Exploring the Influence of IL-8, IL-10, Patient-Reported Pain, and Physical Activity on Endometriosis Severity
Abstract
:1. Introduction
1.1. Endometriosis Development and Biomarkers
- IL-8 (interleukin 8), known as an α-chemokine with chemotactic activity and acting as a strong angiogenic agent, primarily produced by peripheral macrophages, has been detected in high concentrations in peritoneal fluid of women with endometriosis. In addition to its chemotactic and activating properties for granulocytes, IL-8 has recently been found to stimulate the proliferation of endometrial cells [44,45,46,47].
- Vascular endothelial growth factor (VEGF), one of the main angiogenic factors with the ability to stimulate mitogenesis, migration, and differentiation of endothelial cells, is strongly expressed in endometriotic tissue as well as in peritoneal macrophages. Peritoneal-activated macrophages are a major source of VEGF in endometriosis, and this expression is directly regulated by ovarian steroids. E2 acts on various macrophage signaling pathways, especially those related to the support of inflammatory cell recruitment and the remodeling of inflamed tissues, such as mitogen-activated protein kinase (MAPK), phosphatidylinositol-3-kinase/protein kinase B, and Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB). Consequently, dysregulation of ovarian steroid hormone homeostasis could influence the survival of ectopic endometrial cells and promote lesion vascularization. It is well known that hypoxia induces the expression of VEGF. Effects of hypoxia are mainly mediated by a protein complex called hypoxia-inducible factor-1 (HIF-1), which consists of two subunits, HIF-lα, the inducible unit, and HIF-1β, the constitutive unit. In endometriotic lesions, abnormally high levels of HIF-lα complex have been found, clearly proving that hypoxia is involved in the production of VEGF in endometriosis [46,47].
- IL-10 (interleukin 10), secreted largely by macrophages, is known for inhibiting T cell activation but also for reducing expression of co-stimulatory molecules (CD-80 and CD-86) and indoleamine 2,3-dioxygenase. Interestingly, macrophages secrete IL-15 (interleukin 15), a chemoattractant for uNK (uterine natural killer) cells, and downregulate the cytotoxicity of uNK cells [45,46].
- Nerve growth factors (NGF), especially BDNF (Brain-derived neurotrophic factor), are abnormally synthesized and released by activated macrophages, mast cells, NK (natural killer) cells, and leukocytes within endometriotic formations close to sensitive nerve fibers, and in peritoneal fluid. These sensitize or stimulate endings of sensitive nerve fibers, leading to a vicious cycle characterized by nociceptor sensitization, focal neoneurogenesis, and activation of sensitive nerve fibers, resulting in hyperalgesia [47].
1.2. Objectives
2. Materials and Methods
- Patients who did not freely express their consent for enrollment in the study.
- Patients who did not meet the necessary conditions for study enrollment or who did not attend scheduled follow-up visits as per the protocol.
- A form containing general data, family and personal medical history, symptomatology
- Questionnaire regarding the physical activity practiced by the patient.
- BSGE questionnaire and FSFI questionnaire.
2.1. Analysis of Patient Symptoms and Endometriosis Severity
- Unaffected,
- The patient trying to conceive for less than 18 months without success, or
- The patient trying to conceive for more than 18 months without success.
2.2. Analysis of Investigated Biomarkers
- IL 8—Elabscience Human IL-8 (Interleukin 8) Elisa kit, catalog no: E-EL-H6008
- IL 10—Elabscience Human IL-10 (Interleukin 10) Elisa kit, catalog no: E-EL-H6154
- VEGF—Elabscience Human VEGF-A (Vascular Endothelial Cell Growth Factor A) ELISA kit, catalog no: E-EL-H0111
- BDNF—Elabscience Human BDNF (Brain-Derived Neurotrophic Factor) ELISA kit, catalog No: E-EL-H0010.
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
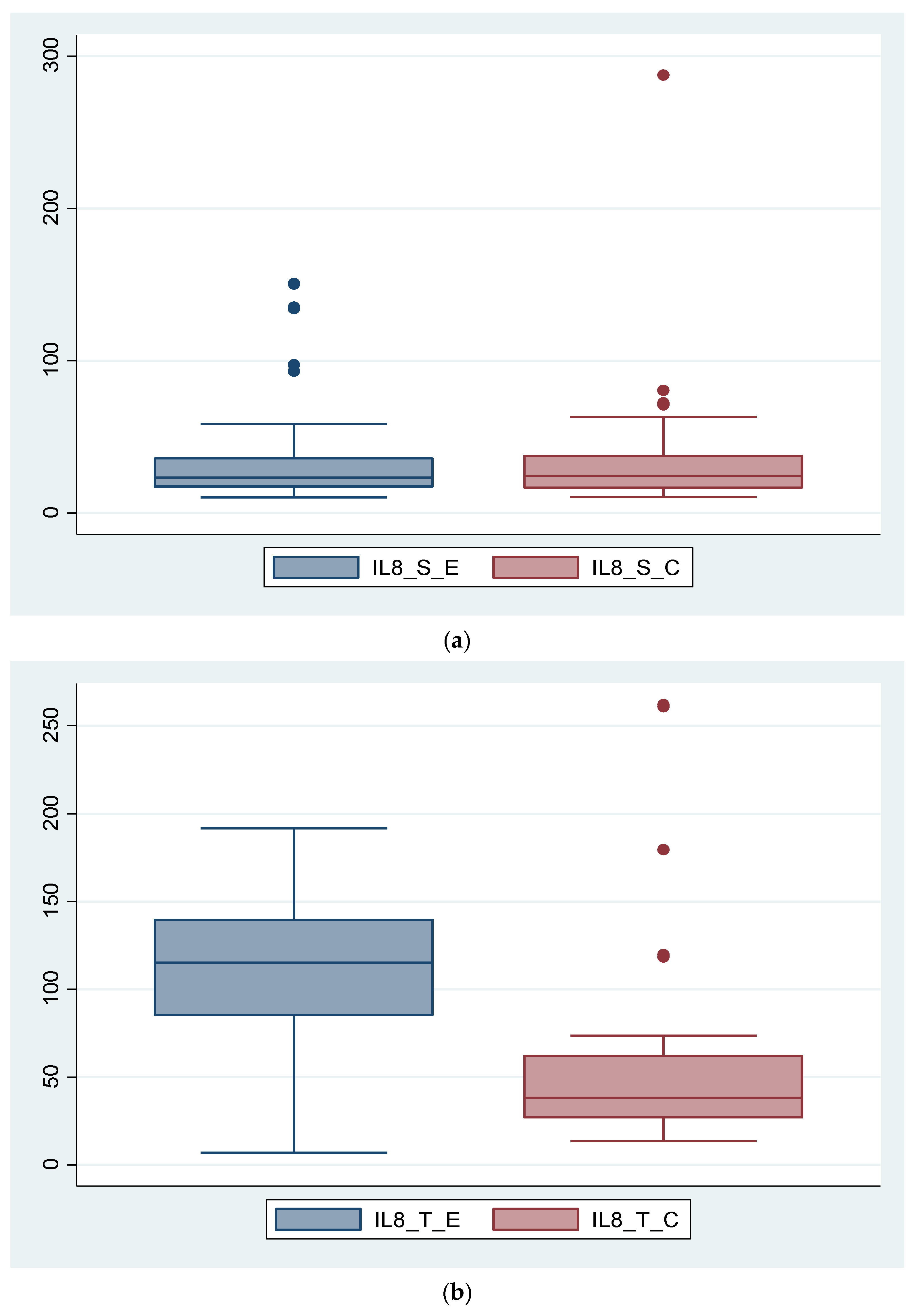
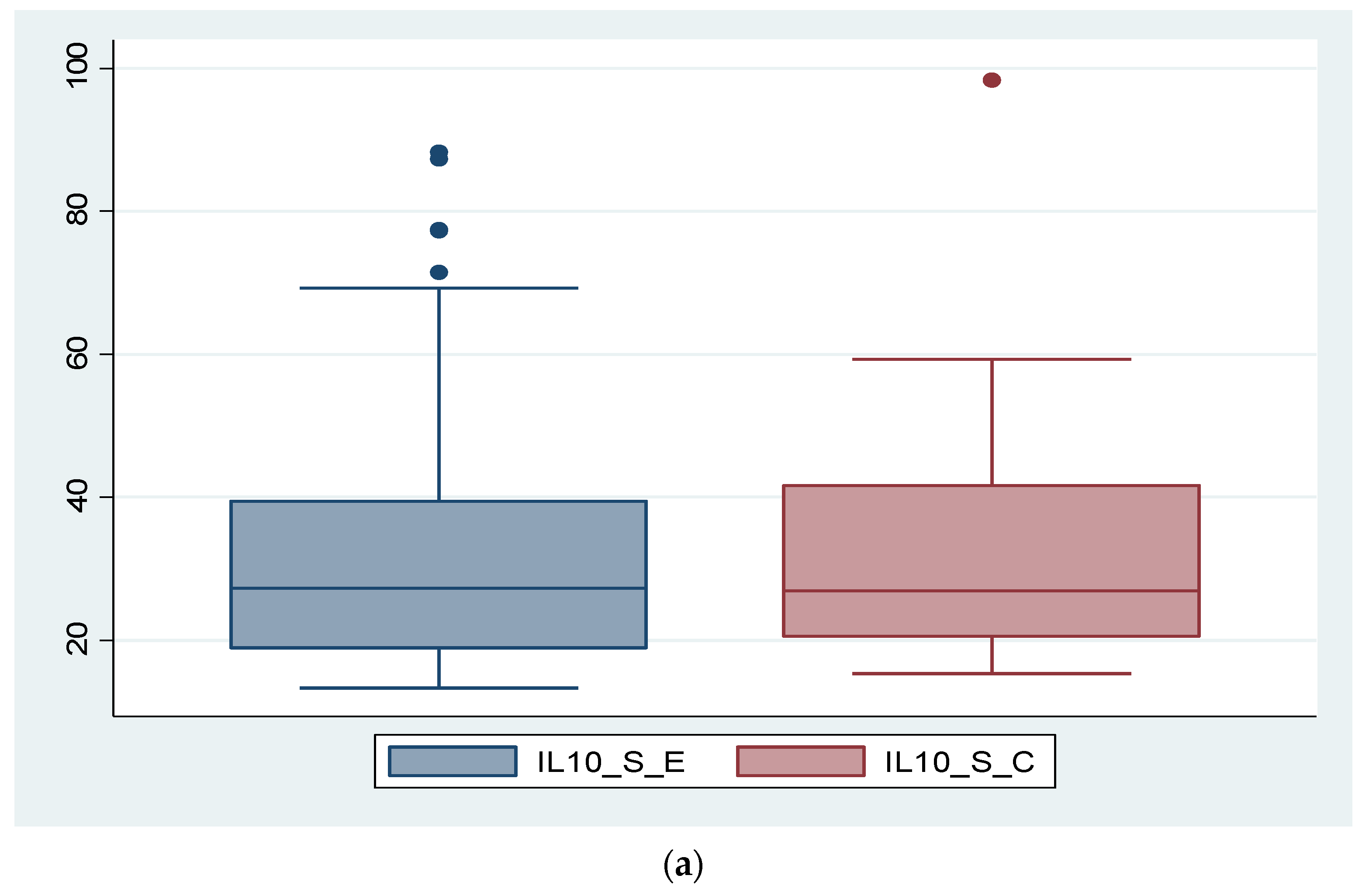
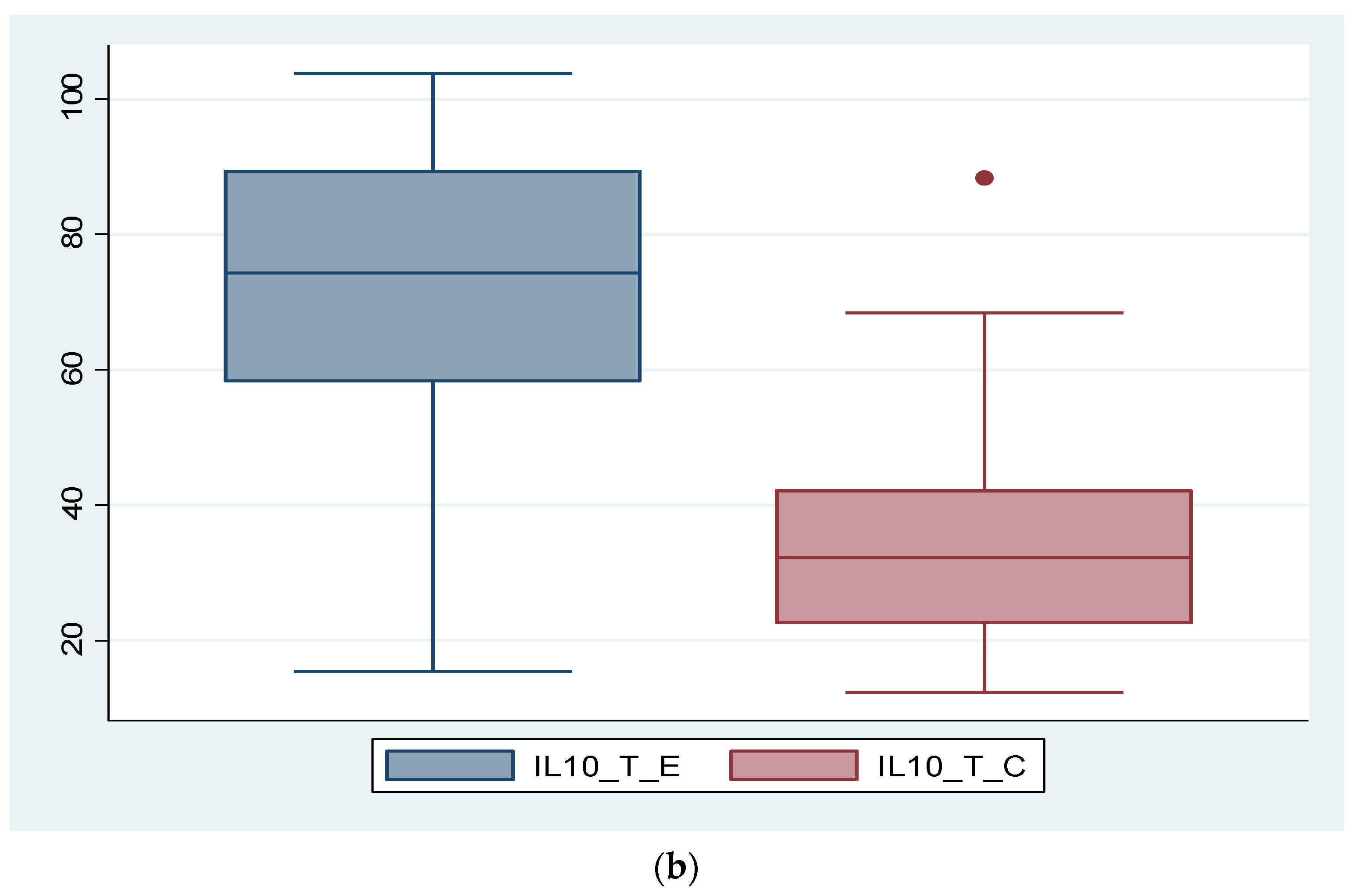
3.2. Expression of Biomarkers at Serum and Tissue Levels
- Uterine fibroid: exhibits elevated IL-8 values similar to endometriosis, with a similar trend for IL-10 but less elevated tissue values compared to endometriosis, higher BDNF values in both serum and tissue and lower serum VEGF-A values but higher tissue values.
- Non-endometriotic ovarian cyst: tissue expression of IL-8 and IL-10 had significantly lower values in cases of non-endometriotic ovarian cysts (43.15 vs. 114.36 pg/mL and 29.53 vs. 71.88 pg/mL, respectively). Regarding BDNF and VEGF-A, only the latter showed higher tissue values.
- Mature ovarian teratoma: shows reduced tissue values of IL-8 and IL-10 but higher values for BDNF and VEGF-A.
3.3. Results of BSGE Questionnaire
3.4. Correlation and Regression of Severity Determinants in Endometriosis
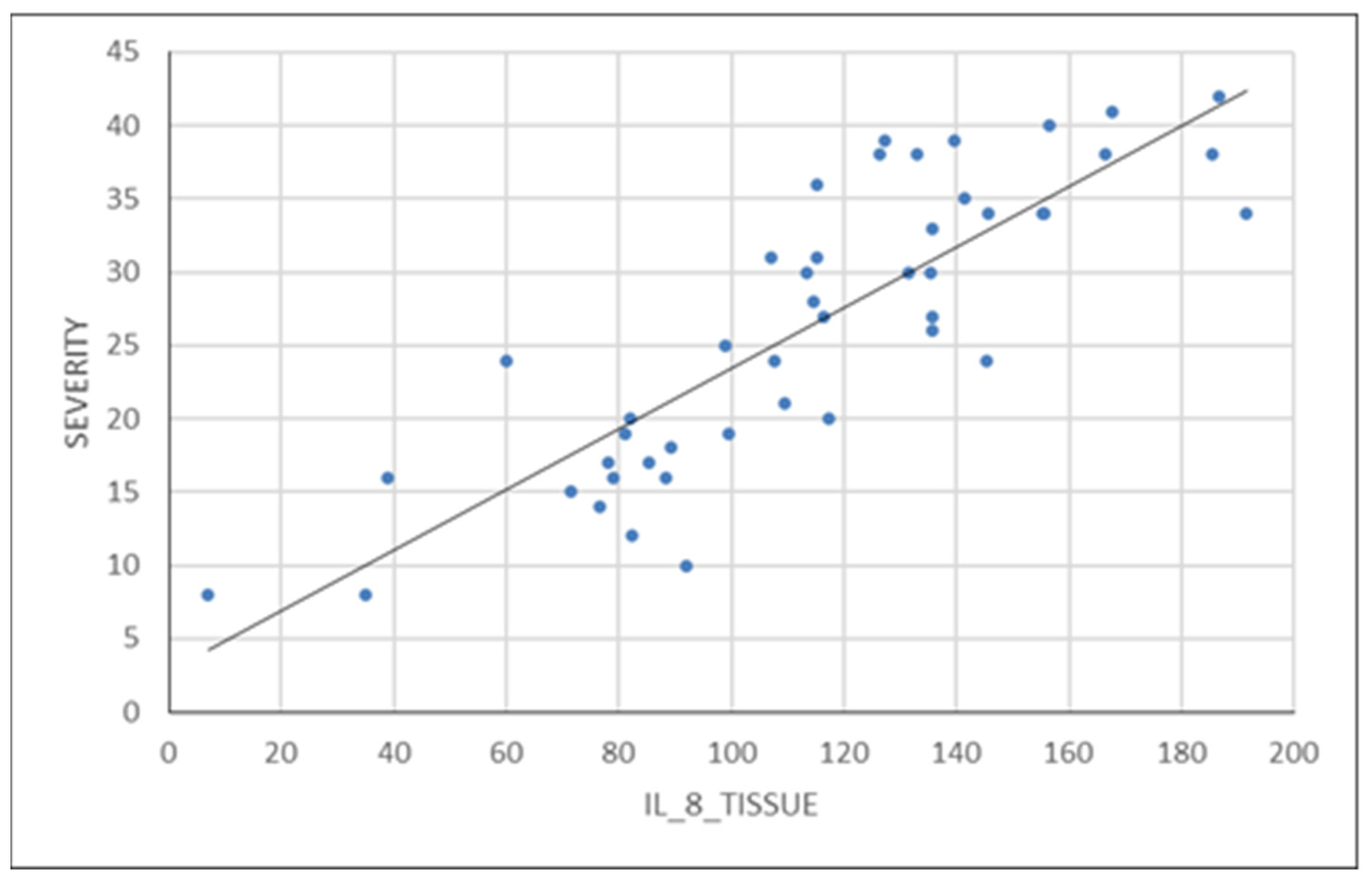
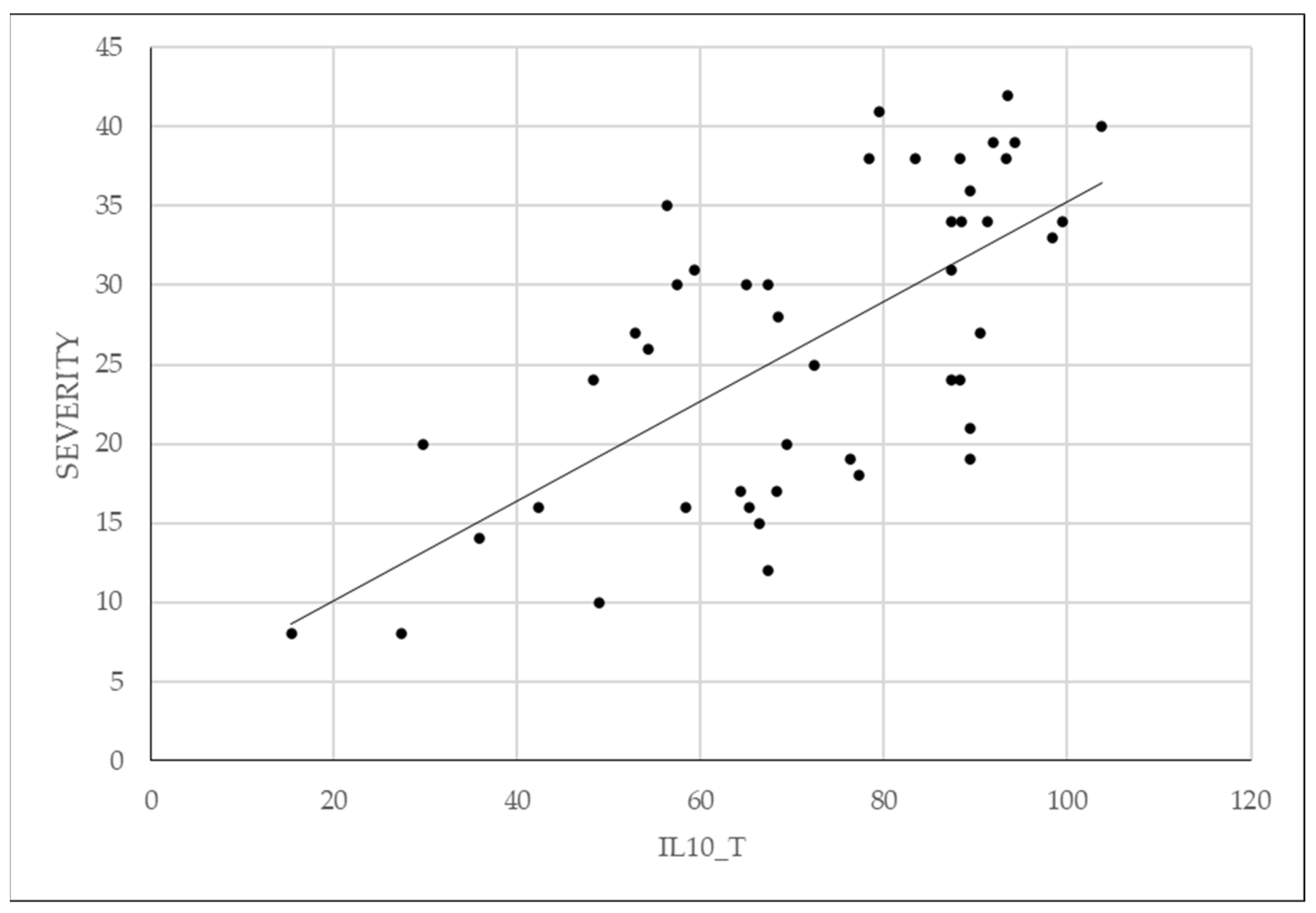
3.5. Analysis of Correlations between IL-8, IL-10 and the Severity of Endometriosis
3.6. Analysis of Linear Regression of Patient-Reported Pain and Physical Activity on Severity of Endometriosis
4. Discussion
- The relatively small number of patients in the two study groups
- The sample of patients exclusively of the Caucasian race
- The heterogeneity of the control group
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Della Corte, L.; Di Filippo, C.; Gabrielli, O.; Reppuccia, S.; La Rosa, V.L.; Ragusa, R.; Fichera, M.; Commodari, E.; Bifulco, G.; Giampaolino, P. The Burden of Endometriosis on Women’s Lifespan: A Narrative Overview on Quality of Life and Psychosocial Wellbeing. Int. J. Environ. Res. Public Health 2020, 17, 4683. [Google Scholar] [CrossRef]
- Škegro, B.; Bjedov, S.; Mikuš, M.; Mustač, F.; Lešin, J.; Matijević, V.; Ćorić, M.; Elveđi Gašparović, V.; Medić, F.; Sokol Karadjole, V. Endometriosis, Pain and Mental Health. Psychiatr. Danub. 2021, 33 (Suppl. S4), 632–636. [Google Scholar]
- Koninckx, P.R.; Fernandes, R.; Ussia, A.; Schindler, L.; Wattiez, A.; Al-Suwaidi, S.; Amro, B.; Al-Maamari, B.; Hakim, Z.; Tahlak, M. Pathogenesis Based Diagnosis and Treatment of Endometriosis. Front. Endocrinol. 2021, 12, 745548. [Google Scholar] [CrossRef]
- Rahmioglu, N.; Mortlock, S.; Ghiasi, M.; Møller, P.L.; Stefansdottir, L.; Galarneau, G.; Turman, C.; Danning, R.; Law, M.H.; Sapkota, Y.; et al. The genetic basis of endometriosis and comorbidity with other pain and inflammatory conditions. Nat Genet. 2023, 55, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E. Endometriosis caused by retrograde menstruation: Now demonstrated by DNA evidence. Fertil. Steril. 2022, 118, 535–536. [Google Scholar] [CrossRef]
- D’Hooghe, T.M.; Debrock, S. Endometriosis, retrograde menstruation and peritoneal inflammation in women and in baboons. Hum. Reprod. Update 2002, 8, 84–88. [Google Scholar] [CrossRef]
- Guo, S.W.; Habiba, M.; Benagiano, G. From Retrograde Menstruation to Endometrial Determinism and a Brave New World of “Root Treatment” of Endometriosis: Destiny or a Fanciful Utopia? Biomolecules 2023, 13, 336. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wu, J.; Wang, W.; Xie, H.; Yao, S. Pro-endometriotic niche in endometriosis. Reprod. Biomed. 2019, 38, 549–559. [Google Scholar] [CrossRef]
- Tong, S.-S.; Yin, X.-Y.; Hu, S.-S.; Cui, Y.; Li, H.-T. Case report of pulmonary endometriosis and review of the literature. J. Int. Med. Res. 2019, 47, 1766–1770. [Google Scholar] [CrossRef]
- Andres, M.P.; Arcoverde, F.V.; Souza, C.C.; Fernandes, L.F.C.; Abrão, M.S.; Kho, R.M. Extrapelvic Endometriosis: A Systematic Review. J. Minim. Invasive Gynecol. 2020, 27, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Fukui, A.; Mai, C.; Saeki, S.; Yamamoto, M.; Takeyama, R.; Kato, T.; Ukita, Y.; Wakimoto, Y.; Yamaya, A.; Shibahara, H. Pelvic endometriosis and natural killer cell immunity. Am. J. Reprod. Immunol. 2021, 85, e13342. [Google Scholar] [CrossRef]
- Szukiewicz, D. Epigenetic regulation and T-cell responses in endometriosis—Something other than autoimmunity. Front. Immunol. 2022, 13, 943839. [Google Scholar] [CrossRef]
- Zutautas, K.B.; Sisnett, D.J.; Miller, J.E.; Lingegowda, H.; Childs, T.; Bougie, O.; Lessey, B.A.; Tayade, C. The dysregulation of leukemia inhibitory factor and its implications for endometriosis pathophysiology. Front. Immunol. 2023, 14, 1089098. [Google Scholar] [CrossRef]
- Ho, H.N.; Wu, M.; Yang, Y. Peritoneal cellular immunity and endometriosis. Am. J. Reprod. Immunol. 1997, 38, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, L.; Li, Y.; Huang, C.; Lian, R.; Wu, T.; Ma, J.; Zhang, Y.; Cheng, Y.; Diao, L.; et al. A History of Endometriosis Is Associated with Decreased Peripheral NK Cytotoxicity and Increased Infiltration of Uterine CD68+ Macrophages. Front. Immunol. 2021, 12, 711231. [Google Scholar] [CrossRef]
- Vinatier, D.; Orazi, G.; Cosson, M.; Dufour, P. Theories of endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 96, 21–34. [Google Scholar] [CrossRef]
- Lamceva, J.; Uljanovs, R.; Strumfa, I. The Main Theories on the Pathogenesis of Endometriosis. Int. J. Mol. Sci. 2023, 24, 4254. [Google Scholar] [CrossRef]
- Hill, C.J.; Fakhreldin, M.; Maclean, A.; Dobson, L.; Nancarrow, L.; Bradfield, A.; Choi, F.; Daley, D.; Tempest, N.; Hapangama, D.K. Endometriosis and the Fallopian Tubes: Theories of Origin and Clinical Implications. J. Clin. Med. 2020, 9, 1905. [Google Scholar] [CrossRef]
- Czyzyk, A.; Podfigurna, A.; Szeliga, A.; Meczekalski, B. Update on endometriosis pathogenesis. Minerva Ginecol. 2017, 69, 447–461. [Google Scholar] [CrossRef]
- Gruber, T.M.; Mechsner, S. Pathogenesis of Endometriosis: The Origin of Pain and Subfertility. Cells 2021, 10, 1381. [Google Scholar] [CrossRef]
- Aredo, J.V.; Heyrana, K.J.; Karp, B.I.; Shah, J.P.; Stratton, P. Relating Chronic Pelvic Pain and Endometriosis to Signs of Sensitization and Myofascial Pain and Dysfunction. Semin. Reprod. Med. 2017, 35, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; Missmer, S.A.; Tu, F.F. Endometriosis and Pelvic Pain for the Gastroenterologist. Gastroenterol. Clin. N. Am. 2022, 51, 195–211. [Google Scholar] [CrossRef]
- Nezhat, C.; Vang, N.; Tanaka, P.P.; Nezhat, C. Optimal Management of Endometriosis and Pain. Obstet. Gynecol. 2019, 134, 834–839. [Google Scholar] [CrossRef]
- Bastu, E.; Celik, H.G.; Kocyigit, Y.; Yozgatli, D.; Yasa, C.; Ozaltin, S.; Tas, S.; Soylu, M.; Durbas, A.; Gorgen, H.; et al. Improvement in quality of life and pain scores after laparoscopic management of deep endometriosis: A retrospective cohort study. Arch. Gynecol. Obstet. 2020, 302, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Tanbo, T.; Fedorcsak, P. Endometriosis-associated infertility: Aspects of pathophysiological mechanisms and treatment options. Acta Obstet. Et Gynecol. Scand. 2017, 96, 659–667. [Google Scholar] [CrossRef]
- Bulletti, C.; Coccia, M.E.; Battistoni, S.; Borini, A. Endometriosis and infertility. J. Assist. Reprod. Genet. 2010, 27, 441–447. [Google Scholar] [CrossRef]
- de Ziegler, D.; Borghese, B.; Chapron, C. Endometriosis and infertility: Pathophysiology and management. Lancet 2010, 376, 730–738. [Google Scholar] [CrossRef]
- Haas, D.; Shebl, O.; Shamiyeh, A.; Oppelt, P. The rASRM score and the Enzian classification for endometriosis: Their strengths and weaknesses. Acta Obstet. Et Gynecol. Scand. 2013, 92, 3–7. [Google Scholar] [CrossRef]
- Nicolaus, K.; Zschauer, S.; Bräuer, D.; Jimenez-Cruz, J.; Lehmann, T.; Rengsberger, M.; Diebolder, H.; Runnebaum, I.B. Extensive endometriosis surgery: rASRM and Enzian score independently relate to post-operative complication grade. Arch. Gynecol. Obstet. 2020, 301, 699–706. [Google Scholar] [CrossRef]
- Fruscalzo, A.; Dayer, A.; Londero, A.P.; Guani, B.; Khomsi, F.; Ayoubi, J.-M.; Feki, A. Endometriosis and Infertility: Prognostic Value of #Enzian Classification Compared to rASRM and EFI Score. J. Pers. Med. 2022, 12, 1623. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Missmer, S.; Abrao, M.S.; Einarsson, J.I.; Horne, A.W.; Johnson, N.P.; Lee, T.T.; Petrozza, J.; Tomassetti, C.; Vermeulen, N.; et al. Endometriosis Classification Systems: An International Survey to Map Current Knowledge and Uptake. J. Minim. Invasive Gynecol. 2022, 29, 716–725.e1. [Google Scholar] [CrossRef]
- Horne, A.W.; Missmer, S.A. Pathophysiology, diagnosis, and management of endometriosis. BMJ 2022, 379, e070750. [Google Scholar] [CrossRef] [PubMed]
- Pascoal, E.; Wessels, J.M.; Aas-Eng, M.K.; Abrao, M.S.; Condous, G.; Jurkovic, D.; Espada, M.; Exacoustos, C.; Ferrero, S.; Guerriero, S.; et al. Strengths and limitations of diagnostic tools for endometriosis and relevance in diagnostic test accuracy research. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2022, 60, 309–327. [Google Scholar] [CrossRef]
- Van den Bosch, T.; Van Schoubroeck, D. Ultrasound diagnosis of endometriosis and adenomyosis: State of the art. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 16–24. [Google Scholar] [CrossRef]
- Ahn, S.H.; Singh, V.; Tayade, C. Biomarkers in endometriosis: Challenges and opportunities. Fertil. Steril. 2017, 107, 523–532. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, X.; Wu, Y.; Zhang, B.; Wei, J.; Li, J.; Huang, Y.; Chen, L.; He, X. Bioinformatics identification and validation of biomarkers and infiltrating immune cells in endometriosis. Front. Immunol. 2022, 13, 944683. [Google Scholar] [CrossRef]
- Králíčková, M.; Vetvicka, V.; Fiala, L.; Laganà, A.S.; Garzon, S. The Search for Biomarkers in Endometriosis: A Long and Windy Road. Reprod. Sci. 2022, 29, 1667–1673. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Wattiez, A.; Donnez, J. Deep endometriosis: Definition, diagnosis, and treatment. Fertil. Steril. 2012, 98, 564–571. [Google Scholar] [CrossRef]
- Kho, R.M.; Andres, M.P.; Borrelli, G.M.; Neto, J.S.; Zanluchi, A.; Abrão, M.S. Surgical treatment of different types of endometriosis: Comparison of major society guidelines and preferred clinical algorithms. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 102–110. [Google Scholar] [CrossRef]
- França, P.R.d.C.; Lontra, A.C.P.; Fernandes, P.D. Endometriosis: A Disease with Few Direct Treatment Options. Molecules 2022, 27, 4034. [Google Scholar] [CrossRef]
- Mira, T.A.A.; Buen, M.M.; Borges, M.G.; Yela, D.A.; Benetti-Pinto, C.L. Systematic review and meta-analysis of complementary treatments for women with symptomatic endometriosis. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2018, 143, 2–9. [Google Scholar] [CrossRef]
- Brichant, G.; Laraki, I.; Henry, L.; Munaut, C.; Nisolle, M. New Therapeutics in Endometriosis: A Review of Hormonal, Non-Hormonal, and Non-Coding RNA Treatments. Int. J. Mol. Sci. 2021, 22, 10498. [Google Scholar] [CrossRef]
- Asghari, S.; Valizadeh, A.; Aghebati-Maleki, L.; Nouri, M.; Yousefi, M. Endometriosis: Perspective, lights, and shadows of etiology. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 106, 163–174. [Google Scholar] [CrossRef]
- Haney, M.; Muscato, J.J.; Weinberg, J.B. Peritoneal fluid cell populations in infertility patients. Fertil. Steril. 1981, 35, 696–698. [Google Scholar] [CrossRef]
- van Furth, R.; Raeburn, J.A.; van Zwet, T.L. Characteristics of human mononuclear phagocytes. Blood 1979, 54, 485–500. [Google Scholar]
- Dmowski, S.; Steele, R.W.; Baker, G.F. Deficient cellular immunity in endometriosis. Am. J. Obstet. Gynecol. 1981, 141, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Genazzani, A.R.; Nisolle, M.; Petraglia, F.; Taylor, R.N. Endometriosis Pathogenesis, Clinical Impact and Management; Volume 9: Frontiers in Gynecological Endocrinology; Springer: Berlin/Heidelberg, Germany, 2021; ISBN 978-3-030-57865-7. [Google Scholar]
- Tseng, J.F.; Ryan, I.P.; Milam, T.D.; Murai, J.T.; Schriock, E.D.; Landers, D.V.; Taylor, R.N. Interleukin-6 secretion in vitro is up-regulated in ectopic and eutopic endometrial stromal cells from women with endometriosis. J. Clin. Endocrinol. Metab. 1996, 81, 1118–1122. [Google Scholar] [CrossRef]
- Khorram, O.; Taylor, R.N.; Ryan, I.P.; Schall, T.J.; Landers, D.V. Peritoneal fluid concentrations of the cytokine RANTES correlate with the severity of endometriosis. Am. J. Obstet. Gynecol. 1993, 169, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef]
- Orisaka, M.; Mizutani, T.; Miyazaki, Y.; Shirafuji, A.; Tamamura, C.; Fujita, M.; Tsuyoshi, H.; Yoshida, Y. Chronic low-grade inflammation and ovarian dysfunction in women with polycystic ovarian syndrome, endometriosis, and aging. Front. Endocrinol. 2023, 14, 1324429. [Google Scholar] [CrossRef]
- Wei, Y.; Liang, Y.; Lin, H.; Dai, Y.; Yao, S. Autonomic nervous system and inflammation interaction in endometriosis-associated pain. J. Neuroinflammation 2020, 17, 80. [Google Scholar] [CrossRef] [PubMed]
- Velho, R.V.; Taube, E.; Sehouli, J.; Mechsner, S. Neurogenic Inflammation in the Context of Endometriosis-What Do We Know? Int. J. Mol. Sci. 2021, 22, 13102. [Google Scholar] [CrossRef] [PubMed]
- Sikora, J.; Smycz-Kubańska, M.; Mielczarek-Palacz, A.; Kondera-Anasz, Z. Abnormal peritoneal regulation of chemokine activation-The role of IL-8 in pathogenesis of endometriosis. Am. J. Reprod. Immunol. 2017, 77, 120482. [Google Scholar] [CrossRef]
- Malhotra, N.; Karmakar, D.; Tripathi, V.; Luthra, K.; Kumar, S. Correlation of angiogenic cytokines-leptin and IL-8 in stage, type and presentation of endometriosis. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2012, 28, 224–227. [Google Scholar] [CrossRef]
- Măluțan, A.M.; Drugan, T.; Ciortea, R.; Bucuri, C.; Rada, M.P.; Mihu, D. Endometriosis-associated changes in serum levels of interferons and chemokines. Turk. J. Med. Sci. 2017, 47, 115–122. [Google Scholar] [CrossRef]
- Rahmawati, N.Y.; Ahsan, F.; Santoso, B.; Mufid, A.F.; Sa’adi, A.; Dwiningsih, S.R.; Tunjungseto, A.; Widyanugraha, M.Y.A. IL-8 and IL-12p70 are associated with pelvic pain among infertile women with endometriosis. Pain Med. 2023, 24, 1262–1269. [Google Scholar] [CrossRef]
- Nishimoto-Kakiuchi, A.; Sato, I.; Nakano, K.; Ohmori, H.; Kayukawa, Y.; Tanimura, H.; Yamamoto, S.; Sakamoto, Y.; Nakamura, G.; Maeda, A.; et al. A long-acting anti-IL-8 antibody improves inflammation and fibrosis in endometriosis. Sci. Transl. Med. 2023, 15, eabq5858. [Google Scholar] [CrossRef]
- Suen, J.-L.; Chang, Y.; Shiu, Y.; Hsu, C.; Sharma, P.; Chiu, C.; Chen, Y.; Hour, T.; Tsai, E. IL-10 from plasmacytoid dendritic cells promotes angiogenesis in the early stage of endometriosis. J. Pathol. 2019, 249, 485–497. [Google Scholar] [CrossRef]
- Zhong, S.; Liang, Y.; Wu, Z.; Wei, L. Association between polymorphisms of cytokine genes and endometriosis: A comprehensive systematic review and meta-analysis. J. Reprod. Immunol. 2023, 158, 103969. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.-J.; Yang, H.-L.; Shao, J.; Mei, J.; Chang, K.-K.; Zhu, R.; Li, M.-Q. Anti-inflammatory cytokines in endometriosis. Cell. Mol. Life Sci. CMLS 2019, 76, 2111–2132. [Google Scholar] [CrossRef]
- Wang, X.-M.; Ma, Z.Y.; Song, N. Inflammatory cytokines IL-6, IL-10, IL-13, TNF-α and peritoneal fluid flora were associated with infertility in patients with endometriosis. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2513–2518. [Google Scholar] [CrossRef]
- Huang, X.; Wu, L.; Pei, T.; Liu, D.; Liu, C.; Luo, B.; Xiao, L.; Li, Y.; Wang, R.; Ouyang, Y.; et al. Single-cell transcriptome analysis reveals endometrial immune microenvironment in minimal/mild endometriosis. Clin. Exp. Immunol. 2023, 212, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Malutan, A.M.; Drugan, C.; Walch, K.; Drugan, T.; Ciortea, R.; Mihu, D. The association between interleukin-10 (IL-10) -592C/A, -819T/C, -1082G/A promoter polymorphisms and endometriosis. Arch. Gynecol. Obstet. 2017, 295, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Pouly, J.-L.; Canis, M. IL-10 is not anti-fibrotic but pro-fibrotic in endometriosis: IL-10 treatment of endometriotic stromal cells in vitro promotes myofibroblast proliferation and collagen type I protein expression. Hum. Reprod. 2023, 38, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Tennfjord, M.K.; Gabrielsen, R.; Tellum, T. Effect of physical activity and exercise on endometriosis-associated symptoms: A systematic review. BMC Women’s Health 2021, 21, 355. [Google Scholar] [CrossRef]
- Abril-Coello, R.; Correyero-León, M.; Ceballos-Laita, L.; Jiménez-Barrio, S. Benefits of physical therapy in improving quality of life and pain associated with endometriosis: A systematic review and meta-analysis. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2023, 162, 233–243. [Google Scholar] [CrossRef]
- Ricci, E.; Viganò, P.; Cipriani, S.; Chiaffarino, F.; Bianchi, S.; Rebonato, G.; Parazzini, F. Physical activity and endometriosis risk in women with infertility or pain: Systematic review and meta-analysis. Medicine 2016, 95, e4957. [Google Scholar] [CrossRef]
- Martire, F.G.; Piccione, E.; Exacoustos, C.; Zupi, E. Endometriosis and Adolescence: The Impact of Dysmenorrhea. J. Clin. Med. 2023, 12, 5624. [Google Scholar] [CrossRef] [PubMed]
- Martire, F.G.; Russo, C.; Selntigia, A.; Nocita, E.; Soreca, G.; Lazzeri, L.; Zupi, E.; Exacoustos, C. Early noninvasive diagnosis of endometriosis: Dysmenorrhea and specific ultrasound findings are important indicators in young women. Fertil. Steril. 2023, 119, 455–464. [Google Scholar] [CrossRef]
- Salmeri, N.; Farina, A.; Candiani, M.; Dolci, C.; Bonavina, G.; Poziello, C.; Viganò, P.; Cavoretto, P.I. Endometriosis and Impaired Placentation: A Prospective Cohort Study Comparing Uterine Arteries Doppler Pulsatility Index in Pregnancies of Patients with and without Moderate-Severe Disease. Diagnostics 2022, 12, 1024. [Google Scholar] [CrossRef]


| IL-8 S 2 | IL 8-T 3 | IL 10-S 2 | IL-10 T 3 | BDNF S 2 | BDNF T 3 | VEGF-A S 2 | VEGF-A T 3 | |
|---|---|---|---|---|---|---|---|---|
| average | 37.85 | 114.36 | 34.77 | 71.88 | 784.60 | 53.80 | 243.12 | 43.94 |
| Std. dev. 1 | 36.93 | 39.5 | 20.75 | 20.69 | 505.77 | 25.32 | 185.53 | 19.86 |
| IL-8 S 2 | IL-8 T 3 | IL-10 S 2 | IL-10 T 3 | BDNF S 2 | BDNF T 3 | VEGF-A S 2 | VEGF-A T 3 | |
|---|---|---|---|---|---|---|---|---|
| average | 34.73 | 56.95 | 33.41 | 34.34 | 958.60 | 65.96 | 314.04 | 148.33 |
| Std. dev. 1 | 42.02 | 54.28 | 18.84 | 15.65 | 639.74 | 48.85 | 378.49 | 120.44 |
| IL 8 S 1 | IL 8 T 2 | IL 10 S 1 | IL 10 T 2 | BDNF S 1 | BDNF T 2 | VEGF-A S 1 | VEGF-A T 2 | |
|---|---|---|---|---|---|---|---|---|
| uterine leiomyoma | 36.42 | 100.99 | 37.97 | 48.89 | 994.31 | 99.05 | 167.81 | 240.58 |
| non-endometriotic ovarian cyst | 33.76 | 43.15 | 34.41 | 29.53 | 901.01 | 76.47 | 318.86 | 114.04 |
| mature ovarian teratoma | 25.29 | 32.91 | 37.57 | 23.78 | 1108.15 | 42.01 | 402.82 | 87.06 |
| uterine septum | 17.79 | 26.61 | 20.32 | 26.74 | 1215.63 | 70.78 | 215.19 | 97.67 |
| Coef. | Std. Err. | p-Value | Coef. | Std. Err. | p-Value | |
|---|---|---|---|---|---|---|
| IL-8_S | −0.027 | 0.036 | 0.464 | |||
| IL-8_T | *** 0.065 | 0.021 | 0.003 | *** 0.082 | 0.025 | 0.002 |
| IL-10_S | 0.035 | 0.065 | 0.591 | |||
| IL-10_T | *** 0.094 | 0.032 | 0.005 | ** 0.087 | 0.038 | 0.029 |
| BDNF_S | 0.001 | 0.001 | 0.388 | |||
| BDNF_T | −0.017 | 0.023 | 0.469 | |||
| VEGF_S | −0.002 | 0.003 | 0.452 | |||
| VEGF_T | 0.042 | 0.030 | 0.175 | |||
| FSFI | ** −0.753 | 0.290 | 0.013 | ** 0.655 | 0.317 | 0.047 |
| AVG_PAIN | *** 2.310 | 0.587 | <0.001 | *** 2.239 | 0.639 | 0.001 |
| PROG_COC | *** −3.370 | 1.049 | 0.003 | ** −2.760 | 1.198 | 0.028 |
| AGE | 0.122 | 0.077 | 0.120 | 0.168 | 0.103 | 0.112 |
| BMI | 0.061 | 0.113 | 0.592 | −0.074 | 0.140 | 0.602 |
| SPORT_PHACT | ** −1.243 | 0.589 | 0.042 | ** −1.682 | 0.718 | 0.026 |
| n = 46; Adj. R2 = 0.907 n = 46; Adj. R2 = 0.907 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nati, I.D.; Malutan, A.; Ciortea, R.; Oancea, M.; Bucuri, C.; Roman, M.; Ormindean, C.; Milon, A.G.; Mihu, D. Exploring the Influence of IL-8, IL-10, Patient-Reported Pain, and Physical Activity on Endometriosis Severity. Diagnostics 2024, 14, 1822. https://doi.org/10.3390/diagnostics14161822
Nati ID, Malutan A, Ciortea R, Oancea M, Bucuri C, Roman M, Ormindean C, Milon AG, Mihu D. Exploring the Influence of IL-8, IL-10, Patient-Reported Pain, and Physical Activity on Endometriosis Severity. Diagnostics. 2024; 14(16):1822. https://doi.org/10.3390/diagnostics14161822
Chicago/Turabian StyleNati, Ionel Daniel, Andrei Malutan, Razvan Ciortea, Mihaela Oancea, Carmen Bucuri, Maria Roman, Cristina Ormindean, Alexandra Gabriela Milon, and Dan Mihu. 2024. "Exploring the Influence of IL-8, IL-10, Patient-Reported Pain, and Physical Activity on Endometriosis Severity" Diagnostics 14, no. 16: 1822. https://doi.org/10.3390/diagnostics14161822





