Objective and Subjective Outcomes Following Radiofrequency of Inferior Turbinates in Patients with Sleep-Disordered Breathing
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants’ Characteristics
2.3. Details of the Surgery
2.4. Objective and Subjective Measurements at Baseline and Follow-Ups
2.5. Statistical Analysis
3. Results
3.1. Breakdown of the Population
3.2. Demographic Data
3.3. Nasal Airflow and Olfactory Function at Baseline
3.4. Other Investigations at Baseline
3.5. Patient-Reported Outcome Measures (PROMs)
3.6. Changes at Follow-up
3.7. Influence of Available Variables on Studied Parameters
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kotecha, B. The nose, snoring and obstructive sleep apnoea. Rhinology 2011, 49, 259–263. [Google Scholar] [CrossRef]
- Kotecha, B.T.; Hannan, S.A.; Khalil, H.M.; Georgalas, C.; Bailey, P. Sleep nasendoscopy: A 10-year retrospective audit study. Eur. Arch. Otorhinolaryngol. 2007, 264, 1361–1367. [Google Scholar] [CrossRef]
- Skatvedt, O. Continuous pressure measurements during sleep to localize obstructions in the upper airways in heavy snorers and patients with obstructive sleep apnea syndrome. Eur. Arch. Otorhinolaryngol. 1995, 252, 11–14. [Google Scholar] [CrossRef]
- Cole, P.; Haight, J.S. Mechanisms of nasal obstruction in sleep. Laryngoscope 1984, 94, 1557–1559. [Google Scholar] [PubMed]
- Friedman, M.; Maley, A.; Kelley, K.; Leesman, C.; Patel, A.; Pulver, T.; Joseph, N.; Catli, T. Impact of nasal obstruction on obstructive sleep apnea. Otolaryngol. Head Neck Surg. 2011, 144, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Morris, L.G.; Burschtin, O.; Lebowitz, R.A.; Jacobs, J.B.; Lee, K.C. Nasal obstruction and sleep-disordered breathing: A study using acoustic rhinometry. Am. J. Rhinol. 2005, 19, 33–39. [Google Scholar] [PubMed]
- Young, T.; Finn, L.; Kim, H. Nasal obstruction as a risk factor for sleep-disordered breathing. The University of Wisconsin Sleep and Respiratory Research Group. J. Allergy Clin. Immunol. 1997, 99, S757–S762. [Google Scholar] [CrossRef]
- Saka, C.; Vuralkan, E.; Firat, I.H.; Alicura, S.; Hucumenoglu, S.; Akin, I.; Ardic, S.; Gokler, A. The effects of CPAP treatment on nasal mucosa in patients with obstructive sleep apnea. Eur. Arch. Otorhinolaryngol. 2012, 269, 2065–2067. [Google Scholar] [CrossRef]
- Lenders, H.; Schaefer, J.; Pirsig, W. Turbinate hypertrophy in habitual snorers and patients with obstructive sleep apnea: Findings of acoustic rhinometry. Laryngoscope 1991, 101, 614–618. [Google Scholar] [CrossRef]
- Silvoniemi, P.; Suonpaa, J.; Sipila, J.; Grenman, R.; Erkinjuntti, M. Sleep disorders in patients with severe nasal obstruction due to septal deviation. Acta Otolaryngol. Suppl. 1997, 529, 199–201. [Google Scholar] [CrossRef]
- Leitzen, K.P.; Brietzke, S.E.; Lindsay, R.W. Correlation between nasal anatomy and objective obstructive sleep apnea severity. Otolaryngol. Head Neck Surg. 2014, 150, 325–331. [Google Scholar] [CrossRef]
- Bousquet, J.; Khaltaev, N.; Cruz, A.A.; Denburg, J.; Fokkens, W.J.; Togias, A.; Zuberbier, T.; Baena-Cagnani, C.E.; Canonica, G.W.; van Weel, C.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 2008, 63 (Suppl. S86), 8–160. [Google Scholar] [CrossRef]
- Kiely, J.L.; Nolan, P.; McNicholas, W.T. Intranasal corticosteroid therapy for obstructive sleep apnoea in patients with co-existing rhinitis. Thorax 2004, 59, 50–55. [Google Scholar] [PubMed]
- Lavigne, F.; Petrof, B.J.; Johnson, J.R.; Lavigne, P.; Binothman, N.; Kassissia, G.O.; Al Samri, M.; Giordano, C.; Dube, N.; Hercz, D.; et al. Effect of topical corticosteroids on allergic airway inflammation and disease severity in obstructive sleep apnoea. Clin. Exp. Allergy 2013, 43, 1124–1133. [Google Scholar] [CrossRef] [PubMed]
- Craig, T.J.; Teets, S.; Lehman, E.B.; Chinchilli, V.M.; Zwillich, C. Nasal congestion secondary to allergic rhinitis as a cause of sleep disturbance and daytime fatigue and the response to topical nasal corticosteroids. J. Allergy Clin. Immunol. 1998, 101, 633–637. [Google Scholar] [CrossRef]
- Pagel, J.M.L.; Mattos, J.L. Allergic Rhinitis and Its Effect on Sleep. Otolaryngol. Clin. N. Am. 2023, 57, 319–328. [Google Scholar] [CrossRef]
- Batra, P.S.; Seiden, A.M.; Smith, T.L. Surgical management of adult inferior turbinate hypertrophy: A systematic review of the evidence. Laryngoscope 2009, 119, 1819–1827. [Google Scholar] [CrossRef]
- Bhandarkar, N.D.; Smith, T.L. Outcomes of surgery for inferior turbinate hypertrophy. Curr. Opin. Otolaryngol. Head Neck Surg. 2010, 18, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.E.; Koch, R.J. Controversies in the management of inferior turbinate hypertrophy: A comprehensive review. Plast. Reconstr. Surg. 1999, 103, 300–312. [Google Scholar] [CrossRef]
- Utley, D.S.; Goode, R.L.; Hakim, I. Radiofrequency energy tissue ablation for the treatment of nasal obstruction secondary to turbinate hypertrophy. Laryngoscope 1999, 109, 683–686. [Google Scholar] [CrossRef]
- Singh, S.; Ramli, R.R.; Wan Mohammad, Z.; Abdullah, B. Coblation versus microdebrider-assisted turbinoplasty for endoscopic inferior turbinates reduction. Auris Nasus Larynx 2020, 47, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Harrill, W.C.; Pillsbury, H.C., 3rd; McGuirt, W.F.; Stewart, M.G. Radiofrequency turbinate reduction: A NOSE evaluation. Laryngoscope 2007, 117, 1912–1919. [Google Scholar] [CrossRef]
- Porter, M.W.; Hales, N.W.; Nease, C.J.; Krempl, G.A. Long-term results of inferior turbinate hypertrophy with radiofrequency treatment: A new standard of care? Laryngoscope 2006, 116, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Nease, C.J.; Krempl, G.A. Radiofrequency treatment of turbinate hypertrophy: A randomized, blinded, placebo-controlled clinical trial. Otolaryngol. Head Neck Surg. 2004, 130, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Garzaro, M.; Pezzoli, M.; Landolfo, V.; Defilippi, S.; Giordano, C.; Pecorari, G. Radiofrequency inferior turbinate reduction: Long-term olfactory and functional outcomes. Otolaryngol. Head Neck Surg. 2012, 146, 146–150. [Google Scholar] [CrossRef]
- Means, C.; Camacho, M.; Capasso, R. Long-Term Outcomes of Radiofrequency Ablation of the Inferior Turbinates. Indian. J. Otolaryngol. Head Neck Surg. 2016, 68, 424–428. [Google Scholar] [CrossRef]
- Pendolino, A.L.; Scarpa, B.; Ottaviano, G. Relationship Between Nasal Cycle, Nasal Symptoms and Nasal Cytology. Am. J. Rhinol. Allergy 2019, 33, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Pendolino, A.L.; Nardello, E.; Lund, V.J.; Maculan, P.; Scarpa, B.; Martini, A.; Ottaviano, G. Comparison between unilateral PNIF and rhinomanometry in the evaluation of nasal cycle. Rhinology 2018, 56, 122–126. [Google Scholar] [CrossRef]
- Pendolino, A.L.; Lund, V.J.; Nardello, E.; Ottaviano, G. The nasal cycle: A comprehensive review. Rhinol. Online 2018, 1, 67–76. [Google Scholar] [CrossRef]
- Ottaviano, G.; Pendolino, A.L.; Nardello, E.; Maculan, P.; Martini, A.; Russo, M.; Lund, V.J. Peak nasal inspiratory flow measurement and visual analogue scale in a large adult population. Clin. Otolaryngol. 2019, 44, 541–548. [Google Scholar] [CrossRef]
- Ottaviano, G.; Scadding, G.K.; Scarpa, B.; Accordi, D.; Staffieri, A.; Lund, V.J. Unilateral peak nasal inspiratory flow, normal values in adult population. Rhinology 2012, 50, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Ottaviano, G.; Fokkens, W.J. Measurements of nasal airflow and patency: A critical review with emphasis on the use of peak nasal inspiratory flow in daily practice. Allergy 2016, 71, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Oleszkiewicz, A.; Schriever, V.A.; Croy, I.; Hahner, A.; Hummel, T. Updated Sniffin’ Sticks normative data based on an extended sample of 9139 subjects. Eur. Arch. Otorhinolaryngol. 2019, 276, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Bordin, A.; Mucignat-Caretta, C.; Gaudioso, P.; Pendolino, A.L.; Leoni, D.; Scarpa, B.; Andrews, P.J.; Cattelan, A.M.; Antonini, A.; Nicolai, P.; et al. Comparison of self-reported symptoms and psychophysical tests in coronavirus disease 2019 (COVID-19) subjects experiencing long-term olfactory dysfunction: A 6-month follow-up study. Int. Forum Allergy Rhinol. 2021, 11, 1592–1595. [Google Scholar] [CrossRef] [PubMed]
- Mattos, J.L.; Edwards, C.; Schlosser, R.J.; Hyer, M.; Mace, J.C.; Smith, T.L.; Soler, Z.M. A brief version of the questionnaire of olfactory disorders in patients with chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2019, 9, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- DeConde, A.S.; Mace, J.C.; Bodner, T.; Hwang, P.H.; Rudmik, L.; Soler, Z.M.; Smith, T.L. SNOT-22 quality of life domains differentially predict treatment modality selection in chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2014, 4, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Ottaviano, G.; Scadding, G.K.; Coles, S.; Lund, V.J. Peak nasal inspiratory flow; normal range in adult population. Rhinology 2006, 44, 32–35. [Google Scholar] [PubMed]
- Hytonen, M.L.; Back, L.J.; Malmivaara, A.V.; Roine, R.P. Radiofrequency thermal ablation for patients with nasal symptoms: A systematic review of effectiveness and complications. Eur. Arch. Otorhinolaryngol. 2009, 266, 1257–1266. [Google Scholar] [CrossRef]
- Cavaliere, M.; Mottola, G.; Iemma, M. Comparison of the effectiveness and safety of radiofrequency turbinoplasty and traditional surgical technique in treatment of inferior turbinate hypertrophy. Otolaryngol. Head Neck Surg. 2005, 133, 972–978. [Google Scholar] [CrossRef]
- Casale, M.; Bottaro, V.; Sabatino, L.; Frari, V.; Bressi, F.; Vespasiani, U.; Baptista, P.; Salvinelli, F. The efficacy of radiofrequency volumetric tissue reduction of hypertrophied inferior turbinate in simple snoring. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2160–2168. [Google Scholar]
- Powell, N.B.; Zonato, A.I.; Weaver, E.M.; Li, K.; Troell, R.; Riley, R.W.; Guilleminault, C. Radiofrequency treatment of turbinate hypertrophy in subjects using continuous positive airway pressure: A randomized, double-blind, placebo-controlled clinical pilot trial. Laryngoscope 2001, 111, 1783–1790. [Google Scholar] [CrossRef]
- Verse, T.; Maurer, J.T.; Pirsig, W. Effect of nasal surgery on sleep-related breathing disorders. Laryngoscope 2002, 112, 64–68. [Google Scholar] [CrossRef]
- Morinaga, M.; Nakata, S.; Yasuma, F.; Noda, A.; Yagi, H.; Tagaya, M.; Sugiura, M.; Teranishi, M.; Nakashima, T. Pharyngeal morphology: A determinant of successful nasal surgery for sleep apnea. Laryngoscope 2009, 119, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Koutsourelakis, I.; Georgoulopoulos, G.; Perraki, E.; Vagiakis, E.; Roussos, C.; Zakynthinos, S.G. Randomised trial of nasal surgery for fixed nasal obstruction in obstructive sleep apnoea. Eur. Respir. J. 2008, 31, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Ishii, L.; Godoy, A.; Ishman, S.L.; Gourin, C.G.; Ishii, M. The nasal obstruction symptom evaluation survey as a screening tool for obstructive sleep apnea. Arch. Otolaryngol. Head Neck Surg. 2011, 137, 119–123. [Google Scholar] [CrossRef]
- Ottaviano, G.; Pendolino, A.L.; Nardello, E.; Pollis, M.; Scarpa, B.; Marchese-Ragona, R. The role of peak nasal and oral inspiratory flow in the evaluation of patients with sleep-related breathing disorders. Rhinology 2020, 58, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Hamerschmidt, R.; Hamerschmidt, R.; Moreira, A.T.; Tenorio, S.B.; Timi, J.R. Comparison of turbinoplasty surgery efficacy in patients with and without allergic rhinitis. Braz. J. Otorhinolaryngol. 2016, 82, 131–139. [Google Scholar] [CrossRef]
- Parthasarathi, K.; Christensen, J.M.; Alvarado, R.; Barham, H.P.; Sacks, R.; Harvey, R.J. Airflow and symptom outcomes between allergic and non-allergic rhinitis patients from turbinoplasty. Rhinology 2017, 55, 332–338. [Google Scholar] [CrossRef]
- Kim, S.D.; Jung, D.W.; Lee, J.W.; Park, J.H.; Mun, S.J.; Cho, K.S. Relationship between allergic rhinitis and nasal surgery success in patients with obstructive sleep apnea. Am. J. Otolaryngol. 2021, 42, 103079. [Google Scholar] [CrossRef]
- Callander, J.K.; Chang, J.L. Treatment of the Nose for Patients with Sleep Apnea. Otolaryngol. Clin. N. Am. 2023, 57, 491–500. [Google Scholar] [CrossRef]
- Cai, Y.; Goldberg, A.N.; Chang, J.L. The Nose and Nasal Breathing in Sleep Apnea. Otolaryngol. Clin. N. Am. 2020, 53, 385–395. [Google Scholar] [CrossRef]
- Holty, J.E.; Guilleminault, C. Surgical options for the treatment of obstructive sleep apnea. Med. Clin. N. Am. 2010, 94, 479–515. [Google Scholar] [CrossRef] [PubMed]
- Schoustra, E.; van Maanen, P.; den Haan, C.; Ravesloot, M.J.L.; de Vries, N. The Role of Isolated Nasal Surgery in Obstructive Sleep Apnea Therapy—A Systematic Review. Brain Sci. 2022, 12, 1446. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, G.; Li, Y.; Zang, H.; Wang, T.; Wang, D.; Han, D. Apnea-hypopnea index decreased significantly after nasal surgery for obstructive sleep apnea: A meta-analysis. Medicine 2017, 96, e6008. [Google Scholar] [CrossRef]
- Iannella, G.; Magliulo, G.; Maniaci, A.; Meccariello, G.; Cocuzza, S.; Cammaroto, G.; Gobbi, R.; Sgarzani, R.; Firinu, E.; Corso, R.M.; et al. Olfactory function in patients with obstructive sleep apnea: A meta-analysis study. Eur. Arch. Otorhinolaryngol. 2021, 278, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.K.; Yuan, X.; Wroblewski, K.E.; McClintock, M.K.; Pinto, J.M. Sleep-Disordered Breathing Is Associated With Impaired Odor Identification in Older U.S. Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 528–533. [Google Scholar] [CrossRef]
- Dong, J.; Zhan, X.; Sun, H.; Fang, F.; Wei, Y. Olfactory dysfunction is associated with cognitive impairment in patients with obstructive sleep apnea: A cross-sectional study. Eur. Arch. Otorhinolaryngol. 2022, 279, 1979–1987. [Google Scholar] [CrossRef]
- Karakurt, S.E.; Karakus, M.F.; Colak, M.; Akbal, S.; Cetin, M.A.; Ikinciogullari, A.; Dere, H.H. Evaluation of olfactory function in patients with obstructive sleep apnea syndrome. Sleep. Breath. 2020, 24, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Walliczek-Dworschak, U.; Cassel, W.; Mittendorf, L.; Pellegrino, R.; Koehler, U.; Guldner, C.; Dworschak, P.O.G.; Hildebrandt, O.; Daniel, H.; Gunzel, T.; et al. Continuous positive air pressure improves orthonasal olfactory function of patients with obstructive sleep apnea. Sleep. Med. 2017, 34, 24–29. [Google Scholar] [CrossRef]
- Assanasen, P.; Choochurn, P.; Banhiran, W.; Bunnag, C. Radiofrequency inferior turbinate reduction improves smell ability of patients with chronic rhinitis and inferior turbinate hypertrophy. Allergy Rhinol. 2014, 5, 12–16. [Google Scholar] [CrossRef]
- Migueis, D.P.; Thuler, L.C.; Lemes, L.N.; Moreira, C.S.; Joffily, L.; Araujo-Melo, M.H. Systematic review: The influence of nasal obstruction on sleep apnea. Braz. J. Otorhinolaryngol. 2016, 82, 223–231. [Google Scholar] [CrossRef]
- Jenkinson, C.; Stewart-Brown, S.; Petersen, S.; Paice, C. Assessment of the SF-36 version 2 in the United Kingdom. J. Epidemiol. Community Health 1999, 53, 46–50. [Google Scholar] [CrossRef]
- Nilsen, A.H.; Helvik, A.S.; Thorstensen, W.M.; Bugten, V. A comparison of symptoms and quality of life before and after nasal septoplasty and radiofrequency therapy of the inferior turbinate. BMC Ear Nose Throat Disord. 2018, 18, 2. [Google Scholar] [CrossRef]
- Li, H.Y.; Lee, L.A.; Wang, P.C.; Fang, T.J.; Chen, N.H. Can nasal surgery improve obstructive sleep apnea: Subjective or objective? Am. J. Rhinol. Allergy 2009, 23, e51–e55. [Google Scholar] [CrossRef] [PubMed]
- Pendolino, A.L.; Ottaviano, G.; Navaratnam, A.V.; Scarpa, B.; Andrews, P.J. Clinical factors influencing olfactory performance in patients with persistent COVID-19 smell loss longer than 1 year. Laryngoscope Investig. Otolaryngol. 2023, 8, 1449–1458. [Google Scholar] [CrossRef]
- Randhawa, P.S.; Watson, N.; Lechner, M.; Ritchie, L.; Choudhury, N.; Andrews, P.J. The outcome of septorhinoplasty surgery on olfactory function. Clin. Otolaryngol. 2016, 41, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Whitcroft, K.L.; Mancini, L.; Yousry, T.; Hummel, T.; Andrews, P.J. Functional septorhinoplasty alters brain structure and function: Neuroanatomical correlates of olfactory dysfunction. Front. Allergy 2023, 4, 1079945. [Google Scholar] [CrossRef]

| n = 17 | |
|---|---|
| Demographics | |
| Age, median [P25–P75], year | 42.0 [35.0–52.0] |
| Sex, No (%) | |
| Female | 7 (41.2%) |
| Male | 10 (58.8%) |
| Smoking status, No (%) | |
| Ex-smoker | 1 (5.9%) |
| Active | 1 (5.9%) |
| No | 15 (88.2%) |
| History of rhinitis, No (%) | |
| Allergic type | 9 (52.9%) |
| Non-allergic type | 8 (47.1%) |
| Sleep symptoms, No (%) | |
| Snoring only | 5 (29.4%) |
| OSA only | 0 (0.0%) |
| Both | 12 (70.6%) |
| Comorbidities, No (%) | |
| None | 7 (41.2%) |
| Asthma | 4 (23.5%) |
| Hypertension | 3 (17.6%) |
| Mental health issues | 3 (17.6%) |
| Other | 5 (29.4%) |
| Medications, No (%) | |
| Nasal douche | 17 (100%) |
| Steroid spray | 8 (47.1%) |
| Steroid + antihistamine spray | 9 (52.9%) |
| Sartan | 2 (11.8%) |
| Beta-2 agonist inhaler | 4 (23.5%) |
| Other | 5 (29.4%) |
| Previous relevant surgery, No (%) | |
| Tonsillectomy | 3 (17.6%) |
| Palatoplasty | 2 (11.8%) |
| Rhinoplasty | 2 (11.8%) |
| Septoplasty | 1 (5.9%) |
| Investigations | |
| Skin prick test, No (%) | |
| Negative | 8 (47.1%) |
| One allergen | 3 (17.6%) |
| Two allergens | 2 (11.8%) |
| Multiple allergens | 4 (23.5%) |
| Nasal endoscopy findings, No (%) | |
| Rhinitis | 17 (100%) |
| IT hypertrophy only | 9 (52.9%) |
| Septal deviation + IT hypertrophy | 8 (47.1%) |
| Baseline (T0) n = 17 | 3-Month (T1) n = 13 | 6-Month (T2) n = 14 | 12-Month (T3) n = 10 | p-Value (T0–T1) | p-Value (T0–T2) | p-Value (T0–T3) | |
|---|---|---|---|---|---|---|---|
| Nasal measurements | |||||||
| Pre-decongestion | |||||||
| PNIF, median [P25–P75], L/min | |||||||
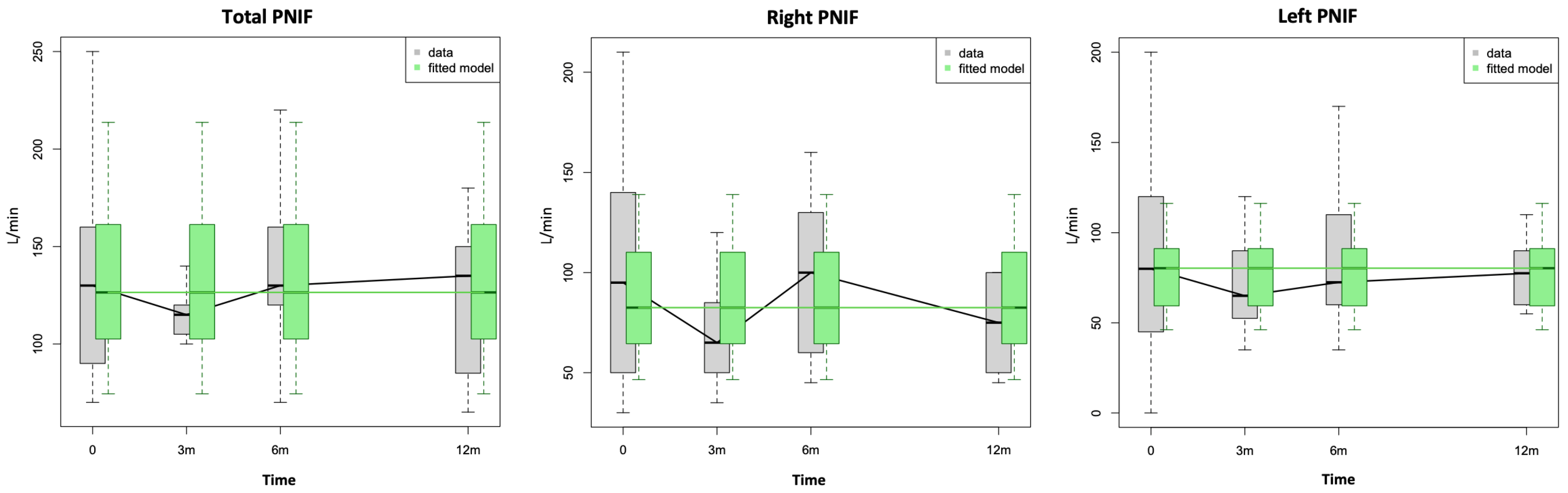
| Bilateral PNIF | 130.0 [90.0–160.0] | 115.0 [107.5–120.0] | 130.0 [120.0–157.5] | 135.0 [93.8–147.5] | 0.80 | 0.83 | 0.83 |
| Right PNIF | 95.0 [50.0–140.0] | 65.0 [50.0–82.5] | 100.0 [60.0–125.0] | 75.0 [52.5–98.8] | 0.50 | 0.70 | 0.68 |
| Left PNIF | 80.0 [45.0–120.0] | 65.0 [53.8–85.0] | 72.5 [60.0–107.5] | 77.5 [61.3–88.8] | 0.66 | 0.92 | 0.76 |
| Acoustic rhinometry, median [P25–P75] | |||||||
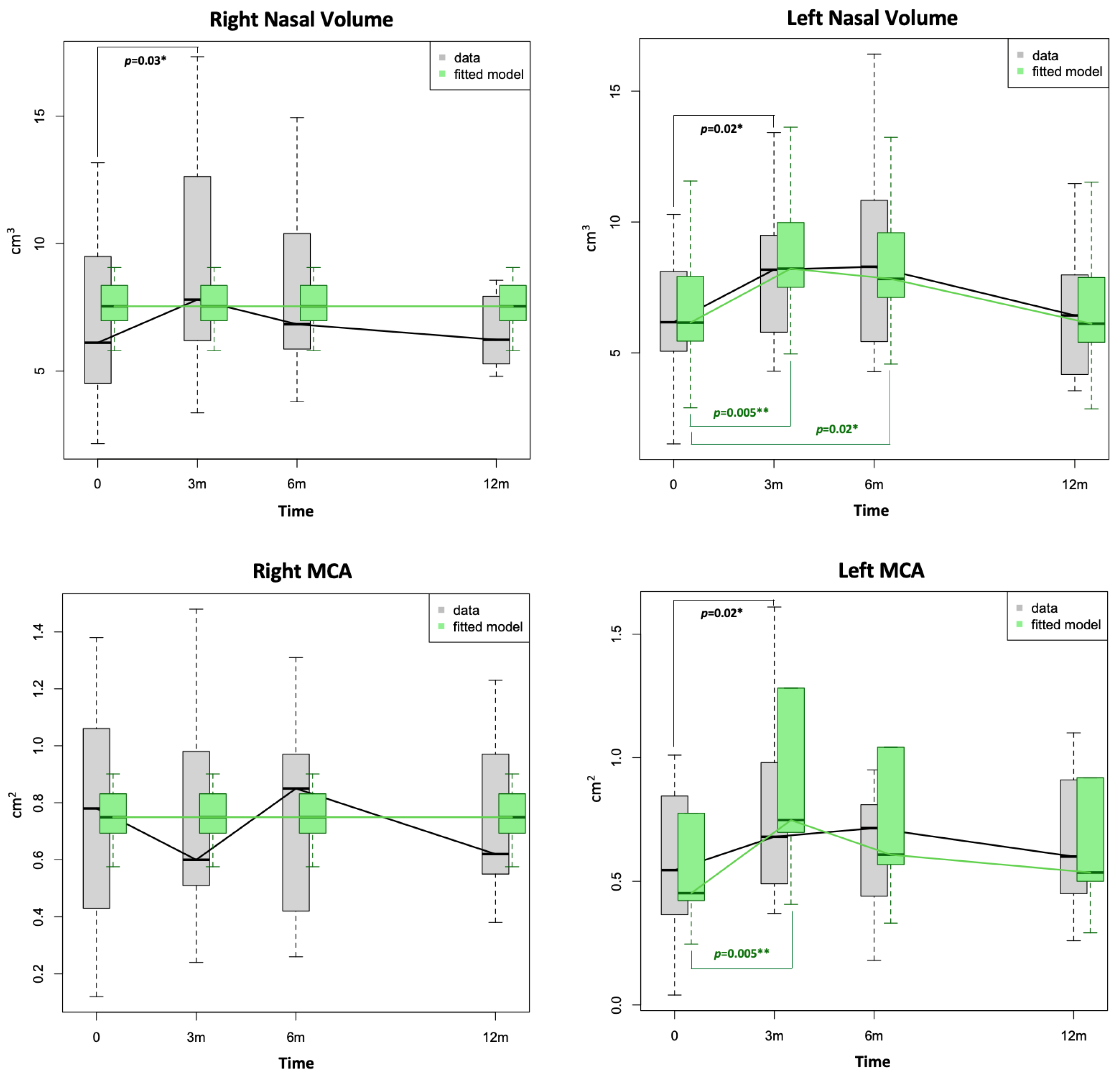
| Right MCA1, cm2 | 0.8 [0.4–1.1] | 0.6 [0.5–1.0] | 0.9 [0.5–1.0] | 0.6 [0.6–0.9] | 0.50 | 0.85 | 0.28 |
| Right nasal volume (0–5), cm3 | 6.1 [4.5–9.5] | 7.8 [6.2–12.6] | 6.8 [5.9–10.0] | 6.2 [5.4–7.8] | 0.03 * | 0.90 | 0.49 |
| Left MCA1, cm2 | 0.5 [0.4–0.8] | 0.7 [0.5–1.0] | 0.7 [0.5–0.8] | 0.6 [0.5–0.9] | 0.02 * | 0.19 | 0.82 |
| Left nasal volume (0–5), cm3 | 6.2 [5.1–8.1] | 8.2 [5.8–9.5] | 8.3 [5.7–10.5] | 6.4 [4.2–8.0] | 0.02 * | 0.09 | 0.50 |
| Post-decongestion | |||||||
| PNIF, median [P25–P75], L/min | |||||||
| Bilateral PNIF | 150.0 [110.0–180.0] | 120.0 [110.0–170.0] | 150.0 [125.0–200.0] | 140.0 [122.5–155.0] | 0.58 | 0.72 | 0.72 |
| Right PNIF | 110.0 [85.0–130.0] | 75.0 [60.0–110.0] | 80.0 [70.0–135.0] | 97.5 [70.0–128.8] | 0.69 | 0.47 | 0.26 |
| Left PNIF | 100.0 [50.0–140.0] | 85.0 [65.0–100.0] | 85.0 [60.0–110.0] | 100.0 [76.3–100.0] | 0.72 | 0.46 | 1.00 |
| Acoustic rhinometry, median [P25–P75] | |||||||
| Right MCA1, cm2 | 1.0 [0.8–1.5] | 0.9 [0.8–1.1] | 0.9 [0.8–1.3] | 1.1 [0.7–1.3] | 0.79 | 0.54 | 0.37 |
| Right nasal volume (0–5), cm3 | 9.4 [6.0–11.9] | 9.3 [7.7–10.9] | 8.2 [7.1–11.2] | 8.7 [7.3–10.5] | 0.24 | 0.95 | 0.84 |
| Left MCA1, cm2 | 0.9 [0.6–1.1] | 1.0 [0.5–1.2] | 1.0 [0.9–1.1] | 1.1 [0.9–1.1] | 0.19 | 0.13 | 0.23 |
| Left nasal volume (0–5), cm3 | 9.5 [6.6–11.6] | 9.1 [6.3–12.1] | 10.1 [6.2–10.7] | 9.9 [8.5–12.0] | 0.78 | 0.79 | 1.00 |
| Baseline (T0) n = 17 | 3 Month (T1) n = 13 | 6 Month (T2) n = 14 | 12 Month (T3) n = 10 | p-Value (T0–T1) | p-Value (T0–T2) | p-Value (T0–T3) | |
|---|---|---|---|---|---|---|---|
| Other measurements | |||||||
| Sniffin’ Sticks Identification, median [P25–P75] | 13.0 [11.0–13.0] | 12.0 [11.0–14.0] | 12.5 [11.3–13.0] | 13.0 [11.0–13.0] | 0.63 | 0.93 | 0.10 |
| Normosmics, n (%) | 13 (76.5%) | 11 (84.6%) | 12 (85.7%) | 9 (90.0%) | 0.38 | 0.20 | 0.10 |
| Hyposmics, n (%) | 4 (23.5%) | 2 (15.4%) | 2 (14.3%) | 1 (10.0%) | 1.00 | 0.37 | N/A + |
| BMI, median [P25–P75], kg/m2 | 30.1 [26.5–32.8] | - | 27.1 [25.5–32.0] | - | - | - | |
| Normal range (18.5–24.9), n (%) | 2 (14.3%) | 3 (23.1%) | 0.47 | ||||
| Overweight, (25–29.9), n (%) | 5 (35.7%) | 5 (38.5%) | 0.93 | ||||
| Obese grade I, (30–34.9), n (%) | 4 (28.6%) | 3 (23.1%) | 1 | ||||
| Obese grade II, (35–39.9), n (%) | 2 (14.3%) | 1 (7.7%) | 1 | ||||
| Obese grade III, (≥40), n (%) | 1 (7.1%) | 1 (7.7%) | 1 | ||||
| Missing | 3 | 4 | 1 | ||||
| Sleep Study | - | - | - | - | |||
| AHI, median [P25–P75] | 12.3 [4.7–17.2] | 11.0 [2.8–16.3] | 0.42 | ||||
| Normal (<5), n (%) | 5 (29.4%) | 5 (33.3%) | 1 | ||||
| Mild OSA (5–14.9), n (%) | 5 (29.4%) | 5 (33.3%) | 1 | ||||
| Moderate OSA, (15–29.9), n (%) | 6 (35.3%) | 4 (26.7%) | 0.89 | ||||
| Severe OSA (≥30), n (%) | 1 (5.9%) | 1 (6.7%) | 1 | ||||
| ODI, median [P25–P75] | 10.5 [3.7–14.6] | 9.2 [2.4–14.3] | 0.48 | ||||
| Snore percentage, median [P25–P75] | 24.3 [5.6–36.5] | 13.8 [2.0–29.7] | 0.89 | ||||
| Missing | 0 | 2 | |||||
| PROMs | |||||||
| SF-36, median [P25–P75], % | |||||||
| Physical functioning | 90.0 [80.0–100] | 85.0 [60.0–95.0] | 90.0 [70.0–100] | 90.0 [85.0–100] | 0.15 | 0.94 | 0.41 |
| Role limitations due to physical health | 100 [25.0–100] | 100 [62.5–100] | 100 [75.0–100] | 100 [50.0–100] | 0.58 | 0.34 | 1.00 |
| Role limitations due to emotional problems | 100 [33.3–100] | 100 [50.0–100] | 100 [100–100] | 100 [100–100] | 0.79 | 0.09 | 0.37 |
| Energy/Fatigue | 50.0 [45.0–65.0] | 45.0 [42.5–65.0] | 45.0 [35.0–70.0] | 50.0 [40.0–65.0] | 0.73 | 0.97 | 0.83 |
| Emotional wellbeing | 80.0 [56.0–88.0] | 76.0 [62.0–76.0] | 76.0 [64.0–84.0] | 84.0 [48.0–88.0] | 0.93 | 0.30 | 0.32 |
| Social functioning | 81.3 [50.0–90.6] | 75.0 [50.0–75] | 75.0 [62.5–100] | 87.5 [62.5–100] | 0.26 | 0.55 | 0.46 |
| Pain | 78.8 [45.0–82.5] | 77.5 [61.3–95.0] | 77.5 [57.5–90.0] | 67.5 [67.5–77.5] | 0.41 | 0.76 | 0.17 |
| General health | 60.0 [40.0–70.0] | 60.0 [35.0–65.0] | 65.0 [55.0–75.0] | 65.0 [35.0–70.0] | 0.33 | 0.08 | 0.80 |
| Health change | 50.0 [25.0–75.0] | 50.0 [50.0–62.5] | 50.0 [50.0–75.0] | 50.0 [50.0–75.0] | 0.17 | 0.85 | 0.42 |
| Epworth sleepiness scale, median [P25–P75] | 8.0 [5.0–13.0] | 9.0 [4.5–15.0] | 4.0 [2.5–10.5] | 4.0 [3.0–10.0] | 0.55 | 0.07 | 0.20 |
| Short-QODNS, median [P25–P75] | 21.0 [15.5–21.0] | 20.0 [15.0–21.0] | 20.0 [15.3–21.0] | 18.5 [15.3–21.0] | 0.78 | 0.85 | 1.00 |
| sVAS, median [P25–P75] | 7.5 [7.0–10] | 8.0 [7.0–10] | 8.0 [7.0–10] | 8.0 [6.5–10.0] | 0.34 | 0.68 | 0.72 |
| SNOT-22, median [P25–P75] | 31.0 [24.5–48.5] | 36.0 [29.5–48.5] | 22.5 [18.8–40.3] | 25.0 [20.0–47.0] | 0.49 | 0.42 | 0.55 |
| NOSE, median [P25–P75] | 14.0 [9.3–18.3] | 14.0 [9.5–15.0] | 7.0 [6.5–9.8] | 10.0 [7.0–13.0] | 0.03 * | 0.09 | 0.83 |
| Age | Sex (Male) | BMI | Septal Deviation | Non-Allergic Rhinitis | Random Effect (Patient) | |
|---|---|---|---|---|---|---|
| PNIF | ||||||
| Bilateral | 1.60 | |||||
| Right | 1.34 | |||||
| Left | −0.10 * | −0.22 *** | −2.42 ** | |||
| Acoustic Rhinometry | ||||||
| Right MCA1 | −0.03 ** | |||||
| Left MCA1 | +0.60 *** | +0.54 *** | ||||
| Right NV | −0.03 ** | |||||
| Left NV | +2.43 * | +2.22 * | 1.56 | |||
| AHI | −19.9 *** | |||||
| Epworth sleepiness scale | −1.30 | −2.63 | −2.41 | 4.87 | ||
| SNOT-22 | −5.42 | +0.92 | +1.29 | −11.28 | 13.65 | |
| NOSE | −6.94 ** | +1.01 | −4.49 | 1.30 | ||
| sVAS | 1.39 | |||||
| Sniffin’ Sticks (Identification) | −2.25 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pendolino, A.L.; Unadkat, S.; Cheong, R.C.T.; Patel, A.; Ferreira, J.; Scarpa, B.; Andrews, P.J. Objective and Subjective Outcomes Following Radiofrequency of Inferior Turbinates in Patients with Sleep-Disordered Breathing. Diagnostics 2024, 14, 1820. https://doi.org/10.3390/diagnostics14161820
Pendolino AL, Unadkat S, Cheong RCT, Patel A, Ferreira J, Scarpa B, Andrews PJ. Objective and Subjective Outcomes Following Radiofrequency of Inferior Turbinates in Patients with Sleep-Disordered Breathing. Diagnostics. 2024; 14(16):1820. https://doi.org/10.3390/diagnostics14161820
Chicago/Turabian StylePendolino, Alfonso Luca, Samit Unadkat, Ryan Chin Taw Cheong, Ankit Patel, Joshua Ferreira, Bruno Scarpa, and Peter J. Andrews. 2024. "Objective and Subjective Outcomes Following Radiofrequency of Inferior Turbinates in Patients with Sleep-Disordered Breathing" Diagnostics 14, no. 16: 1820. https://doi.org/10.3390/diagnostics14161820
APA StylePendolino, A. L., Unadkat, S., Cheong, R. C. T., Patel, A., Ferreira, J., Scarpa, B., & Andrews, P. J. (2024). Objective and Subjective Outcomes Following Radiofrequency of Inferior Turbinates in Patients with Sleep-Disordered Breathing. Diagnostics, 14(16), 1820. https://doi.org/10.3390/diagnostics14161820









