Contrast-Enhanced Sonography of the Liver: How to Avoid Artifacts
Abstract
1. Introduction
2. CEUS: Frame Rate, Image Quality, and Mode Selection
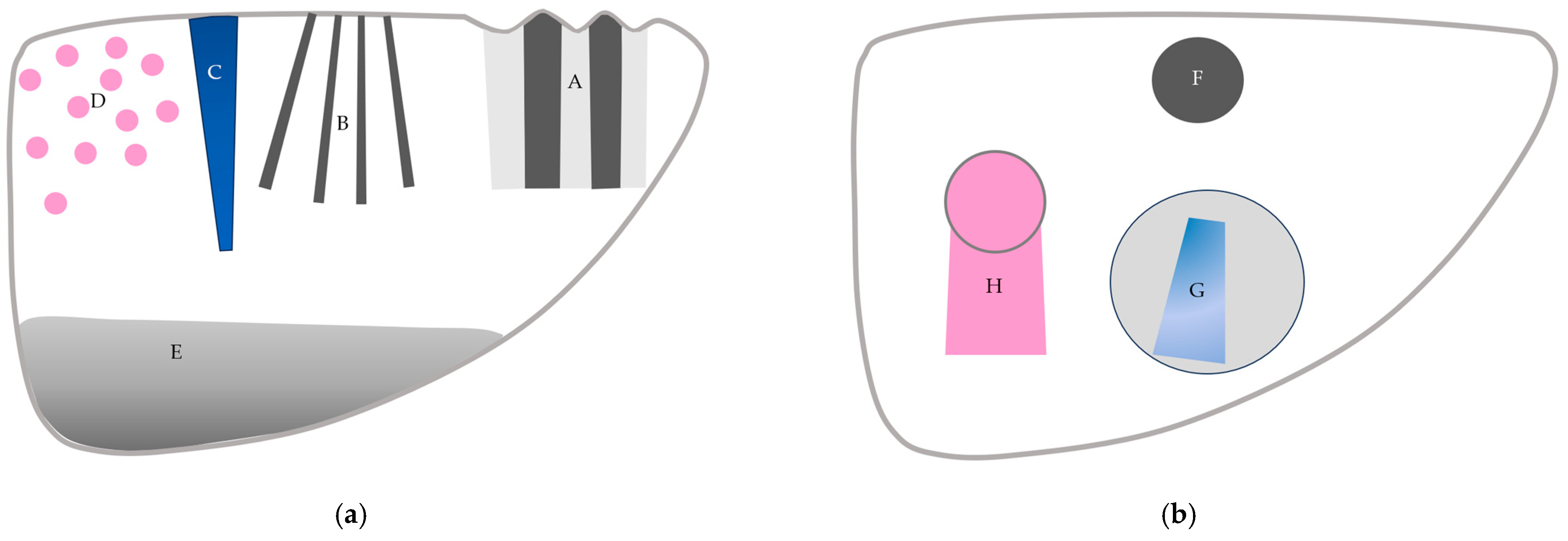
3. CEUS Artifacts: How to Classify and Recognize Them
3.1. B-Mode US-Related Artifacts
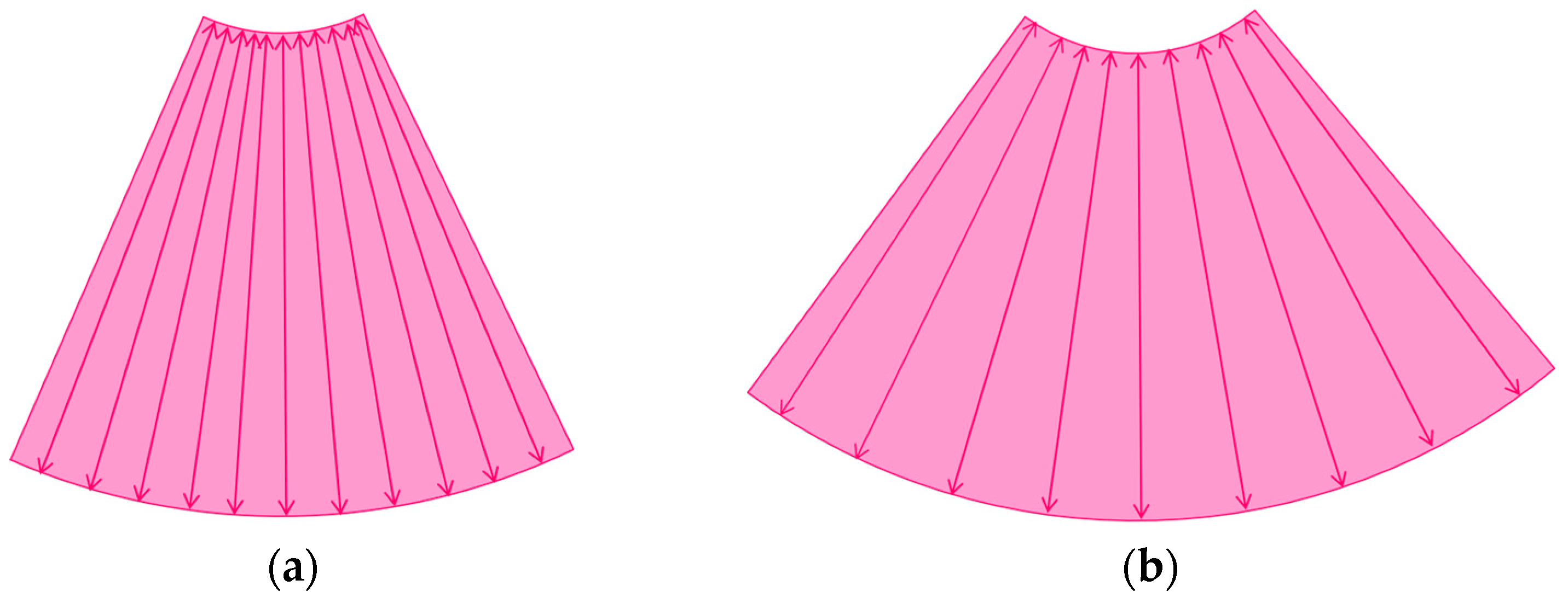
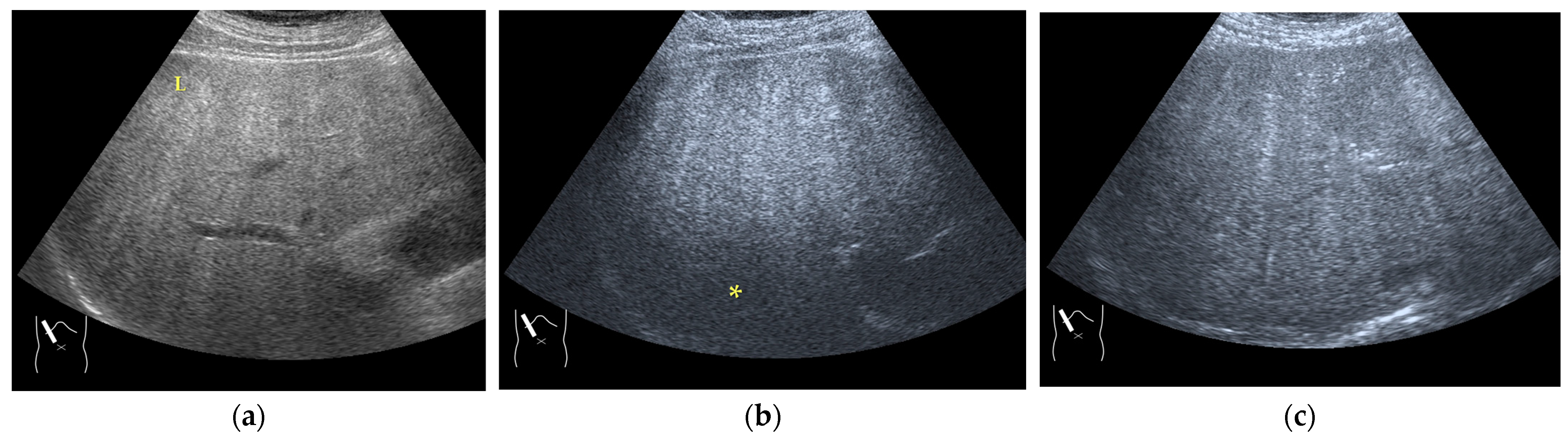
3.1.1. Refraction Artifacts
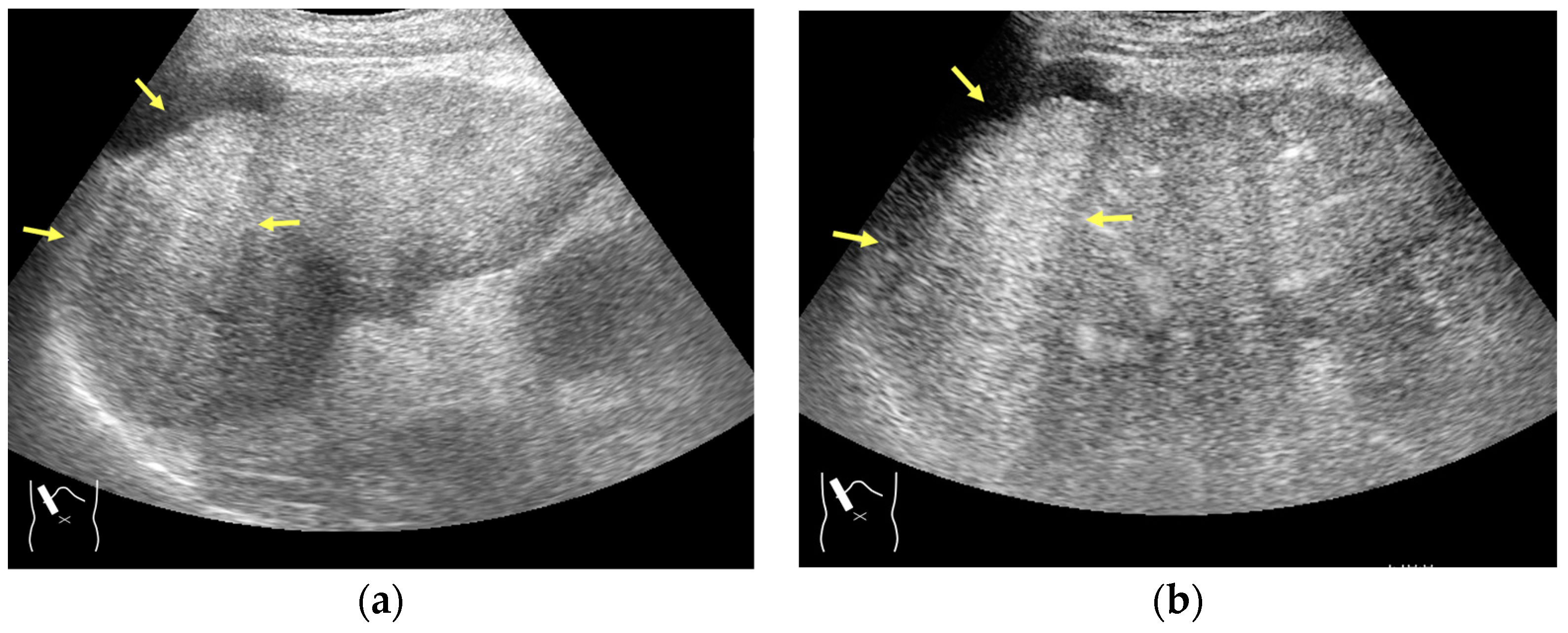
3.1.2. Attenuation Artifacts
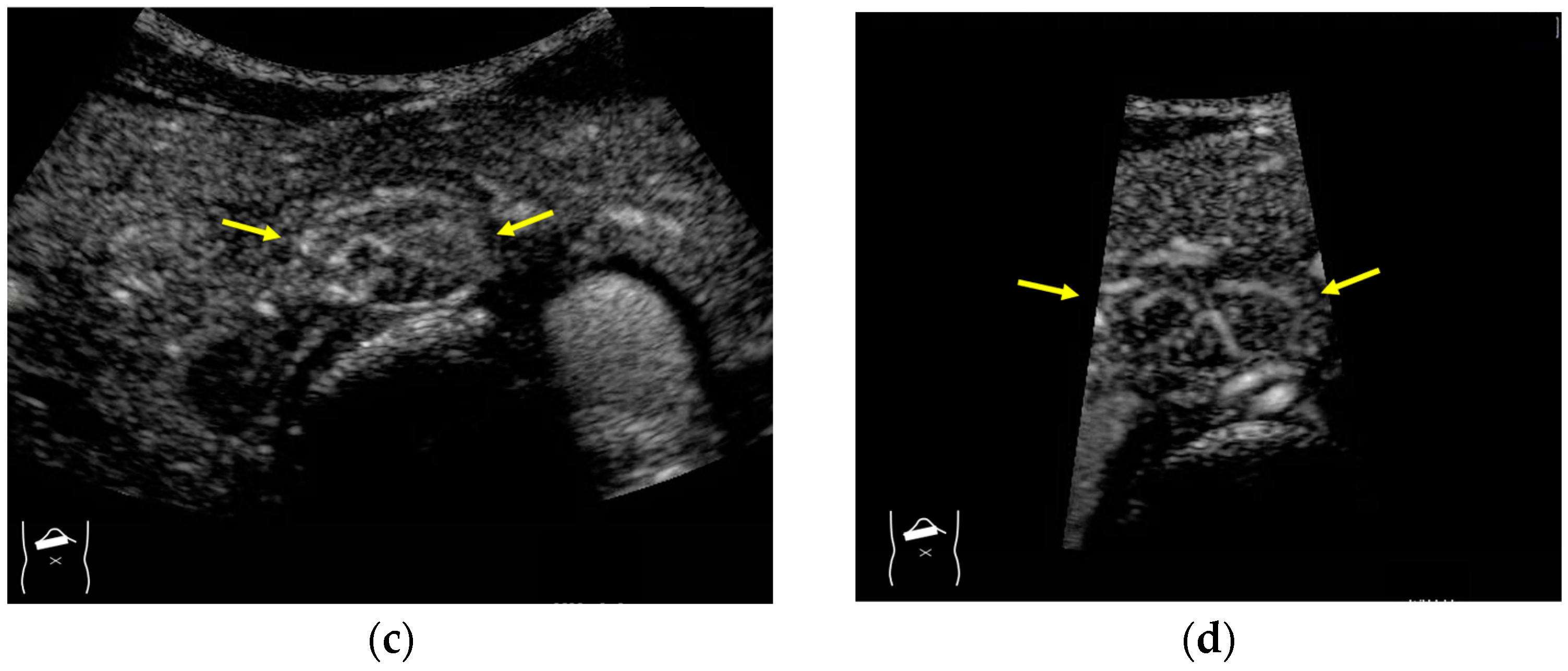
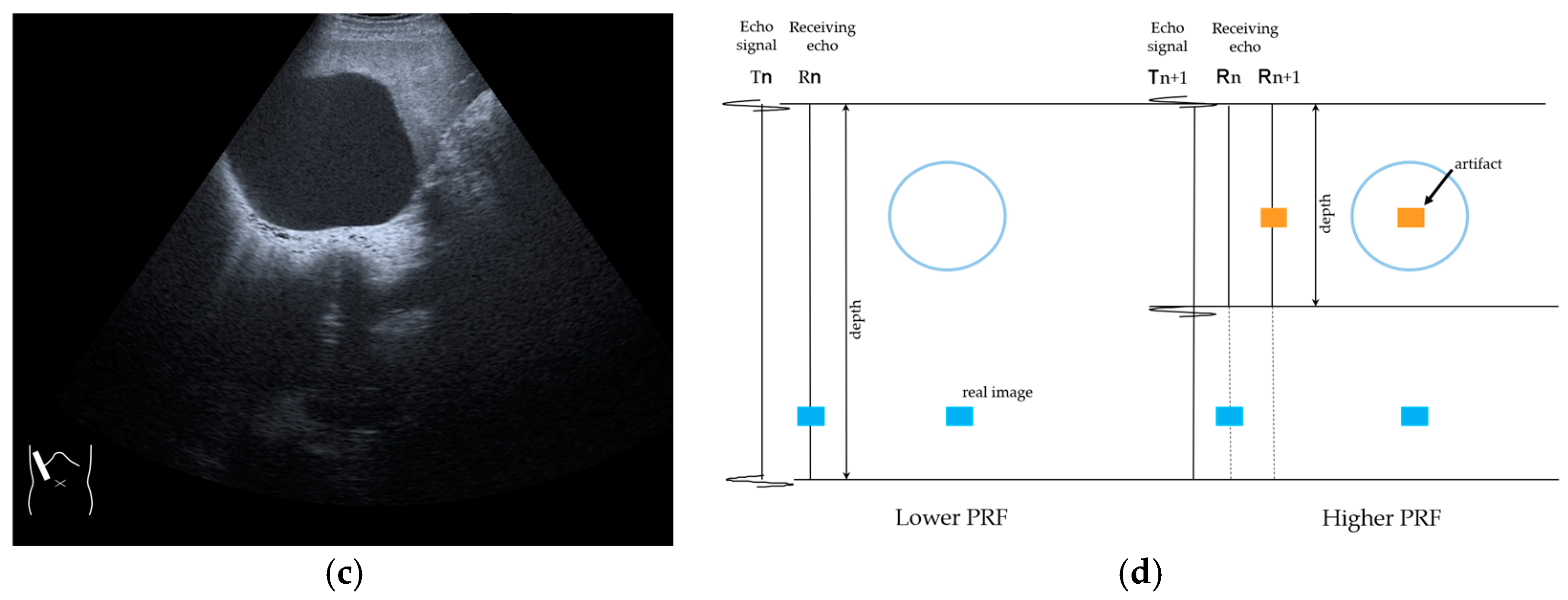
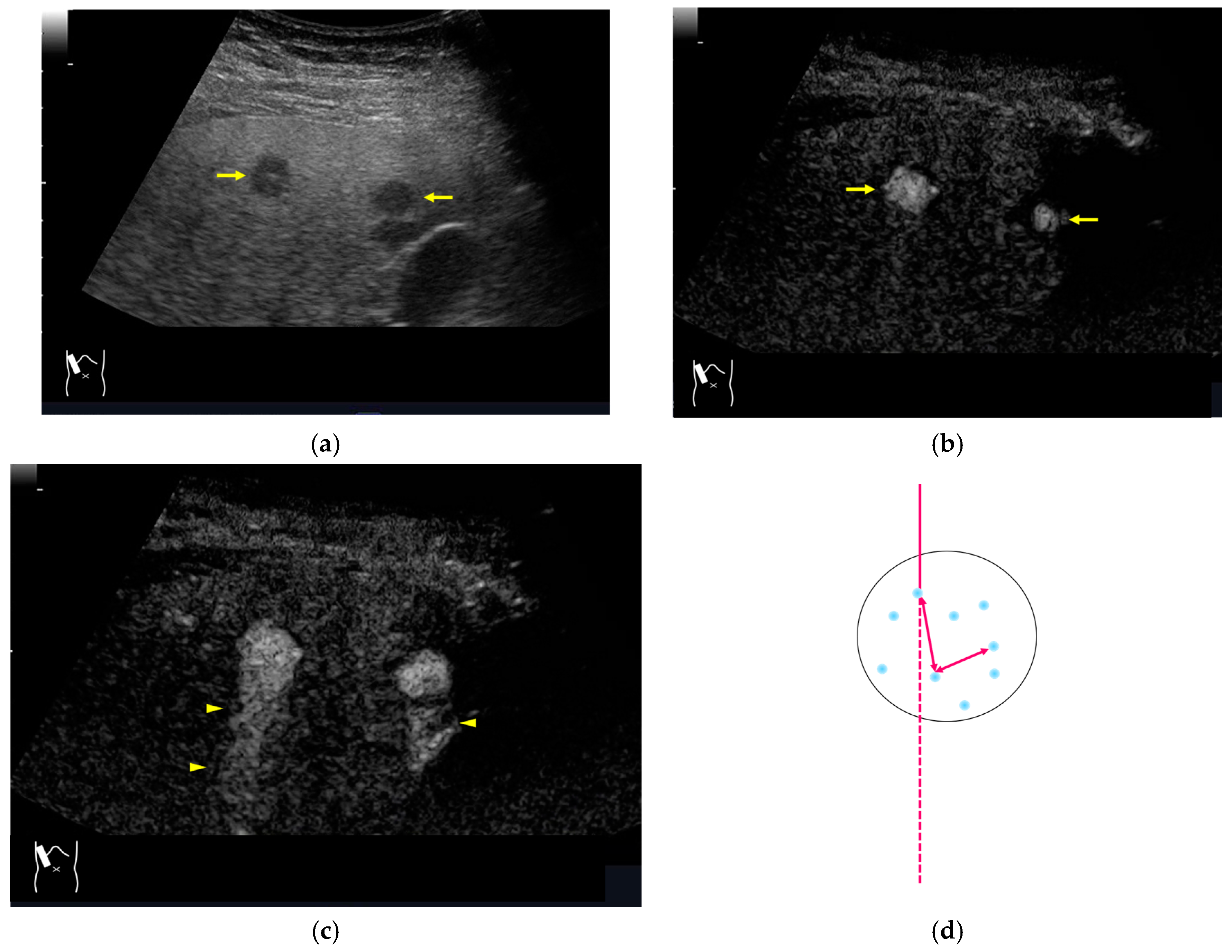
3.1.3. Range-Ambiguity Artifacts (RAAs)
3.2. CEUS-Specific Artifacts
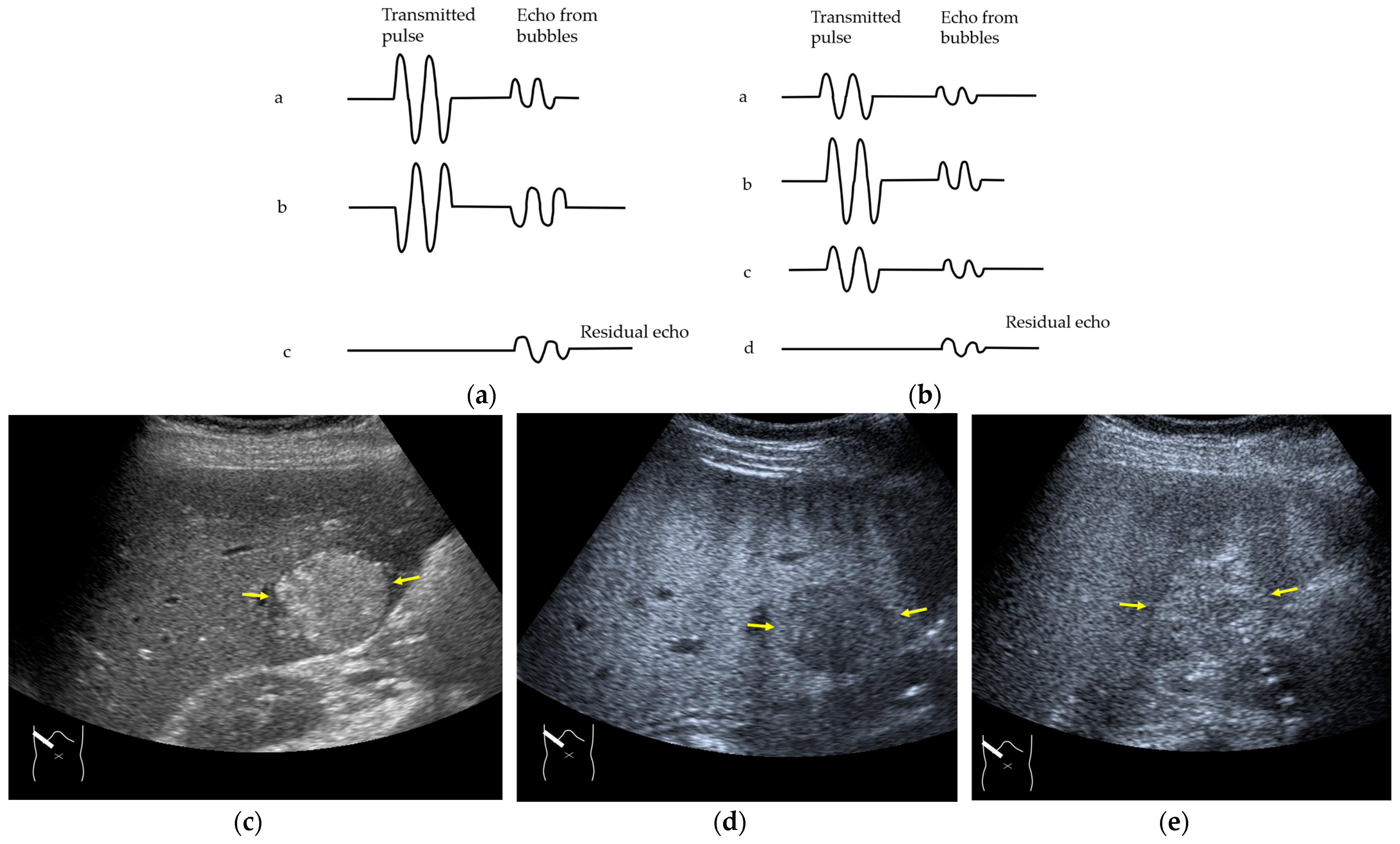
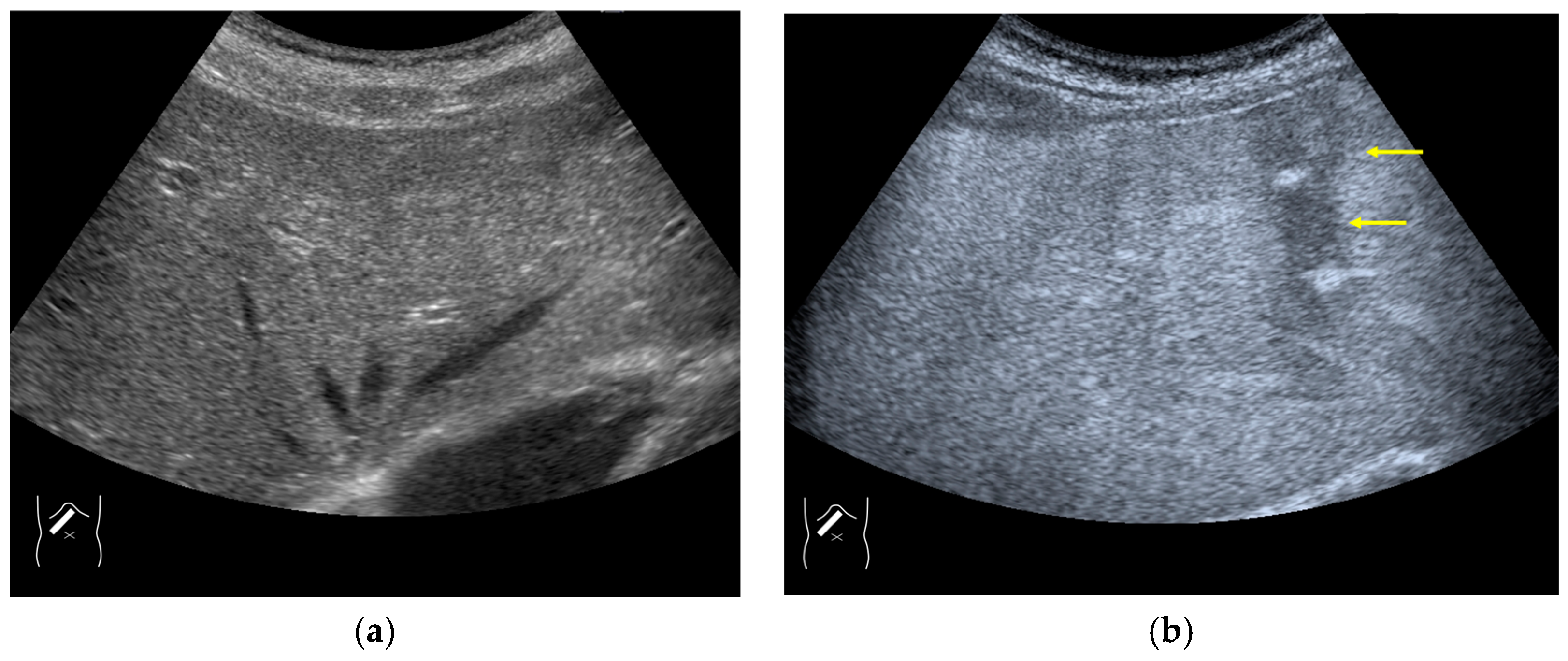
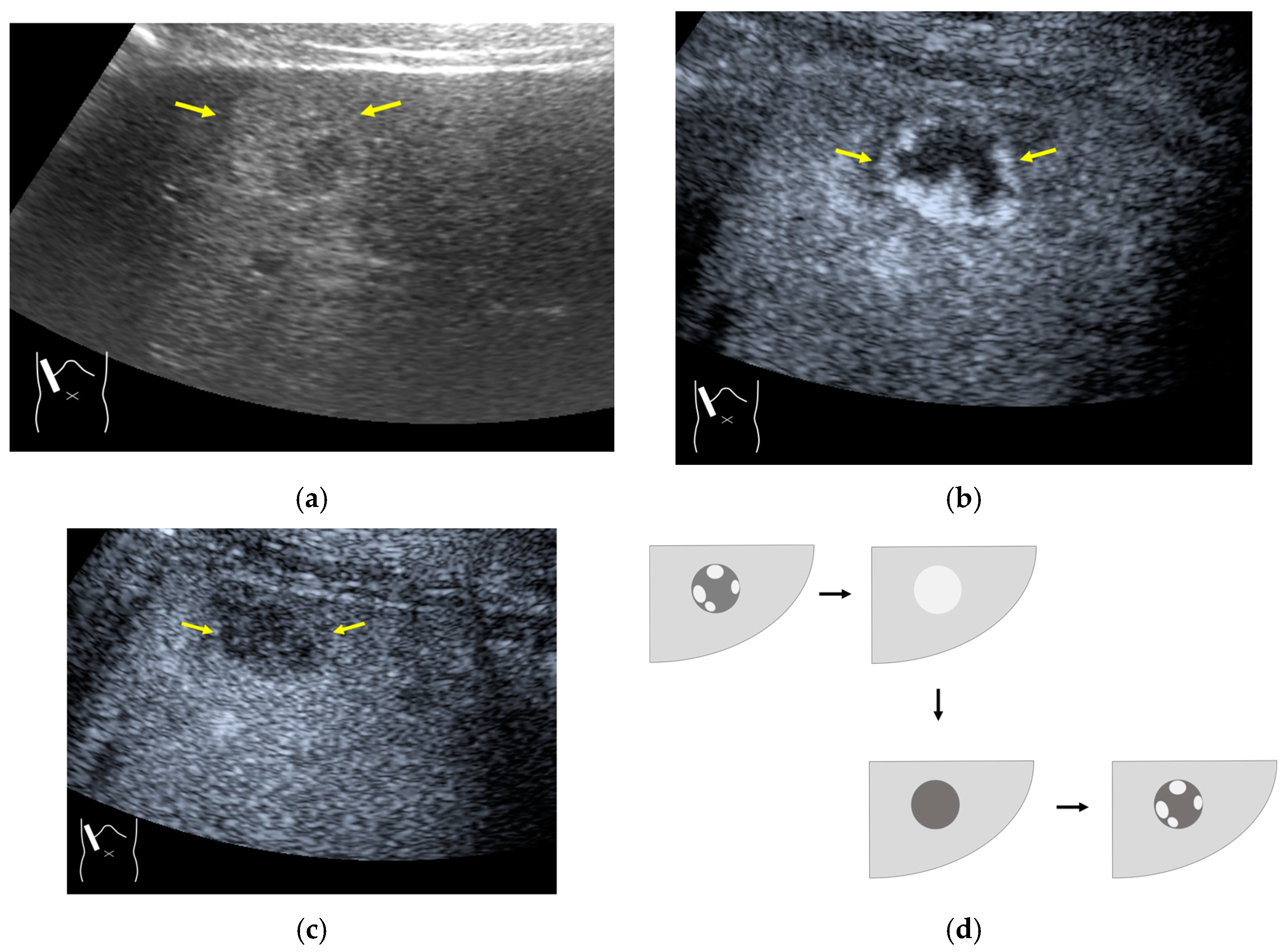
3.2.1. Microbubble Destruction Artifacts
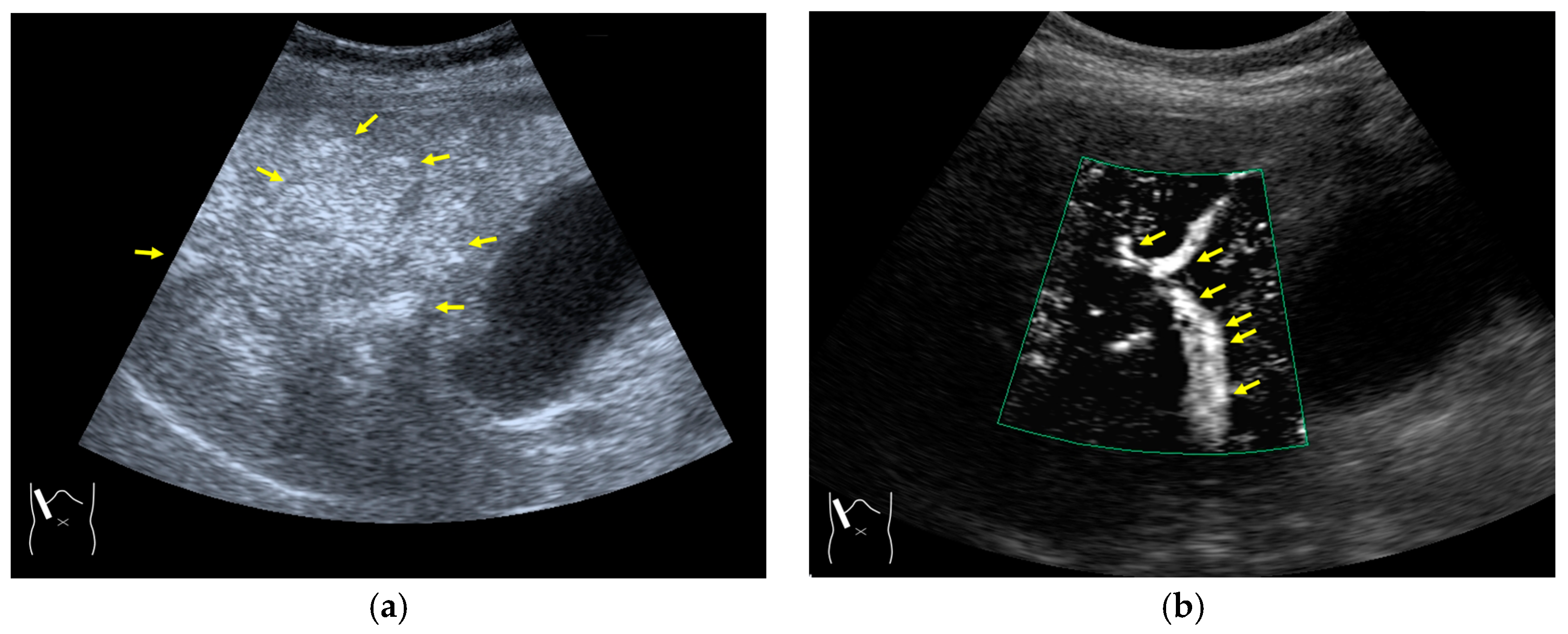
3.2.2. Prolonged Heterogeneous Accumulation Artifacts
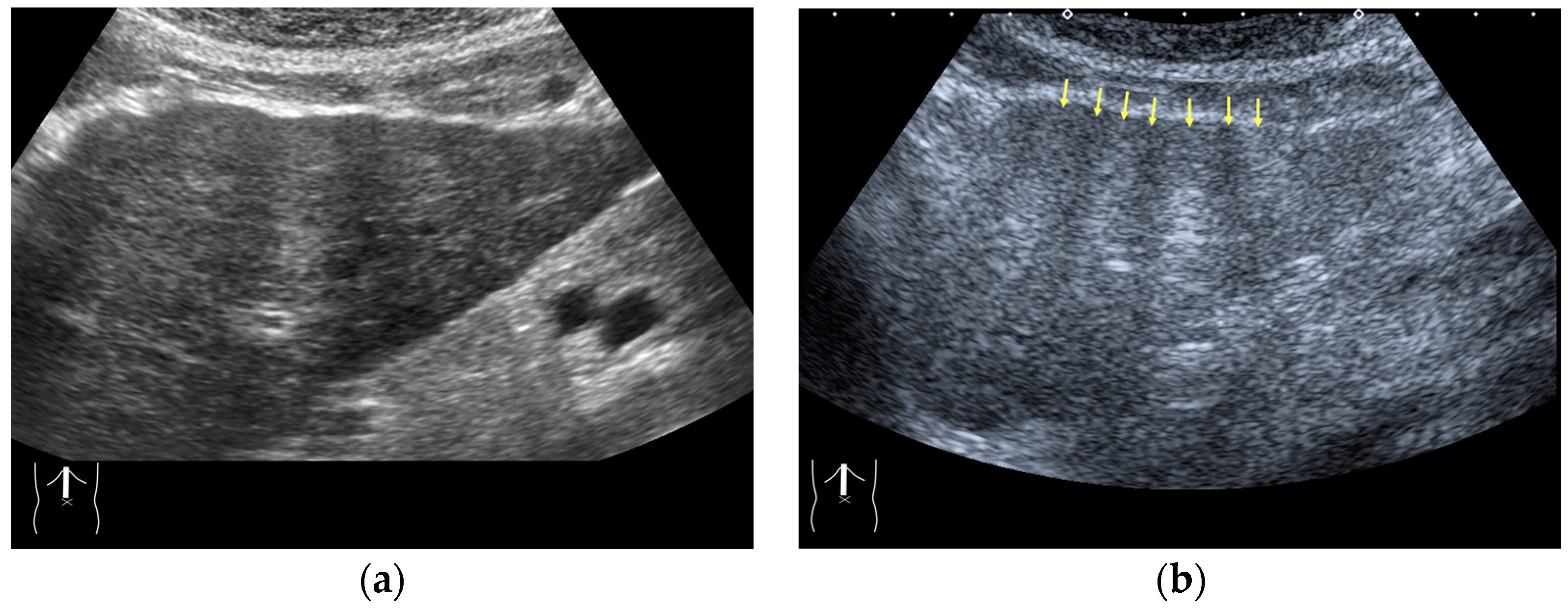
3.2.3. CEUS-Related Posterior Echo Enhancement
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, S.; Qiu, Y.J.; Zuo, D.; Shi, S.N.; Wang, W.P.; Dong, Y. Imaging features of hepatocellular carcinoma in the non-cirrhotic liver with sonazoid-enhanced contrast-enhanced ultrasound. Diagnostics 2022, 12, 2272. [Google Scholar] [CrossRef]
- Huang, W.; Wen, R.; Wu, Y.; Lin, P.; Guo, D.; Peng, Y.; Liu, D.; Mou, M.; Chen, F.; Huang, F.; et al. Can modifications of LR-M criteria improve the diagnostic performance of contrast-enhanced ultrasound LI-RADS for small hepatic lesions up to 3 cm? J. Ultrasound Med. 2023, 42, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yi, H.; Cai, B.; He, Y.; Huang, S.; Zhang, Y. Feasibility of contrast-enhanced ultrasonography (CEUS) in evaluating renal microvascular perfusion in pediatric patients. BMC Med. Imaging 2022, 22, 194. [Google Scholar] [CrossRef]
- Picardi, M.; Giordano, C.; Trastulli, F.; Leone, A.; Pepa, R.D.; Pugliese, N.; Iula, R.; Cave, G.D.; Rascato, M.G.; Esposito, M.; et al. Sulfur exafluoride contrast-enhanced ultrasound showing early wash-out of marked degree identifies lymphoma invasion of spleen with excellent diagnostic accuracy: A monocentric study of 260 splenic nodules. Cancers 2022, 14, 1927. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, G.; Zhang, X.; Ni, T. The role of contrast-enhanced ultrasound in differentiating splenic tuberculosis from splenic lymphoma. Front. Oncol. 2022, 12, 891815. [Google Scholar] [CrossRef]
- Yamashita, Y.; Shimokawa, T.; Ashida, R.; Dietrich, C.F.; D’Onofrio, M.; Hirooka, Y.; Kudo, M.; Mori, H.; Sofuni, A.; Masayuki Kitano, M. Value of low-mechanical-index contrast-enhanced transabdominal ultrasound for diagnosis of pancreatic cancer: A meta-analysis. Ultrasound Med. Biol. 2021, 47, 3315–3322. [Google Scholar] [CrossRef]
- Yu, M.H.; Kim, Y.J.; Park, H.S.; Jung, S.I. Benign gallbladder diseases: Imaging techniques and tips for differentiating with malignant gallbladder diseases. World J. Gastroenterol. 2020, 26, 2967–2986. [Google Scholar] [CrossRef]
- Wang, C.Y.; Fan, X.J.; Wang, F.L.; Ge, Y.Y.; Cai, Z.; Wang, W.; Zhou, X.P.; Du, J.; Dai, D.W. Clinical value of oral contrast-enhanced ultrasonography in diagnosis of gastric tumors. World J. Gastrointest. Oncol. 2024, 16, 110–117. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, T.; Sun, S.; Ye, X.; Wang, Y.; Pan, M.; Shi, H. Preoperative contrast-enhanced ultrasound predicts microvascular invasion in hepatocellular carcinoma as accurately as contrast-enhanced MR. J. Ultrasound Med. 2024, 43, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; Xia, B.; Chen, W.; Gao, X.; Yang, J.; Li, S.; Wang, B.; Mai, H.; Liu, S.; Wen, F.; et al. The Safety and Effectiveness of Intravenous Contrast-Enhanced Sonography in Chinese Children-A Single Center and Prospective Study in China. Front. Pharmacol. 2019, 10, 1447. [Google Scholar] [CrossRef]
- Dobek, A.; Kobierecki, M.; Ciesielski, W.; Grząsiak, O.; Fabisiak, A.; Stefańczyk, L. Usefulness of contrast-enhanced ultrasound in the differentiation between hepatocellular carcinoma and benign liver lesions. Diagnostics 2023, 13, 2025. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Correas, J.M.; Cui, X.W.; Dong, Y.; Havre, R.F.; Jenssen, C.; Jung, E.M.; Krix, M.; Lim, A.; Lassau, N.; et al. EFSUMB technical review—Update 2023: Dynamic contrast-enhanced ultrasound (DCE-CEUS) for the quantification of tumor perfusion. Ultraschall Med. 2024, 45, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.K.; Kang, H.J.; Choi, S.H.; Park, M.S.; Yu, M.H.; Kim, B.; You, M.W.; Lim, S.; Young Seo Cho, Y.S.; Min Woo Lee, M.W.; et al. Diagnosing hepatocellular carcinoma using sonazoid contrast-enhanced ultrasonography: 2023 guidelines from the Korean society of radiology and the Korean society of abdominal radiology. Korean J. Radiol. 2023, 24, 482–497. [Google Scholar] [CrossRef]
- Dawit, H.; Lam, E.; McInnes, M.D.F.; van der Pol, C.B.; Bashir, M.R.; Salameh, J.P.; Levis, B.; Sirlin, C.B.; Chernyak, V.; Choi, S.H.; et al. LI-RADS CT and MRI ancillary feature association with hepatocellular carcinoma and malignancy: An individual participant data meta-analysis. Radiology 2024, 310, e231501. [Google Scholar] [CrossRef]
- Kono, Y.; Piscaglia, F.; Wilson, S.R.; Medellin, A.; Rodgers, S.K.; Planz, V.; Kamaya, A.; Fetzer, D.T.; Berzigotti, A.; Sidhu, P.S.; et al. Clinical impact of CEUS on non-characterizable observations and observations with intermediate probability of malignancy on CT/MRI in patients at risk for HCC. Abdom. Radiol. 2024, 11, 2639–2649. [Google Scholar] [CrossRef]
- Sugimoto, K.; Kakegawa, T.; Takahashi, H.; Tomita, Y.; Abe, M.; Yoshimasu, Y.; Takeuchi, H.; Kasai, Y.; Itoi, T. Usefulness of modified CEUS LI-RADS for the diagnosis of hepatocellular carcinoma using Sonazoid. Diagnostics 2020, 10, 828. [Google Scholar] [CrossRef]
- Quaia, E. Physical bases and principles of action of microbubble-based contrast agents. In Contrast Media in Ultrasonography, 1st ed.; Baert, A.L., Sartor, K., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2005; pp. 15–30. [Google Scholar]
- Lu, Y.; Liu, B.; Zheng, Y.; Luo, J.; Zhang, X.; Huang, G.; Xie, X.; Ye, J.; Wang, W.; Liu, X.; et al. Application of real-time three-dimensional contrast-enhanced ultrasound using SonoVue for the evaluation of focal liver lesions: A prospective single-center study. Am. J. Transl. Res. 2018, 10, 1469–1480. [Google Scholar] [PubMed]
- Li, N.; Nie, F.; Wang, Y.; Pang, W.; Wang, W.; Zhao, Y.; Jia, Y. Contrast-enhanced ultrasound demonstrates greater adjuvant potential: Past, current status, and future applications in Hepatocellular carcinoma early diagnosis. Med. Ultrason. 2023, 25, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Bartolotta, T.V.; Taibbi, A.; Randazzo, A.; Gagliardo, C. New frontiers in liver ultrasound: From mono to multi parametricity. World J. Gastrointest. Oncol. 2021, 13, 1302–1316. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Dai, H.Y.; Li, C.Y.; Jin, Y.; Zhu, L.L.; Zhang, T.F.; Zhang, Y.X.; Mai, W.H. Improved sensitivity and positive predictive value of contrast-enhanced intraoperative ultrasound in colorectal cancer liver metastasis: A systematic review and meta-analysis. J. Gastrointest. Oncol. 2022, 13, 221–230. [Google Scholar] [CrossRef]
- Gheorghiu, M.I.; Seicean, A.; Pojoga, C.; Hagiu, C.; Seicean, R.; Sparchez, Z. Contrast-enhanced guided endoscopic ultrasound procedures. World J. Gastroenterol. 2024, 30, 2311–2320. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, E.M.; Wright, L.; Isaksson, M.; Lambert, G.; Marwick, T.H. Current Applications of Robot-Assisted Ultrasound Examination. JACC Cardiovasc. Imaging 2023, 16, 239–247. [Google Scholar] [CrossRef]
- Lupsor-Platon, M.; Serban, T.; Silion, A.I.; Tirpe, G.R.; Tirpe, A.; Florea, M. Performance of ultrasound techniques and the potential of artificial intelligence in the evaluation of hepatocellular carcinoma and non-alcoholic fatty liver disease. Cancers 2021, 13, 790. [Google Scholar] [CrossRef]
- Huang, Q.; Zeng, Q.; Long, Y.; Tan, L.; Zheng, R.; Xu, E.; Li, K. Fusion imaging techniques and contrast-enhanced ultrasound for thermal ablation of hepatocellular carcinoma—A prospective randomized controlled trial. Int. J. Hyperth. 2019, 36, 1207–1215. [Google Scholar] [CrossRef]
- Karako, K.; Song, P.; Chen, Y.; Tang, W. Realizing 5G- and AI-based doctor-to-doctor remote diagnosis: Opportunities, challenges, and prospects. Biosci. Trends 2020, 14, 314–317. [Google Scholar] [CrossRef]
- Cannella, R.; Pilato, G.; Mazzola, M.; Bartolotta, T.V. New microvascular ultrasound techniques: Abdominal applications. Radiol. Med. 2023, 128, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Ignee, A.; Greis, C.; Cui, X.W.; Schreiber-Dietrich, D.G.; Hocke, M. Artifacts and pitfalls in contrast-enhanced ultrasound of the liver. Ultraschall Med. 2014, 35, 108–125. [Google Scholar] [CrossRef]
- De Muzio, F.; Grassi, F.; Dell’Aversana, F.; Fusco, R.; Danti, G.; Flammia, F.; Chiti, G.; Valeri, T.; Agostini, A.; Palumbo, P.; et al. A narrative review on LI-RADS algorithm in liver tumors: Prospects and pitfalls. Diagnostics 2022, 12, 1655. [Google Scholar] [CrossRef]
- Zagzebski, J.A. Pulse-echo ultrasound instrumentation. In Essentials of Ultrasound Physics, 1st ed.; Zagzebski, J.A., Ed.; Mosby-Year Book: St. Louis, MO, USA, 1996; pp. 46–68. [Google Scholar]
- Hedrick, W.R.; Hykes, D.L.; Starchman, D.E. Image formation in real-time imaging. In Ultrasound Physics and Instrumentation, 4th ed.; Hedrick, W.R., Hykes, D.L., Starchman, D.E., Eds.; Elsevier Mosby: St. Louis, MO, USA, 2005; pp. 97–102. [Google Scholar]
- Giangregorio, F.; Garolfi, M.; Mosconi, E.; Ricevuti, L.; Debellis, M.G.; Mendozza, M.; Esposito, C.; Vigotti, E.; Cadei, D.; Abruzzese, D. High frame-rate contrast enhanced ultrasound (HIFR-CEUS) in the characterization of small hepatic lesions in cirrhotic patients. J. Ultrasound 2023, 26, 71–79. [Google Scholar] [CrossRef]
- Quaia, E. Contrast-specific imaging techniques: Technical perspective. In Contrast Media in Ultrasonography, 1st ed.; Baert, A.L., Sartor, K., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany; New York, NY, USA, 2005; pp. 43–70. [Google Scholar]
- Claudon, M.; Cosgrove, D.; Albrecht, T.; Bolondi, L.; Bosio, M.; Calliada, F.; Correas, J.M.; Darge, K.; Dietrich, C.; D’Onofrio, M.; et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS)—Update 2008. Ultraschall Med. 2008, 29, 28–44. [Google Scholar] [CrossRef]
- Claudon, M.; Dietrich, C.F.; Choi, B.I.; Cosgrove, D.O.; Kudo, M.; Nolsøe, C.P.; Piscaglia, F.; Wilson, S.R.; Barr, R.G.; Chammas, M.C.; et al. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver—Update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med. Biol. 2013, 39, 187–210. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, W.R.; Hykes, D.L.; Starchman, D.E. Image artifacts. In Ultrasound Physics and Instrumentation, 4th ed.; Hedrick, W.R., Hykes, D.L., Starchman, D.E., Eds.; Elsevier Mosby: St. Louis, MO, USA, 2005; pp. 183–196. [Google Scholar]
- Hedrick, W.R.; Hykes, D.L.; Starchman, D.E. Dopler Imaging. In Ultrasound Physics and Instrumentation, 4th ed.; Hedrick, W.R., Hykes, D.L., Starchman, D.E., Eds.; Elsevier Mosby: St. Louis, MO, USA, 2005; pp. 239–258. [Google Scholar]
- Uno, A.; Ishida, H.; Konno, K.; Hamashima, Y.; Naganuma, H.; Komatsuda, T.; Sato, M.; Watanabe, S. Post-tumoral distorted vascular images: Diagnostic problem of sonogram. J. Med. Ultrason. 2001, 28, 89–96. [Google Scholar] [CrossRef]
- Naganuma, H.; Hideaki Ishida, H.; Uno, A.; Nagai, H.; Kuroda, H.; Ogawa, M. Diagnostic problems in two-dimensional shear wave elastography of the liver. World J. Radiol. 2020, 12, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Naganuma, H.; Ishida, H. Factors other than fibrosis that increase measured shear wave velocity. World J. Gastroenterol. 2022, 28, 6512–6521. [Google Scholar] [CrossRef]
- Fetzer, D.T.; Vijay, K.; Caserta, M.P.; Patterson-Lachowicz, A.; Dahiya, N.; Rodgers, S.K. Artifacts and technical considerations at contrast-enhanced US. Radiographics 2023, 4, e220093. [Google Scholar] [CrossRef]
- Naganuma, H.; Ishida, H.; Funaoka, M.; Fujimori, S.; Okuyama, A.; Odashima, M.; Takeuchi, S.; Hanaoka, A. Mobile echoes in liver cysts: A form of range-ambiguity artifact. J. Clin. Ultrasound 2010, 38, 475–479. [Google Scholar] [CrossRef]
- Naganuma, H.; Ishida, H.; Nagai, H.; Ogawa, M.; Ohyama, Y. Range-ambiguity artifact in abdominal ultrasound. J. Med. Ultrason. 2019, 46, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Zagzebski, J.A. Physics of Diagnostic ultrasound. In Essentials of Ultrasound Physics, 1st ed.; Zagzebski, J.A., Ed.; Mosby-Year Book: St. Louis, MO, USA, 1996; pp. 1–19. [Google Scholar]
- Hedrick, W.R.; Hykes, D.L.; Starchman, D.E. Basic ultrasound physics. In Ultrasound Physics and Instrumentation, 4th ed.; Hedrick, W.R., Hykes, D.L., Starchman, D.E., Eds.; Elsevier Mosby: St. Louis, MO, USA, 2005; pp. 1–22. [Google Scholar]
- Ishida, H.; Yagiwsawa, H.; Morikawa, P.; Niizawa, M.; Naganuma, S.; Arakawa, H.; Masamune, O. Signe du Drapeau: Nouvel aspect échographique de la cirrhose macronodulaire. J. Echographie Med. Ultrason 1988, 9, 133–138. [Google Scholar]
- Ozturk, A.; Grajo, J.R.; Gee, M.S.; Benjamin, A.; Zubajlo, R.E.; Thomenius, K.E.; Anthony, B.W.; Samir, A.E.; Dhyani, M. Quantitative hepatic fat quantification in non-alcoholic fatty liver disease using ultrasound-based techniques: A review of literature and their diagnostic performance. Ultrasound Med. Biol. 2018, 44, 2461–2475. [Google Scholar] [CrossRef]
- Wiskin, J.; Malik, B.; Ruoff, C.; Pirshafiey, N.; Lenox, M.; Klock, J. Whole-body imaging using low frequency transmission ultrasound. Acad. Radiol. 2023, 30, 2674–2685. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Zhou, C.; Song, P.; Huang, C.; Lok, U.W.; Tang, S.; Watt, K.; Callstrom, M.; Chen, S. Ultrasound attenuation estimation in harmonic imaging for robust fatty liver detection. Ultrasound Med. Biol. 2020, 46, 3080–3087. [Google Scholar] [CrossRef] [PubMed]
- Cain, J.A.; Visagan, S.; Monti, M.M. S.M.A.R.T. F.U.S: Surrogate model of attenuation and refraction in transcranial focused ultrasound. PLoS ONE 2022, 17, e0264101. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, W.R.; Hykes, D.L.; Starchman, D.E. Attenuation in tissue. In Ultrasound Physics and Instrumentation, 4th ed.; Hedrick, W.R., Hykes, D.L., Starchman, D.E., Eds.; Elsevier Mosby: St. Louis, MO, USA, 2005; pp. 23–30. [Google Scholar]
- Hedrick, W.R.; Hykes, D.L.; Starchman, D.E. Single-element transducers: Properties. In Ultrasound Physics and Instrumentation, 4th ed.; Hedrick, W.R., Hykes, D.L., Starchman, D.E., Eds.; Elsevier Mosby: St. Louis, MO, USA, 2005; pp. 47–64. [Google Scholar]
- Dietrich, C.F.; Mertens, J.C.; Braden, B.; Schuessler, G.; Ott, M.; Ignee, A. Contrast-enhanced ultrasound of histologically proven liver hemangiomas. Hepatology 2007, 45, 1139–1145. [Google Scholar] [CrossRef]
- Kudo, M. Defect Reperfusion Imaging with Sonazoid®: A Breakthrough in Hepatocellular Carcinoma. Liver Cancer 2016, 5, 1–7. [Google Scholar] [CrossRef]
- Cui, X.W.; Ignee, A.; Hocke, M.; Seitz, K.; Schrade, G.; Dietrich, C.F. Prolonged heterogeneous liver enhancement on contrast-enhanced ultrasound. Ultraschall Med. 2014, 35, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Mei, Q.; Yu, M.; Chen, Q. Clinical value of contrast-enhanced ultrasound in early diagnosis of small hepatocellular carcinoma (≤2 cm). World J. Clin. Cases 2022, 10, 8525–8534. [Google Scholar] [CrossRef]
- Naganuma, H.; Ishida, H.; Kuroda, H.; Suzuki, Y.; Ogawa, M. Hereditary hemorrhagic telangiectasia: How to efficiently detect hepatic abnormalities using ultrasonography. J. Med. Ultrason. 2020, 47, 421–433. [Google Scholar] [CrossRef]
- Ijuin, H.; Tokitoku, D.; Atsuchi, Y.; Kosaihira, T.; Nagamine, M.; Nozaki, K.; Arima, T.; Takahama, T.; Ishida, H. Flaming portal vein as a new color Doppler sign of portal gas: Report of two cases. J. Med. Ultrason. 2008, 35, 119–123. [Google Scholar]
- Aziz, M.U.; Eisenbrey, J.R.; Deganello, A.; Zahid, M.; Sharbidre, K.; Sidhu, P.; Robbin, M.L. Microvascular flow imaging: A state-of-the-art Review of clinical use and promise. Radiology 2022, 305, 250–264. [Google Scholar] [CrossRef]
- Sato, M.; Ishida, H.; Konno, K.; Komatsuda, T.; Furukawa, K.; Yamada, M.; Yagisawa, H.; Yoshida, Y.; Watanabe, S. Analysis of posterior echoes using reconstructed vertical ultrasound images. J. Med. Ultrason. 2006, 33, 85–90. [Google Scholar] [CrossRef]
- Maturen, K.E.; Wasnik, A.P.; Bailey, J.E.; Higgins, E.G.; Rubin, J.M. Posterior acoustic enhancement in hepatocellular carcinoma. J. Ultrasound Med. 2011, 30, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Naganuma, H.; Ishida, H.; Uno, A.; Nagai, H.; Ogawa, M.; Kamiyama, N. Refraction artifact on abdominal sonogram. J. Med. Ultrason. 2021, 48, 273–283. [Google Scholar] [CrossRef]
- Kobayashi, N.; Iijima, H.; Tada, T.; Shibata, Y.; Nishimura, T.; Kumada, T.; Hashimoto, M.; Higashiura, A.; Yoshida, M.; Nishimura, J.; et al. A new ultrasonographic “fluttering sign” for hepatic hemangioma. Ultrasound Med. Biol. 2021, 47, 941–946. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naganuma, H.; Ishida, H.; Nagai, H.; Uno, A. Contrast-Enhanced Sonography of the Liver: How to Avoid Artifacts. Diagnostics 2024, 14, 1817. https://doi.org/10.3390/diagnostics14161817
Naganuma H, Ishida H, Nagai H, Uno A. Contrast-Enhanced Sonography of the Liver: How to Avoid Artifacts. Diagnostics. 2024; 14(16):1817. https://doi.org/10.3390/diagnostics14161817
Chicago/Turabian StyleNaganuma, Hiroko, Hideaki Ishida, Hiroshi Nagai, and Atushi Uno. 2024. "Contrast-Enhanced Sonography of the Liver: How to Avoid Artifacts" Diagnostics 14, no. 16: 1817. https://doi.org/10.3390/diagnostics14161817
APA StyleNaganuma, H., Ishida, H., Nagai, H., & Uno, A. (2024). Contrast-Enhanced Sonography of the Liver: How to Avoid Artifacts. Diagnostics, 14(16), 1817. https://doi.org/10.3390/diagnostics14161817



