging 2022, 15, e013745. [Google Scholar] [CrossRef]
- Haaf, P.; Garg, P.; Messroghli, D.R.; Broadbent, D.A.; Greenwood, J.P.; Plein, S. Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: A comprehensive review. J. Cardiovasc. Magn. Reson. 2016, 18, 89. [Google Scholar] [CrossRef]
- Sprinkart, A.M.; Luetkens, J.A.; Träber, F.; Doerner, J.; Gieseke, J.; Schnackenburg, B.; Schmitz, G.; Thomas, D.; Homsi, R.; Block, W.; et al. Gradient Spin Echo (GraSE) imaging for fast myocardial T2 mapping. J. Cardiovasc. Magn. Reson. 2015, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Arai, A.E. Magnetic resonance imaging for area at risk, myocardial infarction, and myocardial salvage. J. Cardiovasc. Pharmacol. Ther. 2011, 16, 313–320. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Kim, H.W.; Kim, R.J. T2-weighted imaging to assess post-infarct myocardium at risk. JACC Cardiovasc. Imaging 2011, 4, 1014–1021. [Google Scholar] [CrossRef]
- Messroghli, D.R.; Walters, K.; Plein, S.; Sparrow, P.; Friedrich, M.G.; Ridgway, J.P.; Sivananthan, M.U. Myocardial T1 mapping: Application to patients with acute and chronic myocardial infarction. Magn. Reson. Med. 2007, 58, 34–40. [Google Scholar] [CrossRef]
- Dall’Armellina, E.; Piechnik, S.K.; Ferreira, V.M.; Si, Q.L.; Robson, M.D.; Francis, J.M.; Cuculi, F.; Kharbanda, R.K.; Banning, A.P.; Choudhury, R.P.; et al. Cardiovascular magnetic resonance by non contrast T1-mapping allows assessment of severity of injury in acute myocardial infarction. J. Cardiovasc. Magn. Reson. 2012, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Borlotti, A.; Viliani, D.; Jerosch-Herold, M.; Alkhalil, M.; De Maria, G.L.; Fahrni, G.; Dawkins, S.; Wijesurendra, R.; Francis, J.; et al. CMR Native T1 Mapping Allows Differentiation of Reversible versus Irreversible Myocardial Damage in ST-Segment-Elevation Myocardial Infarction: An OxAMI Study (Oxford Acute Myocardial Infarction). Circ. Cardiovasc. Imaging 2017, 10, e005986. [Google Scholar] [CrossRef] [PubMed]
- Carrick, D.; Haig, C.; Rauhalammi, S.; Ahmed, N.; Mordi, I.; McEntegart, M.; Petrie, M.C.; Eteiba, H.; Hood, S.; Watkins, S.; et al. Prognostic significance of infarct core pathology revealed by quantitative non-contrast in comparison with contrast cardiac magnetic resonance imaging in reperfused ST-elevation myocardial infarction survivors. Eur. Heart J. 2016, 37, 1044–1059. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.; Duffy, S.J.; White, D.A.; Gao, X.-M.; Du, X.-J.; Ellims, A.H.; Dart, A.M.; Taylor, A.J. Acute left ventricular remodeling following myocardial infarction: Coupling of regional healing with remote extracellular matrix expansion. JACC Cardiovasc. Imaging 2012, 5, 884–893. [Google Scholar] [CrossRef]
- Carberry, J.; Carrick, D.; Haig, C.; Rauhalammi, S.M.; Ahmed, N.; Mordi, I.; McEntegart, M.; Petrie, M.C.; Eteiba, H.; Hood, S.; et al. Remote Zone Extracellular Volume and Left Ventricular Remodeling in Survivors of ST-Elevation Myocardial Infarction. Hypertension 2016, 68, 385–391. [Google Scholar] [CrossRef]
- Kali, A.; Choi, E.-Y.; Sharif, B.; Kim, Y.J.; Bi, X.; Spottiswoode, B.; Cokic, I.; Yang, H.-J.; Tighiouart, M.; Conte, A.H.; et al. Native T1 Mapping by 3-T CMR Imaging for Characterization of Chronic Myocardial Infarctions. JACC Cardiovasc. Imaging 2015, 8, 1019–1030. [Google Scholar] [CrossRef]
- Bauner, K.U.; Biffar, A.; Theisen, D.; Greiser, A.; Zech, C.J.; Nguyen, E.T.; Reiser, M.F.; Wintersperger, B.J. Extracellular volume fractions in chronic myocardial infarction. Investig. Radiol. 2012, 47, 538–545. [Google Scholar] [CrossRef]
- Dastidar, A.G.; Harries, I.; Pontecorboli, G.; Bruno, V.D.; De Garate, E.; Moret, C.; Baritussio, A.; Johnson, T.W.; McAlindon, E.; Bucciarelli-Ducci, C. Native T1 mapping to detect extent of acute and chronic myocardial infarction: Comparison with late gadolinium enhancement technique. Int. J. Cardiovasc. Imaging 2019, 35, 517–527. [Google Scholar] [CrossRef]
- Wamil, M.; Borlotti, A.; Liu, D.; Briosa e Gala, A.; Bracco, A.; Alkhalil, M.; De Maria, G.L.; Piechnik, S.K.; Ferreira, V.M.; Banning, A.P.; et al. Combined T1-mapping and tissue tracking analysis predicts severity of ischemic injury following acute STEMI—An Oxford Acute Myocardial Infarction (OxAMI) study. Int. J. Cardiovasc. Imaging 2019, 35, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Dabir, D.; Luetkens, J.; Kuetting, D.L.R.; Feisst, A.; Isaak, A.; Schild, H.H.; Thomas, D. Cardiac magnetic resonance including parametric mapping in acute Takotsubo syndrome: Preliminary findings. Eur. J. Radiol. 2019, 113, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, Y.; Noguchi, T.; Morita, Y.; Tateishi, E.; Kono, A.; Miura, H.; Komori, Y.; Asaumi, Y.; Fukuda, T.; Yasuda, S. Clinical impact of native T1 mapping for detecting myocardial impairment in takotsubo cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1147–1155. [Google Scholar] [CrossRef]
- Neil, C.; Nguyen, T.H.; Kucia, A.; Crouch, B.; Sverdlov, A.; Chirkov, Y.; Mahadavan, G.; Selvanayagam, J.; Dawson, D.; Beltrame, J.; et al. Slowly resolving global myocardial inflammation/oedema in Tako-Tsubo cardiomyopathy: Evidence from T2-weighted cardiac MRI. Heart 2012, 98, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Scally, C.; Rudd, A.; Mezincescu, A.; Wilson, H.; Srivanasan, J.; Horgan, G.; Broadhurst, P.; Newby, D.E.; Henning, A.; Dawson, D.K. Persistent Long-Term Structural, Functional, and Metabolic Changes after Stress-Induced (Takotsubo) Cardiomyopathy. Circulation 2018, 137, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, K.; Ahearn, T.; Srinivasan, J.; Neil, C.J.; Scally, C.; Rudd, A.; Jagpal, B.; Frenneaux, M.P.; Pislaru, C.; Horowitz, J.D.; et al. Alterations in Cardiac Deformation, Timing of Contraction and Relaxation, and Early Myocardial Fibrosis Accompany the Apparent Recovery of Acute Stress-Induced (Takotsubo) Cardiomyopathy: An End to the Concept of Transience. J. Am. Soc. Echocardiogr. 2017, 30, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Gili, S.; Cammann, V.L.; Schlossbauer, S.A.; Kato, K.; D’Ascenzo, F.; Di Vece, D.; Jurisic, S.; Micek, J.; Obeid, S.; Bacchi, B.; et al. Cardiac arrest in takotsubo syndrome: Results from the InterTAK Registry. Eur. Heart J. 2019, 40, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.G.; Sechtem, U.; Schulz-Menger, J.; Holmvang, G.; Alakija, P.; Cooper, L.T.; White, J.A.; Abdel-Aty, H.; Gutberlet, M.; Prasad, S.; et al. Cardiovascular Magnetic Resonance in Myocarditis: A JACC White Paper. J. Am. Coll. Cardiol. 2009, 53, 1475–1487. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef]
- Lagan, J.; Schmitt, M.; Miller, C.A. Clinical applications of multi-parametric CMR in myocarditis and systemic inflammatory diseases. Int. J. Cardiovasc. Imaging 2018, 34, 35–54. [Google Scholar] [CrossRef]
- Hinojar, R.; Foote, L.; Arroyo Ucar, E.; Jackson, T.; Jabbour, A.; Yu, C.-Y.; McCrohon, J.; Higgins, D.M.; Carr-White, G.; Mayr, M.; et al. Native T1 in discrimination of acute and convalescent stages in patients with clinical diagnosis of myocarditis: A proposed diagnostic algorithm using CMR. JACC Cardiovasc. Imaging 2015, 8, 37–46. [Google Scholar] [CrossRef]
- Lurz, P.; Luecke, C.; Eitel, I.; Föhrenbach, F.; Frank, C.; Grothoff, M.; de Waha, S.; Rommel, K.-P.; Lurz, J.A.; Klingel, K.; et al. Comprehensive Cardiac Magnetic Resonance Imaging in Patients with Suspected Myocarditis: The MyoRacer-Trial. J. Am. Coll. Cardiol. 2016, 67, 1800–1811. [Google Scholar] [CrossRef]
- von Knobelsdorff-Brenkenhoff, F.; Schüler, J.; Dogangüzel, S.; Dieringer, M.A.; Rudolph, A.; Greiser, A.; Kellman, P.; Schulz-Menger, J. Detection and Monitoring of Acute Myocarditis Applying Quantitative Cardiovascular Magnetic Resonance. Circ. Cardiovasc. Imaging 2017, 10, e005242. [Google Scholar] [CrossRef] [PubMed]
- Radunski, U.K.; Lund, G.K.; Säring, D.; Bohnen, S.; Stehning, C.; Schnackenburg, B.; Avanesov, M.; Tahir, E.; Adam, G.; Blankenberg, S.; et al. T1 and T2 mapping cardiovascular magnetic resonance imaging techniques reveal unapparent myocardial injury in patients with myocarditis. Clin. Res. Cardiol. 2017, 106, 10–17. [Google Scholar] [CrossRef]
- Birnie, D.H.; Sauer, W.H.; Bogun, F.; Cooper, J.M.; Culver, D.A.; Duvernoy, C.S.; Judson, M.A.; Kron, J.; Mehta, D.; Cosedis Nielsen, J.; et al. HRS Expert Consensus Statement on the Diagnosis and Management of Arrhythmias Associated with Cardiac Sarcoidosis. Heart Rhythm. 2014, 11, 1304–1323. [Google Scholar] [CrossRef]
- Muser, D.; Santangeli, P.; Pathak, R.K.; Castro, S.A.; Liang, J.J.; Magnani, S.; Hayashi, T.; Garcia, F.C.; Hutchinson, M.D.; Frankel, D.S.; et al. Long-Term Outcomes of Catheter Ablation of Ventricular Tachycardia in Patients with Cardiac Sarcoidosis. Circ. Arrhythmia Electrophysiol. 2016, 9, e004333. [Google Scholar] [CrossRef]
- Muser, D.; Santangeli, P.; Liang, J.; Castro, S.; Hayashi, T.; Magnani, S.; Frankel, D.; Dixit, S.; Zado, E.; Desjardins, B.; et al. Characterization of the Electroanatomic Substrate in Cardiac Sarcoidosis: Correlation with Imaging Findings of Scar and Inflammation. JACC Clin. Electrophysiol. 2018, 4, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Muser, D.; Santangeli, P.; Castro, S.A.; Liang, J.J.; Enriquez, A.; Werner, T.J.; Nucifora, G.; Magnani, S.; Hayashi, T.; Zado, E.S.; et al. Prognostic role of serial quantitative evaluation of 18F-fluorodeoxyglucose uptake by PET/CT in patients with cardiac sarcoidosis presenting with ventricular tachycardia. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1394–1404. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Xu, Q.; Xu, B.; Wang, H. Cardiac Magnetic Resonance Imaging for Diagnosis of Cardiac Sarcoidosis: A Meta-Analysis. Can. Respir. J. 2018, 2018, 7457369. [Google Scholar] [CrossRef] [PubMed]
- Greulich, S.; Kitterer, D.; Latus, J.; Aguor, E.; Steubing, H.; Kaesemann, P.; Patrascu, A.; Greiser, A.; Groeninger, S.; Mayr, A.; et al. Comprehensive Cardiovascular Magnetic Resonance Assessment in Patients with Sarcoidosis and Preserved Left Ventricular Ejection Fraction. Circ. Cardiovasc. Imaging 2016, 9, e005022. [Google Scholar] [CrossRef]
- Crouser, E.D.; Ruden, E.; Julian, M.W.; Raman, S.V. Resolution of abnormal cardiac MRI T2 signal following immune suppression for cardiac sarcoidosis. J. Investig. Med. 2016, 64, 1148–1150. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Isted, A.; Hinojar, R.; Foote, L.; Carr-White, G.; Nagel, E. T1 and T2 Mapping in Recognition of Early Cardiac Involvement in Systemic Sarcoidosis. Radiology 2017, 285, 63–72. [Google Scholar] [CrossRef]
- Ntusi, N.A.B.; Piechnik, S.K.; Francis, J.M.; Ferreira, V.M.; Rai, A.B.S.; Matthews, P.M.; Robson, M.D.; Moon, J.; Wordsworth, P.B.; Neubauer, S.; et al. Subclinical myocardial inflammation and diffuse fibrosis are common in systemic sclerosis—A clinical study using myocardial T1-mapping and extracellular volume quantification. J. Cardiovasc. Magn. Reson. 2014, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Barison, A.; Gargani, L.; De Marchi, D.; Aquaro, G.D.; Guiducci, S.; Picano, E.; Cerinic, M.M.; Pingitore, A. Early myocardial and skeletal muscle interstitial remodelling in systemic sclerosis: Insights from extracellular volume quantification using cardiovascular magnetic resonance. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 74–80. [Google Scholar] [CrossRef]
- Thuny, F.; Lovric, D.; Schnell, F.; Bergerot, C.; Ernande, L.; Cottin, V.; Derumeaux, G.; Croisille, P. Quantification of myocardial extracellular volume fraction with cardiac MR imaging for early detection of left ventricle involvement in systemic sclerosis. Radiology 2014, 271, 373–380. [Google Scholar] [CrossRef]
- Hinojar, R.; Foote, L.; Sangle, S.; Marber, M.; Mayr, M.; Carr-White, G.; D’Cruz, D.; Nagel, E.; Puntmann, V.O. Native T1 and T2 mapping by CMR in lupus myocarditis: Disease recognition and response to treatment. Int. J. Cardiol. 2016, 222, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Corona-Villalobos, C.P.; Kiani, A.N.; Eng, J.; Kamel, I.R.; Zimmerman, S.L.; Petri, M. Myocardial T2 mapping by cardiovascular magnetic resonance reveals subclinical myocardial inflammation in patients with systemic lupus erythematosus. Int. J. Cardiovasc. Imaging 2015, 31, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Winau, L.; Hinojar Baydes, R.; Braner, A.; Drott, U.; Burkhardt, H.; Sangle, S.; D’Cruz, D.P.; Carr-White, G.; Marber, M.; Schnoes, K.; et al. High-sensitive troponin is associated with subclinical imaging biosignature of inflammatory cardiovascular involvement in systemic lupus erythematosus. Ann. Rheum. Dis. 2018, 77, 1590–1598. [Google Scholar] [CrossRef]
- Mavrogeni, S.; Bratis, K.; Markussis, V.; Spargias, C.; Papadopoulou, E.; Papamentzelopoulos, S.; Constadoulakis, P.; Matsoukas, E.; Kyrou, L.; Kolovou, G. The diagnostic role of cardiac magnetic resonance imaging in detecting myocardial inflammation in systemic lupus erythematosus. Differentiation from viral myocarditis. Lupus 2013, 22, 34–43. [Google Scholar] [CrossRef]
- Abdel-Aty, H.; Boyé, P.; Zagrosek, A.; Wassmuth, R.; Kumar, A.; Messroghli, D.; Bock, P.; Dietz, R.; Friedrich, M.G.; Schulz-Menger, J. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: Comparison of different approaches. J. Am. Coll. Cardiol. 2005, 45, 1815–1822. [Google Scholar] [CrossRef]
- Vermes, E.; Pantaléon, C.; Auvet, A.; Cazeneuve, N.; Machet, M.C.; Delhommais, A.; Bourguignon, T.; Aupart, M.; Brunereau, L. Cardiovascular magnetic resonance in heart transplant patients: Diagnostic value of quantitative tissue markers: T2 mapping and extracellular volume fraction, for acute rejection diagnosis. J. Cardiovasc. Magn. Reson. 2018, 20, 59. [Google Scholar] [CrossRef]
- Dolan, R.S.; Rahsepar, A.A.; Blaisdell, J.; Suwa, K.; Ghafourian, K.; Wilcox, J.E.; Khan, S.S.; Vorovich, E.E.; Rich, J.D.; Anderson, A.S.; et al. Multiparametric Cardiac Magnetic Resonance Imaging Can Detect Acute Cardiac Allograft Rejection after Heart Transplantation. JACC Cardiovasc. Imaging 2019, 12, 1632–1641. [Google Scholar] [CrossRef]
- Anthony, C.; Imran, M.; Pouliopoulos, J.; Emmanuel, S.; Iliff, J.; Liu, Z.; Moffat, K.; Ru Qiu, M.; McLean, C.A.; Stehning, C.; et al. Cardiovascular Magnetic Resonance for Rejection Surveillance After Cardiac Transplantation. Circulation 2022, 145, 1811–1824. [Google Scholar] [CrossRef]
- Halliday, B.P.; Baksi, A.J.; Gulati, A.; Ali, A.; Newsome, S.; Izgi, C.; Arzanauskaite, M.; Lota, A.; Tayal, U.; Vassiliou, V.S.; et al. Outcome in Dilated Cardiomyopathy Related to the Extent, Location, and Pattern of Late Gadolinium Enhancement. JACC Cardiovasc. Imaging 2019, 12, 1645–1655. [Google Scholar] [CrossRef]
- Gulati, A.; Jabbour, A.; Ismail, T.F.; Guha, K.; Khwaja, J.; Raza, S.; Morarji, K.; Brown, T.D.; Ismail, N.A.; Dweck, M.R.; et al. ASsociation of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013, 309, 896–908. [Google Scholar] [CrossRef]
- Nakamori, S.; Dohi, K.; Ishida, M.; Goto, Y.; Imanaka-Yoshida, K.; Omori, T.; Goto, I.; Kumagai, N.; Fujimoto, N.; Ichikawa, Y.; et al. Native T1 Mapping and Extracellular Volume Mapping for the Assessment of Diffuse Myocardial Fibrosis in Dilated Cardiomyopathy. JACC Cardiovasc. Imaging 2018, 11, 48–59. [Google Scholar] [CrossRef]
- Nakamori, S.; Bui, A.H.; Jang, J.; El-Rewaidy, H.A.; Kato, S.; Ngo, L.H.; Josephson, M.E.; Manning, W.J.; Nezafat, R. Increased myocardial native T1 relaxation time in patients with nonischemic dilated cardiomyopathy with complex ventricular arrhythmia. J. Magn. Reson. Imaging. 2017, 47, 779–786. [Google Scholar] [CrossRef]
- Chen, Z.; Sohal, M.; Voigt, T.; Sammut, E.; Tobon-Gomez, C.; Child, N.; Jackson, T.; Shetty, A.; Bostock, J.; Cooklin, M.; et al. Myocardial tissue characterization by cardiac magnetic resonance imaging using T1 mapping predicts ventricular arrhythmia in ischemic and non–ischemic cardiomyopathy patients with implantable cardioverter-defibrillators. Heart Rhythm. 2015, 12, 792–801. [Google Scholar] [CrossRef]
- Nakamori, S.; Ngo, L.H.; Rodriguez, J.; Neisius, U.; Manning, W.J.; Nezafat, R. T1 Mapping Tissue Heterogeneity Provides Improved Risk Stratification for ICDs without Needing Gadolinium in Patients with Dilated Cardiomyopathy. JACC Cardiovasc. Imaging 2020, 13, 1917–1930. [Google Scholar] [CrossRef]
- Kiaos, A.; Antonakaki, D.; Bazmpani, M.-A.; Karvounis, C.; Rimoldi, O.; Karamitsos, T.D. Prognostic value of cardiovascular magnetic resonance T1 mapping techniques in non-ischemic dilated cardiomyopathy: A systematic review and meta-analysis. Int. J. Cardiol. 2020, 312, 110–116. [Google Scholar] [CrossRef]
- Køber, L.; Thune, J.J.; Nielsen, J.C.; Haarbo, J.; Videbæk, L.; Korup, E.; Jensen, G.; Hildebrandt, P.; Steffensen, F.H.; Bruun, N.E.; et al. Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure. N. Engl. J. Med. 2016, 375, 1221–1230. [Google Scholar] [CrossRef]
- Selvanayagam, J.B.; Hartshorne, T.; Billot, L.; Grover, S.; Hillis, G.S.; Jung, W.; Krum, H.; Prasad, S.; McGavigan, A.D. Cardiovascular magnetic resonance-GUIDEd management of mild to moderate left ventricular systolic dysfunction (CMR GUIDE): Study protocol for a randomized controlled trial. Ann. Noninvasive Electrocardiol. 2017, 22, e12420. [Google Scholar] [CrossRef]
- Doeblin, P.; Hashemi, D.; Tanacli, R.; Lapinskas, T.; Gebker, R.; Stehning, C.; Motzkus, L.A.; Blum, M.; Tahirovic, E.; Dordevic, A.; et al. CMR Tissue Characterization in Patients with HFmrEF. J. Clin. Med. 2019, 8, 1877. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Bertrand, P.B.; Willems, E.; Gielen, E.; Mullens, W.; Giri, S.; Tang, W.H.W.; Raman, S.V.; Verhaert, D. Global myocardial oedema in advanced decompensated heart failure. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 787–794. [Google Scholar] [CrossRef]
- Bohnen, S.; Radunski, U.K.; Lund, G.K.; Kandolf, R.; Stehning, C.; Schnackenburg, B.; Adam, G.; Blankenberg, S.; Muellerleile, K. Performance of t1 and t2 mapping cardiovascular magnetic resonance to detect active myocarditis in patients with recent-onset heart failure. Circ. Cardiovasc. Imaging 2015, 8, e003073. [Google Scholar] [CrossRef]
- Emrich, T.; Hahn, F.; Fleischmann, D.; Halfmann, M.C.; Düber, C.; Varga-Szemes, A.; Escher, F.; Pefani, E.; Münzel, T.; Schultheiss, H.; et al. T1 and T2 mapping to detect chronic inflammation in cardiac magnetic resonance imaging in heart failure with reduced ejection fraction. ESC Heart Fail. 2020, 7, 2544–2552. [Google Scholar] [CrossRef]
- Muser, D.; Nucifora, G.; Gianfagna, E.; Pavoni, D.; Rebellato, L.; Facchin, D.; Daleffe, E.; Proclemer, A. Clinical Spectrum of Isolated Left Ventricular Noncompaction: Thromboembolic Events, Malignant Left Ventricular Arrhythmias, and Refractory Heart Failure. J. Am. Coll. Cardiol. 2014, 63, e39. [Google Scholar] [CrossRef]
- Zhou, H.; Lin, X.; Fang, L.; Zhao, X.; Ding, H.; Chen, W.; Xu, R.; Bai, X.; Wang, Y.; Fang, Q. Characterization of Compacted Myocardial Abnormalities by Cardiac Magnetic Resonance with Native T1 Mapping in Left Ventricular Non-Compaction Patients—A Comparison with Late Gadolinium Enhancement. Circ. J. 2016, 80, 1210–1216. [Google Scholar] [CrossRef]
- Araujo-Filho, J.A.B.; Assuncao, A.N.; Tavares de Melo, M.D.; Bière, L.; Lima, C.R.; Dantas, R.N.; Nomura, C.H.; Salemi, V.M.C.; Jerosch-Herold, M.; Parga, J.R. Myocardial T1 mapping and extracellular volume quantification in patients with left ventricular non-compaction cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 888–895. [Google Scholar] [CrossRef]
- Muser, D.; Liang, J.J.; Witschey, W.R.; Pathak, R.K.; Castro, S.; Magnani, S.; Zado, E.S.; Garcia, F.C.; Desjardins, B.; Callans, D.J.; et al. Ventricular arrhythmias associated with left ventricular noncompaction: Electrophysiologic characteristics, mapping, and ablation. Heart Rhythm. 2017, 14, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.Y.; Abbasi, S.A.; Neilan, T.G.; Shah, R.V.; Chen, Y.; Heydari, B.; Cirino, A.L.; Lakdawala, N.K.; Orav, E.J.; González, A.; et al. T1 measurements identify extracellular volume expansion in hypertrophic cardiomyopathy sarcomere mutation carriers with and without left ventricular hypertrophy. Circ. Cardiovasc. Imaging 2013, 6, 415–422. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Yang, F.; Wang, J.; Xu, Y.; Fang, T.; Pu, L.; Zhou, X.; Han, Y.; Chen, Y. Prognostic value of myocardial extracellular volume fraction evaluation based on cardiac magnetic resonance T1 mapping with T1 long and short in hypertrophic cardiomyopathy. Eur. Radiol. 2021, 31, 4557–4567. [Google Scholar] [CrossRef] [PubMed]
- McDiarmid, A.K.; Swoboda, P.P.; Erhayiem, B.; Lancaster, R.E.; Lyall, G.K.; Broadbent, D.A.; Dobson, L.E.; Musa, T.A.; Ripley, D.P.; Garg, P.; et al. Athletic Cardiac Adaptation in Males Is a Consequence of Elevated Myocyte Mass. Circ. Cardiovasc. Imaging 2016, 9, e003579. [Google Scholar] [CrossRef] [PubMed]
- Swoboda, P.P.; McDiarmid, A.K.; Erhayiem, B.; Broadbent, D.A.; Dobson, L.E.; Garg, P.; Ferguson, C.; Page, S.P.; Greenwood, J.P.; Plein, S. Assessing Myocardial Extracellular Volume by T1 Mapping to Distinguish Hypertrophic Cardiomyopathy from Athlete’s Heart. J. Am. Coll. Cardiol. 2016, 67, 2189–2190. [Google Scholar] [CrossRef]
- Austin, B.A.; Tang, W.H.W.; Rodriguez, E.R.; Tan, C.; Flamm, S.D.; Taylor, D.O.; Starling, R.C.; Desai, M.Y. Delayed hyper-enhancement magnetic resonance imaging provides incremental diagnostic and prognostic utility in suspected cardiac amyloidosis. JACC Cardiovasc. Imaging 2009, 2, 1369–1377. [Google Scholar] [CrossRef]
- Maceira, A.M.; Joshi, J.; Prasad, S.K.; Moon, J.C.; Perugini, E.; Harding, I.; Sheppard, M.N.; Poole-Wilson, P.A.; Hawkins, P.N.; Pennell, D.J. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation 2005, 111, 186–193. [Google Scholar] [CrossRef]
- Fontana, M.; Pica, S.; Reant, P.; Abdel-Gadir, A.; Treibel, T.A.; Banypersad, S.M.; Maestrini, V.; Barcella, W.; Rosmini, S.; Bulluck, H.; et al. Prognostic Value of Late Gadolinium Enhancement Cardiovascular Magnetic Resonance in Cardiac Amyloidosis. Circulation 2015, 132, 1570–1579. [Google Scholar] [CrossRef]
- Richards, D.B.; Cookson, L.M.; Berges, A.C.; Barton, S.V.; Lane, T.; Ritter, J.M.; Fontana, M.; Moon, J.C.; Pinzani, M.; Gillmore, J.D.; et al. Therapeutic Clearance of Amyloid by Antibodies to Serum Amyloid P Component. N. Engl. J. Med. 2015, 373, 1106–1114. [Google Scholar] [CrossRef]
- Banypersad, S.M.; Sado, D.M.; Flett, A.S.; Gibbs, S.D.J.; Pinney, J.H.; Maestrini, V.; Cox, A.T.; Fontana, M.; Whelan, C.J.; Wechalekar, A.D.; et al. Quantification of myocardial extracellular volume fraction in systemic AL amyloidosis: An equilibrium contrast cardiovascular magnetic resonance study. Circ. Cardiovasc. Imaging 2013, 6, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M.; Banypersad, S.M.; Treibel, T.A.; Abdel-Gadir, A.; Maestrini, V.; Lane, T.; Gilbertson, J.A.; Hutt, D.F.; Lachmann, H.J.; Whelan, C.J.; et al. Differential Myocyte Responses in Patients with Cardiac Transthyretin Amyloidosis and Light-Chain Amyloidosis: A Cardiac MR Imaging Study. Radiology 2015, 277, 388–397. [Google Scholar] [CrossRef]
- Putko, B.N.; Wen, K.; Thompson, R.B.; Mullen, J.; Shanks, M.; Yogasundaram, H.; Sergi, C.; Oudit, G.Y. Anderson-Fabry cardiomyopathy: Prevalence, pathophysiology, diagnosis and treatment. Heart Fail. Rev. 2015, 20, 179–191. [Google Scholar] [CrossRef]
- Thompson, R.B.; Chow, K.; Khan, A.; Chan, A.; Shanks, M.; Paterson, I.; Oudit, G.Y. T1 mapping with cardiovascular MRI is highly sensitive for Fabry disease independent of hypertrophy and sex. Circ. Cardiovasc. Imaging 2013, 6, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Halliday, B.P.; Pennell, D.J. Cardiovascular Magnetic Resonance to Guide and Monitor the Myocardial Response to Treatment. Circ. Cardiovasc. Imaging 2019, 12, e010045. [Google Scholar] [CrossRef]
- Anderson, L.J.; Holden, S.; Davis, B.; Prescott, E.; Charrier, C.C.; Bunce, N.H.; Firmin, D.N.; Wonke, B.; Porter, J.; Walker, J.M.; et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur. Heart J. 2001, 22, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Kirk, P.; Roughton, M.; Porter, J.B.; Walker, J.M.; Tanner, M.A.; Patel, J.; Wu, D.; Taylor, J.; Westwood, M.A.; Anderson, L.J.; et al. Cardiac T2* magnetic resonance for prediction of cardiac complications in thalassemia major. Circulation 2009, 120, 1961–1968. [Google Scholar] [CrossRef] [PubMed]
- Krittayaphong, R.; Zhang, S.; Saiviroonporn, P.; Viprakasit, V.; Tanapibunpon, P.; Komoltri, C.; Wangworatrakul, W. Detection of cardiac iron overload with native magnetic resonance T1 and T2 mapping in patients with thalassemia. Int. J. Cardiol. 2017, 248, 421–426. [Google Scholar] [CrossRef]
- Meloni, A.; Pistoia, L.; Positano, V.; De Luca, A.; Martini, N.; Spasiano, A.; Fotzi, I.; Bitti, P.P.; Visceglie, D.; Alberini, G.; et al. Increased myocardial extracellular volume is associated with myocardial iron overload and heart failure in thalassemia major. Eur. Radiol. 2023, 33, 1266–1276. [Google Scholar] [CrossRef]
- Hong, Y.J.; Park, H.S.; Park, J.K.; Han, K.; Park, C.H.; Kim, T.K.; Yoo, S.J.; Lee, J.Y.; Kim, P.K.; Hur, J.; et al. Early Detection and Serial Monitoring of Anthracycline-Induced Cardiotoxicity Using T1-mapping Cardiac Magnetic Resonance Imaging: An Animal Study. Sci. Rep. 2017, 7, 2663. [Google Scholar] [CrossRef]
- Galán-Arriola, C.; Lobo, M.; Vílchez-Tschischke, J.P.; López, G.J.; de Molina-Iracheta, A.; Pérez-Martínez, C.; Agüero, J.; Fernández-Jiménez, R.; Martín-García, A.; Oliver, E.; et al. Serial Magnetic Resonance Imaging to Identify Early Stages of Anthracycline-Induced Cardiotoxicity. J. Am. Coll. Cardiol. 2019, 73, 779–791. [Google Scholar] [CrossRef]
- Dweck, M.R.; Boon, N.A.; Newby, D.E. Calcific aortic stenosis: A disease of the valve and the myocardium. J. Am. Coll. Cardiol. 2012, 60, 1854–1863. [Google Scholar] [CrossRef]
- Kockova, R.; Kacer, P.; Pirk, J.; Maly, J.; Sukupova, L.; Sikula, V.; Kotrc, M.; Barciakova, L.; Honsova, E.; Maly, M.; et al. Native T1 Relaxation Time and Extracellular Volume Fraction as Accurate Markers of Diffuse Myocardial Fibrosis in Heart Valve Disease—Comparison with Targeted Left Ventricular Myocardial Biopsy. Circ. J. 2016, 80, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Bull, S.; White, S.K.; Piechnik, S.K.; Flett, A.S.; Ferreira, V.M.; Loudon, M.; Francis, J.M.; Karamitsos, T.D.; Prendergast, B.D.; Robson, M.D.; et al. Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart 2013, 99, 932–937. [Google Scholar] [CrossRef]
- de Meester de Ravenstein, C.; Bouzin, C.; Lazam, S.; Boulif, J.; Amzulescu, M.; Melchior, J.; Pasquet, A.; Vancraeynest, D.; Pouleur, A.-C.; Vanoverschelde, J.-L.J.; et al. Histological Validation of measurement of diffuse interstitial myocardial fibrosis by myocardial extravascular volume fraction from Modified Look-Locker imaging (MOLLI) T1 mapping at 3 T. J. Cardiovasc. Magn. Reson. 2015, 17, 48. [Google Scholar] [CrossRef] [PubMed]
- Pradella, S.; Grazzini, G.; Brandani, M.; Calistri, L.; Nardi, C.; Mori, F.; Miele, V.; Colagrande, S. Cardiac magnetic resonance in patients with mitral valve prolapse: Focus on late gadolinium enhancement and T1 mapping. Eur. Radiol. 2019, 29, 1546–1554. [Google Scholar] [CrossRef]
- Eyre, K.; Lindsay, K.; Razzaq, S.; Chetrit, M.; Friedrich, M. Simultaneous multi-parametric acquisition and reconstruction techniques in cardiac magnetic resonance imaging: Basic concepts and status of clinical development. Front. Cardiovasc. Med. 2022, 9, 953823. [Google Scholar] [CrossRef] [PubMed]
- Salerno, M.; Kramer, C.M. Advances in Parametric Mapping with Cardiac Magnetic Resonance Imaging. JACC Cardiovasc. Imaging 2013, 6, 806–822. [Google Scholar] [CrossRef]
- Burrage, M.K.; Shanmuganathan, M.; Masi, A.; Hann, E.; Zhang, Q.; Popescu, I.A.; Soundarajan, R.; Leal Pelado, J.; Chow, K.; Neubauer, S.; et al. Cardiovascular magnetic resonance stress and rest T1-mapping using regadenoson for detection of ischemic heart disease compared to healthy controls. Int. J. Cardiol. 2021, 333, 239–245. [Google Scholar] [CrossRef]
- Levelt, E.; Piechnik, S.K.; Liu, A.; Wijesurendra, R.S.; Mahmod, M.; Ariga, R.; Francis, J.M.; Greiser, A.; Clarke, K.; Neubauer, S.; et al. Adenosine stress CMR T1-mapping detects early microvascular dysfunction in patients with type 2 diabetes mellitus without obstructive coronary artery disease. J. Cardiovasc. Magn. Reson. 2017, 19, 81. [Google Scholar] [CrossRef]
- Mahmod, M.; Piechnik, S.K.; Levelt, E.; Ferreira, V.M.; Francis, J.M.; Lewis, A.; Pal, N.; Dass, S.; Ashrafian, H.; Neubauer, S.; et al. Adenosine stress native T1 mapping in severe aortic stenosis: Evidence for a role of the intravascular compartment on myocardial T1 values. J. Cardiovasc. Magn. Reson. 2014, 16, 92. [Google Scholar] [CrossRef]
- Soto-Iglesias, D.; Penela, D.; Jáuregui, B.; Acosta, J.; Fernández-Armenta, J.; Linhart, M.; Zucchelli, G.; Syrovnev, V.; Zaraket, F.; Terés, C.; et al. Cardiac Magnetic Resonance–Guided Ventricular Tachycardia Substrate Ablation. J. Am. Coll. Cardiol. 2020, 6, 436–447. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muser, D.; Chahal, A.A.; Selvanayagam, J.B.; Nucifora, G. Clinical Applications of Cardiac Magnetic Resonance Parametric Mapping. Diagnostics 2024, 14, 1816. https://doi.org/10.3390/diagnostics14161816
Muser D, Chahal AA, Selvanayagam JB, Nucifora G. Clinical Applications of Cardiac Magnetic Resonance Parametric Mapping. Diagnostics. 2024; 14(16):1816. https://doi.org/10.3390/diagnostics14161816
Chicago/Turabian StyleMuser, Daniele, Anwar A. Chahal, Joseph B. Selvanayagam, and Gaetano Nucifora. 2024. "Clinical Applications of Cardiac Magnetic Resonance Parametric Mapping" Diagnostics 14, no. 16: 1816. https://doi.org/10.3390/diagnostics14161816
APA StyleMuser, D., Chahal, A. A., Selvanayagam, J. B., & Nucifora, G. (2024). Clinical Applications of Cardiac Magnetic Resonance Parametric Mapping. Diagnostics, 14(16), 1816. https://doi.org/10.3390/diagnostics14161816






