Clinical Significance of Combined Density and Deep-Learning-Based Texture Analysis for Stratifying the Risk of Short-Term and Long-Term Breast Cancer in Screening
Abstract
:1. Introduction
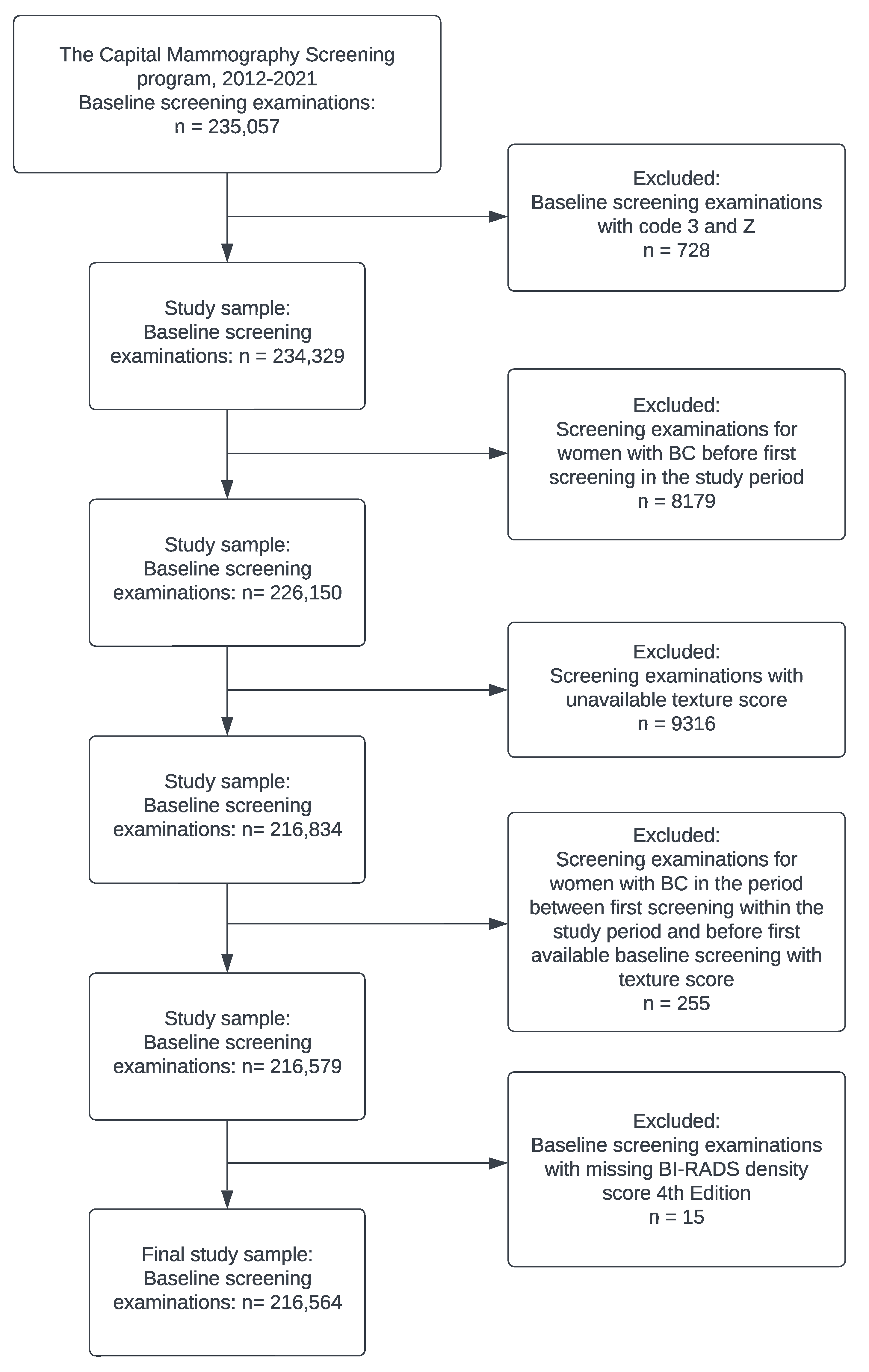
2. Materials and Methods
2.1. Study Population
2.2. Image Technique and Interpretation
2.3. Breast Density
2.4. Deep-Learning-Based Texture Model
2.5. Statistical Analysis
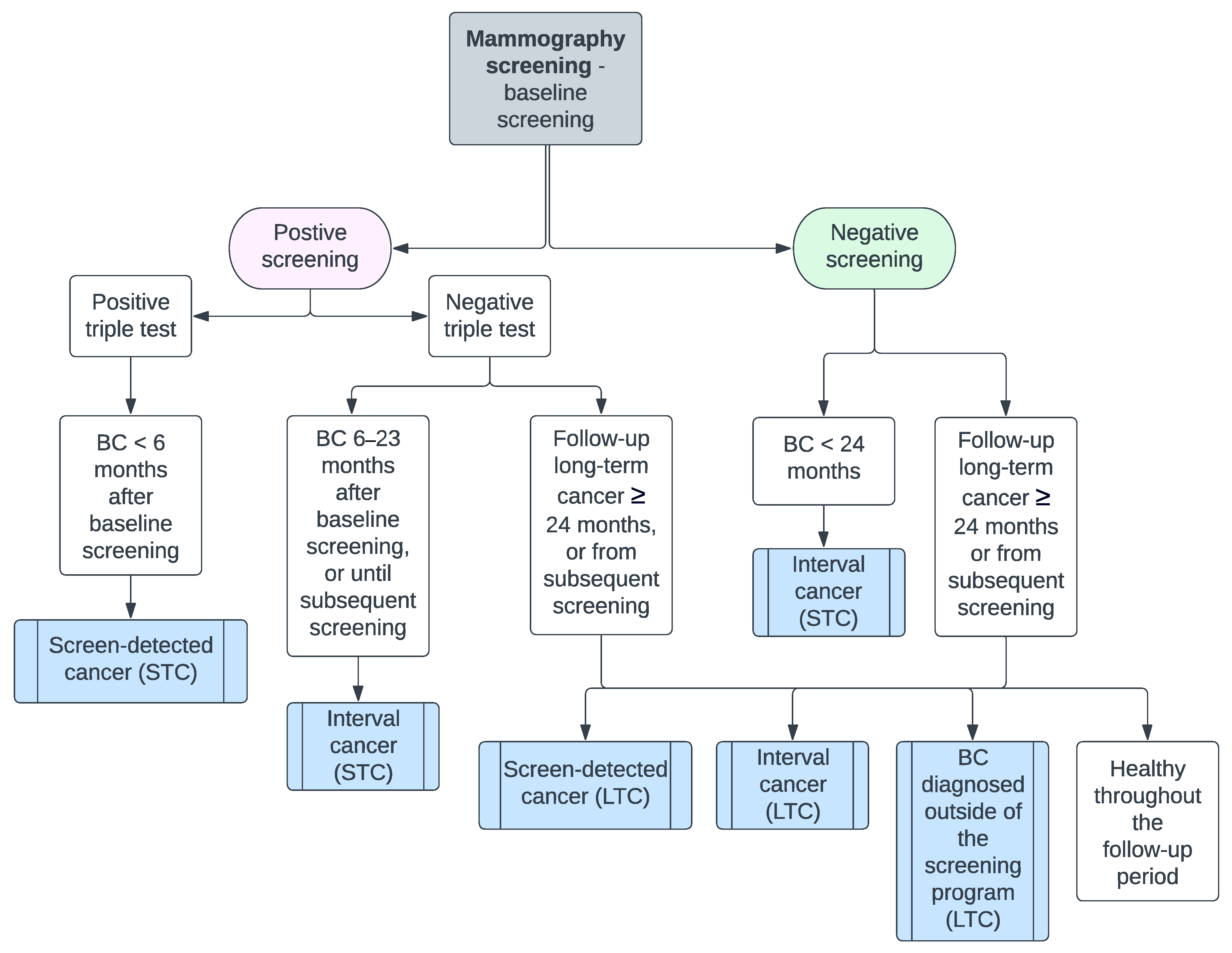
3. Results
3.1. Short-Term Cancer
3.2. Long-Term Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Number of Women with Baseline Screening n (%) | Person-Years SDC | SDC Rate Per 1000 Person-Years | Person-Years IC | IC Rate Per 1000 Person-Years | Person-Years LTC | LTC Rate Per 1000 Person-Years | |
|---|---|---|---|---|---|---|---|
| Total | 216,564 (100%) | 106,105.3 | 14 | 424,558.94 | 1.3 | 1,158,577 | 4.05 |
| B1 + T1 | 33,392 (15.4%) | 16,413.47 | 6.09 | 65,708.38 | 0.27 | 186,106 | 1.63 |
| B1 + T2 | 18,469 (8.5%) | 9064.02 | 9.93 | 362,573 | 0.61 | 102,546 | 2.76 |
| B1 + T3 | 11,275 (5.2%) | 5518.79 | 16.49 | 22,059.63 | 0.5 | 63,114 | 4.52 |
| B1 + T4 | 4535 (2.1%) | 2214.66 | 22.13 | 8843.84 | 1.13 | 25,763 | 5.28 |
| B2 + T1 | 14,118 (6.5%) | 6937.89 | 6.77 | 27,796.89 | 0.36 | 67,106 | 2.16 |
| B2 + T2 | 21,892 (10.1%) | 10,737.66 | 11.55 | 42989 | 1.07 | 112,563 | 3.18 |
| B2 + T3 | 23,374 (10.8%) | 11,447.95 | 15.02 | 45,782.36 | 1.2 | 129,549 | 3.87 |
| B2 + T4 | 22,375 (10.3%) | 10,898.48 | 28.08 | 43,531.16 | 2.16 | 132,586 | 6.32 |
| B3 + T1 | 4789 (2.2%) | 2356 | 4.24 | 9442.34 | 1.06 | 19,923 | 1.96 |
| B3 + T2 | 11,791 (5.4%) | 5787.83 | 9.85 | 23,205.86 | 0.99 | 52,183 | 3.35 |
| B3 + T3 | 16,344 (7.5%) | 8012.83 | 12.48 | 32,090.63 | 1.78 | 79,078 | 4.83 |
| B3 + T4 | 21,994 (10.2%) | 10,729.64 | 24.14 | 42,920.38 | 2.47 | 130,195 | 6.81 |
| B4 + T1 | 1842 (0.9%) | 906.02 | 3.31 | 3633.15 | 1.38 | 8591 | 4.42 |
| B4 + T2 | 1989 (0.9%) | 977.77 | 4.09 | 3911.26 | 4.09 | 8790 | 4.55 |
| B4 + T3 | 3148 (1.5%) | 1541.7 | 15.57 | 6162.52 | 3.73 | 13,600 | 5.29 |
| B4 + T4 | 5237 (2.4%) | 2560.6 | 19.14 | 10,224.23 | 4.3 | 26,884 | 7.59 |
| BI-RADS density and Texture categories | |||||||
| B1 | 67,671 (31.2%) | 33,210.93 | 9.94 | 132,869.14 | 0.46 | 377,529 | 2.67 |
| B2 | 81,759 (37.8%) | 40,021.98 | 16.22 | 160,099.42 | 1.28 | 441,803 | 4.17 |
| B3 | 54,918 (25.4%) | 26,886.29 | 15.84 | 107,659.21 | 1.82 | 281,379 | 5.27 |
| B4 | 12,216 (5.6%) | 5986.09 | 13.36 | 23,931.16 | 3.68 | 57,865 | 6.12 |
| T1 | 54,141 (25%) | 26,613.39 | 6.01 | 106,580.77 | 0.4 | 281,726 | 1.87 |
| T2 | 54,141 (25%) | 26,567.27 | 10.35 | 106,363.41 | 1.01 | 276,083 | 3.1 |
| T3 | 54,141 (25%) | 26,521.28 | 14.59 | 106,095.15 | 1.38 | 285,341 | 4.35 |
| T4 | 54,141 (25%) | 26,403.37 | 25.11 | 105,519.61 | 2.41 | 315,427 | 6.55 |
| 50–55 | 55–60 | 60–65 | 65- | Total | |
|---|---|---|---|---|---|
| Total | 98,516 | 41,413 | 36,985 | 39,650 | 216,564 |
| B1 + T1 | 12,412 | 7321 | 6594 | 7065 | 33,392 |
| B1 + T2 | 6317 | 3717 | 3916 | 4519 | 18,469 |
| B1 + T3 | 3394 | 2115 | 2485 | 3281 | 11,275 |
| B1 + T4 | 1312 | 783 | 1042 | 1398 | 4535 |
| B2 + T1 | 7874 | 2718 | 1948 | 1578 | 14,118 |
| B2 + T2 | 10,519 | 4331 | 3585 | 3457 | 21,892 |
| B2 + T3 | 9310 | 4849 | 4385 | 4830 | 23,374 |
| B2 + T4 | 7494 | 4583 | 4710 | 5588 | 22,375 |
| B3 + T1 | 3297 | 699 | 433 | 360 | 4789 |
| B3 + T2 | 7787 | 1789 | 1182 | 1033 | 11,791 |
| B3 + T3 | 9871 | 2713 | 2008 | 1752 | 16,344 |
| B3 + T4 | 10,377 | 4190 | 3663 | 3764 | 21,994 |
| B4 + T1 | 1227 | 278 | 177 | 160 | 1842 |
| B4 + T2 | 1476 | 222 | 148 | 143 | 1989 |
| B4 + T3 | 2349 | 374 | 205 | 220 | 3148 |
| B4 + T4 | 3500 | 731 | 504 | 502 | 5237 |
| BI-RADS density and Texture categories | |||||
| B1 | 23,435 | 13,936 | 14,037 | 16,263 | 67,671 |
| B2 | 35,197 | 16,481 | 14,628 | 15,453 | 81,759 |
| B3 | 31,332 | 9391 | 7286 | 6909 | 54,918 |
| B4 | 8552 | 1605 | 1034 | 1025 | 12,216 |
| T1 | 24,810 | 11,016 | 9152 | 9163 | 54,141 |
| T2 | 26,099 | 10,059 | 8831 | 9152 | 54,141 |
| T3 | 24,924 | 10,051 | 9083 | 10,083 | 54,141 |
| T4 | 22,683 | 10,287 | 9919 | 11,252 | 54,141 |
| Screen-Detected Cancer | Interval Cancer | Long-Term Cancer | ||||
|---|---|---|---|---|---|---|
| Model 1 (95% CI) | Model 2 (95% CI) | Model 1 (95% CI) | Model 2 (95% CI) | Model 1 (95% CI) | Model 2 (95% CI) | |
| B1 + T1 | Ref (1.00) | Ref (1.00) | Ref (1.00) | Ref (1.00) | Ref (1.00) | Ref (1.00) |
| B1 + T2 | 1.63 (1.22–2.17) | 1.58 (1.19–2.1) | 2.22 (1.19–4.18) | 2.19 (1.18–4.14) | 1.69 (1.44–1.99) | 1.68 (1.43–1.97) |
| B1 + T3 | 2.71 (2.04–3.59) | 2.54 (1.91–3.38) | 1.82 (0.83–3.8) | 1.78 (0.81–3.71) | 2.76 (2.35–3.25) | 2.71 (2.31–3.19) |
| B1 + T4 | 3.63 (2.56–5.08) | 3.37 (2.37–4.71) | 4.13 (1.83–8.77) | 4.02 (1.78–8.54) | 3.23 (2.63–3.95) | 3.16 (2.57–3.86) |
| B2 + T1 | 1.11 (0.78–1.56) | 1.21 (0.85–1.71) | 1.31 (0.58–2.79) | 1.37 (0.61–2.92) | 1.32 (1.08–1.61) | 1.39 (1.14–1.69) |
| B2 + T2 | 1.9 (1.46–2.47) | 1.98 (1.52–2.56) | 3.91 (2.31–6.91) | 4 (2.36–7.08) | 1.95 (1.67–2.27) | 2 (1.72–2.34) |
| B2 + T3 | 2.47 (1.93–3.16) | 2.47 (1.94–3.17) | 4.39 (2.63–7.68) | 4.41 (2.64–7.71) | 2.37 (2.05–2.73) | 2.39 (2.07–2.75) |
| B2 + T4 | 4.61 (3.69–5.8) | 4.46 (3.57–5.62) | 7.88 (4.89–13.48) | 7.79 (4.83–13.32) | 3.87 (3.4–4.42) | 3.84 (3.37–4.39) |
| B3 + T1 | 0.7 (0.34–1.27) | 0.79 (0.39–1.44) | 3.87 (1.72–8.22) | 3.87 (1.72–8.22) | 1.2 (0.85–1.65) | 1.31 (0.93–1.81) |
| B3 + T2 | 1.62 (1.16–2.23) | 1.81 (1.3–2.5) | 3.61 (1.96–6.79) | 3.85 (2.08–7.24) | 2.05 (1.7–2.47) | 2.24 (1.85–2.69) |
| B3 + T3 | 2.05 (1.55–2.7) | 2.24 (1.7–2.96) | 6.48 (3.9–11.33) | 6.82 (4.09–11.92) | 2.96 (2.54–3.44) | 3.16 (2.57–3.86) |
| B3 + T4 | 3.96 (3.16–5.01) | 4.1 (3.27–5.19) | 9.02 (5.62–15.35) | 9.2 (5.73–15.67) | 4.17 (3.67–4.76) | 4.32 (3.79–4.92) |
| B4 + T1 | 0.54 (0.13–1.44) | 0.61 (0.15–1.62) | 5.02 (1.66–12.59) | 5.35 (1.77–13.43) | 2.71 (1.9–3.74) | 2.71 (1.9–3.74) |
| B4 + T2 | 0.67 (0.21–1.6) | 0.76 (0.23–1.81) | 14.93 (7.53–29.34) | 16.13 (8.11–31.78) | 2.79 (1.97–3.82) | 3.12 (2.21–4.29) |
| B4 + T3 | 2.56 (1.6–3.92) | 2.91 (1.82–4.47) | 13.62 (7.37–25.57) | 14.74 (7.95–27.76) | 3.24 (2.49–4.16) | 3.65 (2.8–4.7) |
| B4 + T4 | 3.4 (2.21–4.39) | 3.49 (2.45–4.89) | 15.71 (9.24–27.87) | 16.71 (9.81–29.71) | 4.65 (3.89–5.54) | 5.11 (4.27–6.11) |
| Number of Women with Baseline Screening n (%) | All STC, n | Screen-Detected Cancer, n (%) | Interval Cancer, n (%) | |
|---|---|---|---|---|
| Total | 216,564 (100%) | 2035 | 1485 (100%) | 550 (100%) |
| B1 + T1 | 33,392 (15.4%) | 118 | 100 (6.7%) | 18 (3.3%) |
| B1 + T2 | 18,469 (8.5%) | 112 | 90 (6.6%) | 22 (4%) |
| B1 + T3 | 11,275 (5.2%) | 102 | 91 (6.1%) | 11 (2%) |
| B1 + T4 | 4535 (2.1%) | 59 | 49 (3.3%) | 10 (1.8%) |
| B2 + T1 | 14,118 (6.5%) | 57 | 47 (3.2%) | 10 (1.8%) |
| B2 + T2 | 21,892 (10.1%) | 170 | 124 (8.4%) | 46 (8.4%) |
| B2 + T3 | 23,374 (10.8%) | 227 | 172 (11.6%) | 55 (10%) |
| B2 + T4 | 22,375 (10.3%) | 400 | 306 (20.6%) | 94 (17.1%) |
| B3 + T1 | 4789 (2.2%) | 20 | 10 (0.7%) | 10 (1.8%) |
| B3 + T2 | 11,791 (5.4%) | 80 | 57 (3.8%) | 23 (4.2%) |
| B3 + T3 | 16,344 (7.5%) | 157 | 100 (6.7%) | 57 (10.4%) |
| B3 + T4 | 21,994 (10.2%) | 365 | 259 (17.4%) | 106 (19.3%) |
| B4 + T1 | 1842 (0.9%) | 8 | 3 (0.2%) | 5 (0.9%) |
| B4 + T2 | 1989 (0.9%) | 20 | 4 (0.3%) | 16 (2.9%) |
| B4 + T3 | 3148 (1.5%) | 47 | 24 (1.6%) | 23 (4.2%) |
| B4 + T4 | 5237 (2.4%) | 93 | 49 (3.3%) | 44 (8%) |
| BI-RADS density and Texture categories | ||||
| B1 | 67,671 (31.2%) | 391 | 330 (22.2%) | 61 (11.1%) |
| B2 | 81,759 (37.8%) | 854 | 649 (43.7%) | 205 (37.3%) |
| B3 | 54,918 (25.4%) | 622 | 426 (28.7% | 196 (35.6%) |
| B4 | 12,216 (5.6%) | 168 | 80 (5.4%) | 88 (16%) |
| T1 | 54,141 (25%) | 203 | 160 (10.8%) | 43 (7.8%) |
| T2 | 54,141 (25%) | 382 | 275 (18.5%) | 107 (19.5%) |
| T3 | 54,141 (25%) | 533 | 387 (26.1%) | 146 (26.6%) |
| T4 | 54,141 (25%) | 917 | 663 (44.7%) | 254 (46.2%) |
| Number of Women with Baseline Screening, n (%) | LTC, n | LTC, of Which Invasive Carcinoma, n | LTC, of Which DCIS, n | Median Time to LTC, Counted from Baseline, Months (Q1–Q3) | |
|---|---|---|---|---|---|
| Total | 214,501 (100%) | 4687 (100%) | 4088 | 599 | 56.3 (36.3–80.2) |
| B1 + T1 | 33,271 (15.5%) | 304 (6.4%) | 276 | 28 | 52.8 (34.2–78.3) |
| B1 + T2 | 18,357 (8.6%) | 283 (6%) | 255 | 28 | 54.1 (32.7–78.3) |
| B1 + T3 | 11,171 (5.2%) | 285 (6.1%) | 252 | 33 | 53.4 (35.6–80.3) |
| B1 + T4 | 4476 (2.1%) | 136 (2.9%) | 120 | 16 | 52.9 (31.9–79) |
| B2 + T1 | 14,056 (6.6%) | 145 (3.1%) | 125 | 20 | 61.8 (34.2–83.2) |
| B2 + T2 | 21,717 (10.1%) | 358 (7.6%) | 309 | 49 | 56 (35.8–79.3) |
| B2 + T3 | 23,146 (10.8%) | 501 (10.7%) | 439 | 62 | 54.8 (34.5–81.1) |
| B2 + T4 | 21,975 (10.2%) | 838 (17.9%) | 746 | 92 | 58.1 (38.7–82.8) |
| B3 + T1 | 4767 (2.2%) | 39 (0.8%) | 30 | 9 | 60.3 (32.2–82.6) |
| B3 + T2 | 11,706 (5.5%) | 175 (3.7%) | 141 | 34 | 51.6 (31.4–75.3) |
| B3 + T3 | 16,184 (7.5%) | 382 (8.2%) | 319 | 63 | 53.5 (33.5–77.4) |
| B3 + T4 | 21,628 (10.1%) | 887 (18.9%) | 777 | 110 | 60.6 (44.1–83.6) |
| B4 + T1 | 1834 (0.9%) | 38 (0.8%) | 34 | 4 | 57 (42.4–78) |
| B4 + T2 | 1968 (0.9%) | 40 (0.9%) | 31 | 9 | 57.5 (38.2–75.5) |
| B4 + T3 | 3101 (1.4%) | 72 (1.5%) | 58 | 14 | 54.5 (32–78.3) |
| B4 + T4 | 5144 (2.5%) | 204 (4.4%) | 176 | 28 | 58.8 (41–79.8) |
| BI-RADS density and Texture categories | |||||
| B1 | 67,275 (31.4%) | 1008 (21.5%) | 903 | 105 | 53 (34.2–78.6) |
| B2 | 80,894 (37.7%) | 1842 (39.3%) | 1619 | 223 | 57.3 (36.1–81.9) |
| B3 | 54,285 (25.3%) | 1483 (31.6%) | 1267 | 216 | 57.5 (36.8–80.4) |
| B4 | 12,047 (5.6%) | 354 (7.6%) | 299 | 55 | 57.1 (39.1–78.6) |
| T1 | 53,928 (25.1%) | 526 (11.2%) | 465 | 61 | 55.6 (34.3–80.1) |
| T2 | 53,748 (25.1%) | 856 (18.3%) | 736 | 120 | 53.3 (34–78.2) |
| T3 | 53,602 (25%) | 1240 (26.5%) | 1068 | 172 | 54.1 (34.2–78.9) |
| T4 | 53,223 (24.8%) | 2065 (44.1%) | 1819 | 246 | 59.4 (40.6–82.7) |
References
- Lynge, E.; Vejborg, I.; Lillholm, M.; Nielsen, M.; Napolitano, G.; von Euler-Chelpin, M. Breast Density and Risk of Breast Cancer. Int. J. Cancer 2023, 152, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.F.; Guo, H.; Martin, L.J.; Sun, L.; Stone, J.; Fishell, E.; Jong, R.A.; Hislop, G.; Chiarelli, A.; Minkin, S.; et al. Mammographic Density and the Risk and Detection of Breast Cancer. N. Engl. J. Med. 2007, 356, 227–236. [Google Scholar] [CrossRef]
- Weigel, S.; Heindel, W.; Heidrich, J.; Hense, H.W.; Heidinger, O. Digital Mammography Screening: Sensitivity of the Programme Dependent on Breast Density. Eur. Radiol. 2017, 27, 2744–2751. [Google Scholar] [CrossRef]
- Boyd, N.F.; Huszti, E.; Melnichouk, O.; Martin, L.J.; Hislop, G.; Chiarelli, A.; Yaffe, M.J.; Minkin, S. Mammographic Features Associated with Interval Breast Cancers in Screening Programs. Breast Cancer Res. 2014, 16, 417. [Google Scholar] [CrossRef]
- Kerlikowske, K.; Zhu, W.; Tosteson, A.N.A.; Sprague, B.L.; Tice, J.A.; Lehman, C.D.; Miglioretti, D.L. Identifying Women with Dense Breasts at Highi Risk of Interval Cancers. Ann. Intern. Med. 2015, 162, 673. [Google Scholar] [CrossRef] [PubMed]
- Posso, M.; Louro, J.; Sánchez, M.; Román, M.; Vidal, C.; Sala, M.; Baré, M.; Castells, X. Mammographic Breast Density: How It Affects Performance Indicators in Screening Programmes? Eur. J. Radiol. 2019, 110, 81–87. [Google Scholar] [CrossRef]
- McCormack, V.A.; Dos Santos Silva, I. Breast Density and Parenchymal Patterns as Markers of Breast Cancer Risk: A Meta-Analysis. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1159–1169. [Google Scholar] [CrossRef]
- Schousboe, J.T.; Kerlikowske, K.; Loh, A.; Cummings, S.R. Personalizing Mammography by Breast Density and Other Risk Factors for Breast Cancer: Analysis of Health Benefits and Cost-Effectiveness. Ann. Intern. Med. 2011, 155, 10. [Google Scholar] [CrossRef] [PubMed]
- Tyrer, J.; Duffy, S.W.; Cuzick, J. A Breast Cancer Prediction Model Incorporating Familial and Personal Risk Factors. Stat. Med. 2004, 23, 1111–1130. [Google Scholar] [CrossRef]
- Mann, R.M.; Athanasiou, A.; Baltzer, P.A.T.; Camps-Herrero, J.; Clauser, P.; Fallenberg, E.M.; Forrai, G.; Fuchsjäger, M.H.; Helbich, T.H.; Killburn-Toppin, F.; et al. Breast Cancer Screening in Women with Extremely Dense Breasts Recommendations of the European Society of Breast Imaging (EUSOBI). Eur. Radiol. 2022, 32, 4036–4045. [Google Scholar] [CrossRef]
- European Guidelines on Breast Cancer Screening and Diagnosis|European Commission Initiative on Breast, Cervical and Colorectal Cancerr. Available online: https://cancer-screening-and-care.jrc.ec.europa.eu/en/ecibc/european-breast-cancer-guidelines?topic=61&usertype=60&updatef2=0 (accessed on 23 May 2024).
- Gastounioti, A.; Conant, E.F.; Kontos, D. Beyond Breast Density: A Review on the Advancing Role of Parenchymal Texture Analysis in Breast Cancer Risk Assessment. Breast Cancer Res. 2016, 18, 91. [Google Scholar] [CrossRef] [PubMed]
- Warner, E.T.; Rice, M.S.; Zeleznik, O.A.; Fowler, E.E.; Murthy, D.; Vachon, C.M.; Bertrand, K.A.; Rosner, B.A.; Heine, J.; Tamimi, R.M. Automated Percent Mammographic Density, Mammographic Texture Variation, and Risk of Breast Cancer: A Nested Case-Control Study. NPJ Breast Cancer 2021, 7, 68. [Google Scholar] [CrossRef]
- Wang, C.; Brentnall, A.R.; Cuzick, J.; Harkness, E.F.; Evans, D.G.; Astley, S. A Novel and Fully Automated Mammographic Texture Analysis for Risk Prediction: Results from Two Case-Control Studies. Breast Cancer Res. 2017, 19, 114. [Google Scholar] [CrossRef]
- Nielsen, M.; Vachon, C.M.; Scott, C.G.; Chernoff, K.; Karemore, G.; Karssemeijer, N.; Lillholm, M.; Karsdal, M.A. Mammographic Texture Resemblance Generalizes as an Independent Risk Factor for Breast Cancer. Breast Cancer Res. 2014, 16, R37. [Google Scholar] [CrossRef] [PubMed]
- Malkov, S.; Shepherd, J.A.; Scott, C.G.; Tamimi, R.M.; Ma, L.; Bertrand, K.A.; Couch, F.; Jensen, M.R.; Mahmoudzadeh, A.P.; Fan, B.; et al. Mammographic Texture and Risk of Breast Cancer by Tumor Type and Estrogen Receptor Status. Breast Cancer Res. 2016, 18, 22. [Google Scholar] [CrossRef]
- Wanders, J.O.P.; van Gils, C.H.; Karssemeijer, N.; Holland, K.; Kallenberg, M.; Peeters, P.H.M.; Nielsen, M.; Lillholm, M. The Combined Effect of Mammographic Texture and Density on Breast Cancer Risk: A Cohort Study. Breast Cancer Res. 2018, 20, 36. [Google Scholar] [CrossRef] [PubMed]
- Kufel, J.; Bargieł-Łączek, K.; Kocot, S.; Koźlik, M.; Bartnikowska, W.; Janik, M.; Czogalik, Ł.; Dudek, P.; Magiera, M.; Lis, A.; et al. What Is Machine Learning, Artificial Neural Networks and Deep Learning?—Examples of Practical Applications in Medicine. Diagnostics 2023, 13, 2582. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, A.D.; von Euler-Chelpin, M.C.; Lynge, E.; Vejborg, I.; Nielsen, M.; Karssemeijer, N.; Lillholm, M. Assessing Breast Cancer Risk by Combining AI for Lesion Detection and Mammographic Texture. Radiology 2023, 308, 2. [Google Scholar] [CrossRef]
- Lauritzen, A.D.; von Euler-Chelpin, M.C.; Lynge, E.; Vejborg, I.; Nielsen, M.; Karssemeijer, N.; Lillholm, M. Robust Cross-Vendor Mammographic Texture Models Using Augmentation-Based Domain Adaptation for Long-Term Breast Cancer Risk. J. Med. Imaging 2023, 10, 054003. [Google Scholar] [CrossRef]
- Von Euler-Chelpin, M.; Lillholm, M.; Vejborg, I.; Nielsen, M.; Lynge, E. Sensitivity of Screening Mammography by Density and Texture: A Cohort Study from a Population-Based Screening Program in Denmark. Breast Cancer Res. 2019, 17, 111. [Google Scholar] [CrossRef]
- D’orsi, C.J.; Mendelson, E.B.; Ikeda, D.M. Breast Imaging Reporting and Data System: ACR BI-RADS-Breast Imaging Atlas; American college of Radiology: Reston, VA, USA, 2003. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Vachon, C.M.; van Gils, C.H.; Sellers, T.A.; Ghosh, K.; Pruthi, S.; Brandt, K.R.; Pankratz, V.S. Mammographic Density, Breast Cancer Risk and Risk Prediction. Breast Cancer Res. 2007, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Bodewes, F.; van Asselt, A.; Greuter, M.; de Bock, G. Mammographic Breast Density and the Risk of Breast Cancer: A Systematic Review and Meta-Analysis. Breast 2022, 66, 62–68. [Google Scholar] [CrossRef]
- Home|Are You Dense|Education about Breast Density. Available online: https://www.areyoudense.org/ (accessed on 15 April 2024).
- Tan, M.; Zheng, B.; Leader, J.K.; Gur, D. Association between Changes in Mammographic Image Features and Risk for Near-Term Breast Cancer Development. IEEE Trans. Med. Imaging 2016, 35, 1719. [Google Scholar] [CrossRef]
- Anandarajah, A.; Chen, Y.; Colditz, G.A.; Hardi, A.; Stoll, C.; Jiang, S. Studies of Parenchymal Texture Added to Mammographic Breast Density and Risk of Breast Cancer: A Systematic Review of the Methods Used in the Literature. Breast Cancer Res. 2022, 24, 101. [Google Scholar] [CrossRef] [PubMed]
- Winkel, R.R.; von Euler-Chelpin, M.; Nielsen, M.; Petersen, K.; Lillholm, M.; Nielsen, M.B.; Lynge, E.; Uldall, W.Y.; Vejborg, I. Mammographic Density and Structural Features Can Individually and Jointly Contribute to Breast Cancer Risk Assessment in Mammography Screening: A Case-Control Study. BMC Cancer 2016, 16, 414. [Google Scholar] [CrossRef] [PubMed]
- Gierach, G.L.; Li, H.; Loud, J.T.; Greene, M.H.; Chow, C.K.; Lan, L.; Prindiville, S.A.; Eng-Wong, J.; Soballe, P.W.; Giambartolomei, C.; et al. Relationships between Computer-Extracted Mammographic Texture Pattern Features and BRCA1/2mutation Status: A Cross-Sectional Study. Breast Cancer Res. 2014, 16, 424. [Google Scholar] [CrossRef] [PubMed]
- Kallenberg, M.; Petersen, K.; Nielsen, M.; Ng, A.Y.; Diao, P.; Igel, C.; Vachon, C.M.; Holland, K.; Winkel, R.R.; Karssemeijer, N.; et al. Unsupervised Deep Learning Applied to Breast Density Segmentation and Mammographic Risk Scoring. IEEE Trans. Med. Imaging 2016, 35, 1322–1331. [Google Scholar] [CrossRef]
- Schopf, C.M.; Ramwala, O.A.; Lowry, K.P.; Hofvind, S.; Marinovich, M.L.; Houssami, N.; Elmore, J.G.; Dontchos, B.N.; Lee, J.M.; Lee, C.I. Artificial Intelligence-Driven Mammography-Based Future Breast Cancer Risk Prediction: A Systematic Review. J. Am. Coll. Radiol. 2024, 21, 319–328. [Google Scholar] [CrossRef]
- Mainprize, J.G.; Alonzo-Proulx, O.; Alshafeiy, T.I.; Patrie, J.T.; Harvey, J.A.; Yaffe, M.J. Prediction of Cancer Masking in Screening Mammography Using Density and Textural Features. Acad. Radiol. 2019, 26, 608–619. [Google Scholar] [CrossRef]
- Alonzo-Proulx, O.; Mainprize, J.G.; Harvey, J.A.; Yaffe, M.J. Investigating the Feasibility of Stratified Breast Cancer Screening Using a Masking Risk Predictor. Breast Cancer Res. 2019, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Hinton, B.; Ma, L.; Pasha Mahmoudzadeh, A.; Malkov, S.; Fan, B.; Greenwood, H.; Joe, B.; Lee, V.; Strand, F.; Kerlikowske, K.; et al. Derived Mammographic Masking Measures Based on Simulated Lesions Predict the Risk of Interval Cancer after Controlling for Known Risk Factors: A Case-Case Analysis. Med. Phys. 2019, 46, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Mainprize, J.G.; Alonzo-Proulx, O.; Alshafeiy, T.; Patrie, J.T.; Harvey, J.A.; Yaffe, M.J. Masking Risk Predictors in Screening Mammography. In Proceedings of the The Fourteenth International Workshop on Breast Imaging 2018, Atlanta, GA, USA, 8–11 July 2018; Volume 10718, pp. 89–94. [Google Scholar] [CrossRef]
- Brentnall, A.R.; Cuzick, J.; Buist, D.S.M.; Bowles, E.J.A. Long-Term Accuracy of Breast Cancer Risk Assessment Combining Classic Risk Factors and Breast Density. JAMA Oncol. 2018, 4, e180174. [Google Scholar] [CrossRef] [PubMed]
- Santen, R.J.; Boyd, N.F.; Chlebowski, R.T.; Cummings, S.; Cuzick, J.; Dowsett, M.; Easton, D.; Forbes, J.F.; Key, T.; Hankinson, S.E.; et al. Critical Assessment of New Risk Factors for Breast Cancer: Considerations for Development of an Improved Risk Prediction Model. Endocr. Relat. Cancer 2007, 14, 169–187. [Google Scholar] [CrossRef] [PubMed]


| Women at Risk of Short-Term Cancer (Invasive + DCIS) | Women at Risk of Long-Term Cancer (Invasive + DCIS) | |
|---|---|---|
| No. of women with baseline screenings | 216,564 | 214,501 |
| Median age, years (Q1–Q3) | 56.2 (51.7–63.0) | 56.1 (51.7–63.0) |
| SDC, n | 1485 | - |
| Of which invasive | 1226 | - |
| Of which DCIS | 259 | - |
| IC, n | 550 | - |
| LTC, n | - | 4687 |
| BI-RADS 1, no. of women (%) | 67,671 (31.2%) | 67,275 (31.4%) |
| BI-RADS 2, no. of women (%) | 81,759 (37.8%) | 80,894 (37.7%) |
| BI-RADS 3, no. of women (%) | 54,918 (25.4%) | 54,285 (25.3%) |
| BI-RADS 4, no. of women (%) | 12,216 (5.6%) | 12,047 (5.6%) |
| Texture 1, no. of women (%) (0.000696–0.0513) | 54,141 (25%) | 53,928 (25.1%) |
| Texture 2, no. of women (%) (0.0513–0.0925) | 54,141 (25%) | 53,748 (25.1%) |
| Texture 3, no. of women (%) (0.0925–0.157) | 54,141 (25%) | 53,602 (25%) |
| Texture 4, no. of women (%) (0.157–0.929) | 54,141 (25%) | 53,223 (24.8%) |
| Screen-Detected Cancer Rate Per 1000 Women | BI-RADS 1 | BI-RADS 2 | BI-RADS 3 | BI-RADS 4 | Any BI-RADS |
|---|---|---|---|---|---|
| Texture 1 | 2.99 | 3.33 | 2.09 | 1.63 | 4.88 |
| Texture 2 | 4.87 | 5.66 | 4.83 | 2.01 | 7.94 |
| Texture 3 | 8.07 | 7.36 | 6.12 | 7.62 | 7.76 |
| Texture 4 | 10.8 | 13.68 | 11.78 | 9.36 | 6.55 |
| Any Texture | 2.96 | 5.08 | 7.15 | 12.25 | 6.86 |
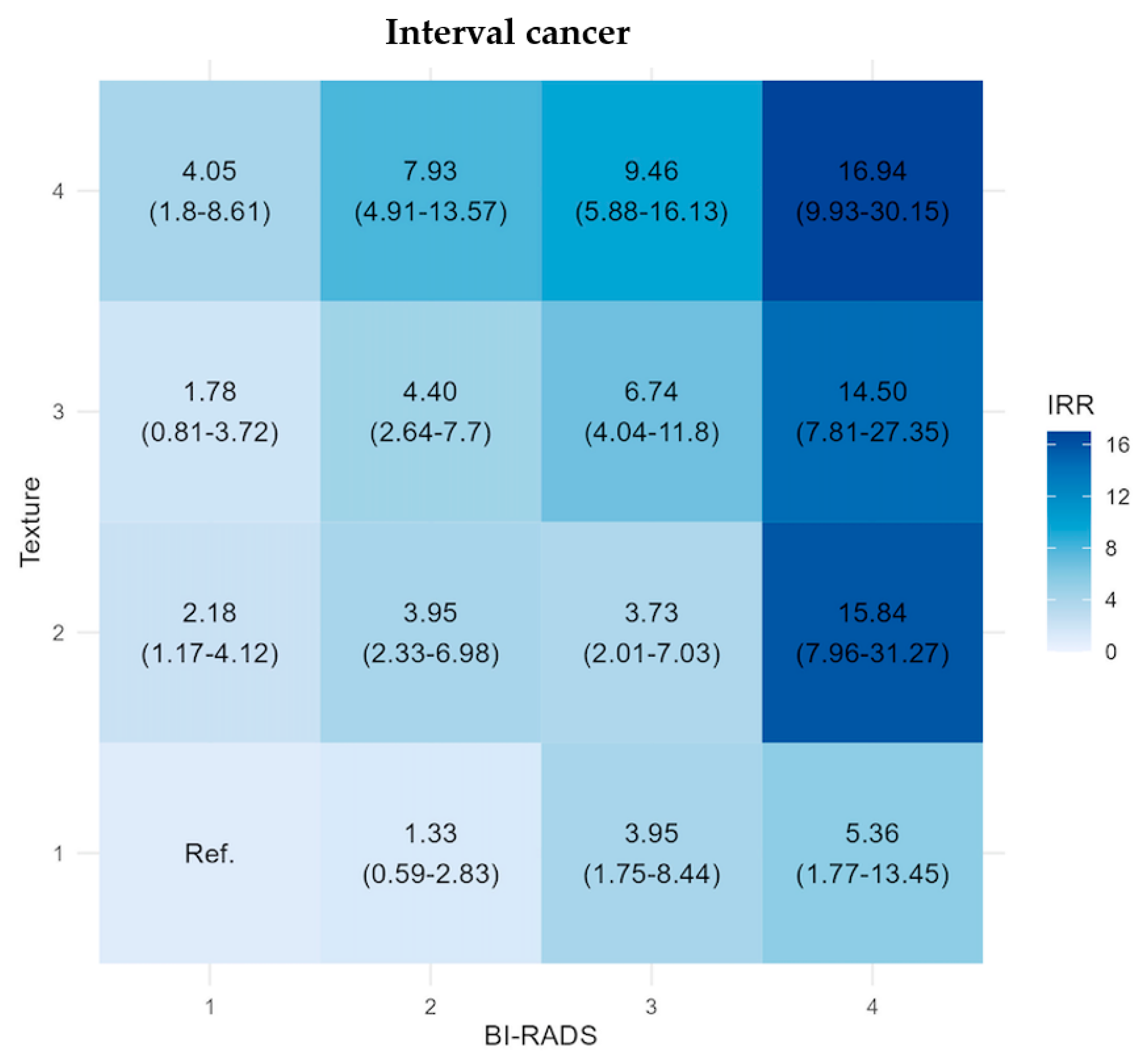
| Interval Cancer Rate Per 1000 Women | BI-RADS 1 | BI-RADS 2 | BI-RADS 3 | BI-RADS 4 | Any BI-RADS |
|---|---|---|---|---|---|
| Texture 1 | 0.54 | 0.71 | 2.09 | 2.71 | 0.9 |
| Texture 2 | 1.19 | 2.1 | 1.95 | 8.04 | 2.51 |
| Texture 3 | 0.98 | 2.35 | 3.49 | 7.31 | 3.57 |
| Texture 4 | 2.21 | 4.2 | 4.82 | 8.4 | 7.2 |
| Any Texture | 0.79 | 1.98 | 2.7 | 4.69 | 2.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilmun, B.M.; Napolitano, G.; Lauritzen, A.; Lynge, E.; Lillholm, M.; Nielsen, M.B.; Vejborg, I. Clinical Significance of Combined Density and Deep-Learning-Based Texture Analysis for Stratifying the Risk of Short-Term and Long-Term Breast Cancer in Screening. Diagnostics 2024, 14, 1823. https://doi.org/10.3390/diagnostics14161823
Vilmun BM, Napolitano G, Lauritzen A, Lynge E, Lillholm M, Nielsen MB, Vejborg I. Clinical Significance of Combined Density and Deep-Learning-Based Texture Analysis for Stratifying the Risk of Short-Term and Long-Term Breast Cancer in Screening. Diagnostics. 2024; 14(16):1823. https://doi.org/10.3390/diagnostics14161823
Chicago/Turabian StyleVilmun, Bolette Mikela, George Napolitano, Andreas Lauritzen, Elsebeth Lynge, Martin Lillholm, Michael Bachmann Nielsen, and Ilse Vejborg. 2024. "Clinical Significance of Combined Density and Deep-Learning-Based Texture Analysis for Stratifying the Risk of Short-Term and Long-Term Breast Cancer in Screening" Diagnostics 14, no. 16: 1823. https://doi.org/10.3390/diagnostics14161823





