Kidney Dysfunction, Hepatic Impairment, and Lipid Metabolism Abnormalities in Patients with Precapillary Pulmonary Hypertension
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. ESC/ERS Scientific Document Group. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Humbert, M.; Souza, R.; Idrees, M.; Kawut, S.M.; Sliwa-Hahnle, K.; Jing, Z.C.; Gibbs, J.S. A global view of pulmonary hypertension. Lancet Respir. Med. 2016, 4, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Frost, A.; Badesch, D.; Gibbs, J.S.R.; Gopalan, D.; Khanna, D.; Manes, A.; Oudiz, R.; Satoh, T.; Torres, F.; Torbicki, A. Diagnosis of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801904. [Google Scholar] [CrossRef] [PubMed]
- Nickel, N.P.; Galura, G.M.; Zuckerman, M.J.; Hakim, M.N.; Alkhateeb, H.; Mukherjee, D.; Austin, E.D.; Heresi, G.A. Liver abnormalities in pulmonary arterial hypertension. Pulm. Circ. 2021, 11, 20458940211054304. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Rathinasabapathy, A.; Luo, J.; Yang, X.; Luo, P.; Chen, Y.; Li, Z.; Li, J. Differential serum lipid distribution in IPAH and CHD-PAH patients. Respir. Med. 2021, 191, 106711. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, S.D.; Wehbe, E.; Heresi, G.A.; Gaur, V.; Minai, O.A.; Arrigain, S.; Nally, J.V., Jr.; Schold, J.D.; Rahman, M.; Dweik, R.A. Presence and outcomes of kidney disease in patients with pulmonary hypertension. Clin. J. Am. Soc. Nephrol. 2014, 9, 855–863. [Google Scholar] [CrossRef]
- Di Lullo, L.; Floccari, F.; Rivera, R.; Barbera, V.; Granata, A.; Otranto, G.; Mudoni, A.; Malaguti, M.; Santoboni, A.; Ronco, C. Pulmonary Hypertension and Right Heart Failure in Chronic Kidney Disease: New Challenge for 21st-Century Cardionephrologists. Cardiorenal Med. 2013, 3, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Batal, O.; Khatib, O.F.; Dweik, R.A.; Hammel, J.P.; McCarthy, K.; Minai, O.A. Comparison of baseline predictors of prognosis in pulmonary arterial hypertension in patients surviving ≤2 years and those surviving ≥5 years after baseline right-sided cardiac catheterization. Am. J. Cardiol. 2012, 109, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Haddad, F.; Fuh, E.; Peterson, T.; Skhiri, M.; Kudelko, K.T.; De Jesus Perez, V.; Winkelmayer, W.C.; Doyle, R.L.; Chertow, G.M.; Zamanian, R.T. Incidence, correlates, and consequences of acute kidney injury in patients with pulmonary arterial hypertension hospitalized with acute right-side heart failure. J. Card. Fail. 2011, 17, 533–539. [Google Scholar] [CrossRef]
- Howard, L.S. Prognostic factors in pulmonary arterial hypertension: Assessing the course of the disease. Eur. Respir. Rev. 2011, 20, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Haapio, M.; House, A.A.; Anavekar, N.; Bellomo, R. Cardiorenal syndrome. J. Am. Coll. Cardiol. 2008, 52, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- Afsar, B.; Ortiz, A.; Covic, A.; Gaipov, A.; Esen, T.; Goldsmith, D.; Kanbay, M. Phosphodiesterase type 5 inhibitors and kidney disease. Int. Urol. Nephrol. 2015, 47, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Goddard, J.; Johnston, N.R.; Hand, M.F.; Cumming, A.D.; Rabelink, T.J.; Rankin, A.J.; Webb, D.J. Endothelin-A receptor antagonism reduces blood pressure and increases renal blood flow in hypertensive patients with chronic renal failure: A comparison of selective and combined endothelin receptor blockade. Circulation 2004, 109, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Stasch, J.P.; Pacher, P.; Evgenov, O.V. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 2011, 123, 2263–2273. [Google Scholar] [CrossRef] [PubMed]
- Khaybullina, D.; Patel, A.; Zerilli, T. Riociguat (adempas): A novel agent for the treatment of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Pharm. Ther. 2014, 39, 749–758. [Google Scholar]
- Nickel, N.P.; O’Leary, J.M.; Brittain, E.L.; Fessel, J.P.; Zamanian, R.T.; West, J.D.; Austin, E.D. Kidney dysfunction in patients with pulmonary arterial hypertension. Pulm. Circ. 2017, 7, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, M.; Laurenzi, M.; Mancini, M.; Zanchetti, A.; Lombardi, C.; De Santo, N.G. Low glomerular filtration in the population: Prevalence, associated disorders, and awareness. Kidney Int. 2006, 70, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Coresh, J.; Byrd-Holt, D.; Astor, B.C.; Briggs, J.P.; Eggers, P.W.; Lacher, D.A.; Hostetter, T.H. Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J. Am. Soc. Nephrol. 2005, 16, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Pływaczewska, M.; Pruszczyk, P.; Kostrubiec, M. Does kidney function matter in pulmonary thromboembolism management? Cardiol. J. 2022, 29, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Nickel, N.P.; Yuan, K.; Dorfmuller, P.; Provencher, S.; Lai, Y.C.; Bonnet, S.; Austin, E.D.; Koch, C.D.; Morris, A.; Perros, F.; et al. Beyond the Lungs: Systemic Manifestations of Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2020, 201, 148–157. [Google Scholar] [CrossRef]
- Bolignano, D.; Rastelli, S.; Agarwal, R.; Fliser, D.; Massy, Z.; Ortiz, A.; Wiecek, A.; Martinez-Castelao, A.; Covic, A.; Goldsmith, D.; et al. Pulmonary hypertension in CKD. Am. J. Kidney Dis. 2013, 61, 612–622. [Google Scholar] [CrossRef]
- Chakinala, M.M.; Coyne, D.W.; Benza, R.L.; Frost, A.E.; McGoon, M.D.; Hartline, B.K.; Frantz, R.P.; Selej, M.; Mink, D.R.; Farber, H.W. Predicting outcomes in pulmonary arterial hypertension based on estimated glomerular filtration rate. In C106. Wrecking Ball: New Paradigms in Understanding and Treatment of PH; American Thoracic Society: New York, NY, USA, 2016; Available online: https://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2016.193.1_MeetingAbstracts.A6316 (accessed on 10 April 2024).
- Samsky, M.D.; Patel, C.B.; DeWald, T.A.; Smith, A.D.; Felker, G.M.; Rogers, J.G.; Hernandez, A.F. Cardiohepatic interactions in heart failure: An overview and clinical implications. J. Am. Coll. Cardiol. 2013, 61, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Lightsey, J.M.; Rockey, D.C. Current concepts in ischemic hepatitis. Curr. Opin. Gastroenterol. 2017, 33, 158–163. [Google Scholar] [CrossRef]
- Coz Yataco, A.; Aguinaga Meza, M.; Buch, K.P.; Disselkamp, M.A. Hospital and intensive care unit management of decompensated pulmonary hypertension and right ventricular failure. Heart Fail Rev. 2016, 21, 323–346. [Google Scholar] [CrossRef]
- Ess, M.; Mussner-Seeber, C.; Mariacher, S.; Lorsbach-Koehler, A.; Pachinger, O.; Frick, M.; Ulmer, H.; Poelzl, G. γ-Glutamyltransferase rather than total bilirubin predicts outcome in chronic heart failure. J. Card. Fail. 2011, 17, 577–584. [Google Scholar] [CrossRef]
- Van Deursen, V.M.; Damman, K.; Hillege, H.L.; van Beek, A.P.; van Veldhuisen, D.J.; Voors, A.A. Abnormal liver function in relation to hemodynamic profile in heart failure patients. J. Card. Fail. 2010, 16, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Poelzl, G.; Ess, M.; Mussner-Seeber, C.; Pachinger, O.; Frick, M.; Ulmer, H. Liver dysfunction in chronic heart failure: Prevalence, characteristics and prognostic significance. Eur. J. Clin. Investig. 2012, 42, 153–163. [Google Scholar] [CrossRef]
- Benza, R.L.; Miller, D.P.; Gomberg-Maitland, M.; Frantz, R.P.; Foreman, A.J.; Coffey, C.S.; Frost, A.; Barst, R.J.; Badesch, D.B.; Elliott, C.G.; et al. Predicting survival in pulmonary arterial hypertension: Insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010, 122, 164–172. [Google Scholar] [CrossRef]
- Stepnowska, E.; Lewicka, E.; Dąbrowska-Kugacka, A.; Daniłowicz-Szymanowicz, L.; Zagożdżon, P.; Kamiński, R.; Lewicka-Potocka, Z.; Miękus, P.; Kozłowski, D.; Potocki, W.; et al. Predictors of poor outcome in patients with pulmonary arterial hypertension: A single center study. PLoS ONE 2018, 13, e0193245. [Google Scholar] [CrossRef]
- Takeda, Y.; Takeda, Y.; Tomimoto, S.; Tani, T.; Narita, H.; Kimura, G. Bilirubin as a prognostic marker in patients with pulmonary arterial hypertension. BMC Pulm. Med. 2010, 10, 22. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wang, N.; Gu, Z.C.; Wei, A.H.; Cheng, A.N.; Fang, S.S.; Du, H.L.; Wang, L.Z.; Zhang, G.Q. A network meta-analysis for safety of endothelin receptor antagonists in pulmonary arterial hypertension. Cardiovasc. Diagn. Ther. 2019, 9, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Matos, W.F.; Intriago, C.; Thakkar, K.; Jahan, N.; Shah, H.; Nishu, R.I.; Marzban, S. Use of Epoprostenol in the Treatment of Pulmonary Arterial Hypertension. Cureus 2021, 13, e18191. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, P.; Cruz, H.G.; Krause, A.; Ulč, I.; Halabi, A.; Dingemanse, J. Pharmacokinetics of the novel oral prostacyclin receptor agonist selexipag in subjects with hepatic or renal impairment. Br. J. Clin. Pharmacol. 2016, 82, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Graziano, S.; Montana, A.; Zaami, S.; Rotolo, M.C.; Minutillo, A.; Busardò, F.P.; Marinelli, E. Sildenafil-associated hepatoxicity: A review of the literature. Eur. Rev. Med. Pharmacol. Sci. 2017, 21 (Suppl. S1), 17–22. [Google Scholar] [PubMed]
- Chaumais, M.C.; Guignabert, C.; Savale, L.; Jaïs, X.; Boucly, A.; Montani, D.; Simonneau, G.; Humbert, M.; Sitbon, O. Clinical pharmacology of endothelin receptor antagonists used in the treatment of pulmonary arterial hypertension. Am. J. Cardiovasc. Drugs 2015, 15, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Segal, E.S.; Valette, C.; Oster, L.; Bouley, L.; Edfjall, C.; Herrmann, P.; Raineri, M.; Kempff, M.; Beacham, S.; van Lierop, C. Risk management strategies in the postmarketing period: Safety experience with the US and European bosentan surveillance programmes. Drug Saf. 2005, 28, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Bowman, L.; D’Arsigny, C.L.; Archer, S.L. Soluble guanylate cyclase: A new therapeutic target for pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Clin. Pharmacol. Ther. 2015, 97, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.H.; Peng, F.H.; Wei, H.; He, J.; Chen, F.D.; Di, R.M.; Jiang, X.; Jiang, R.; Chen, Y.J.; Heresi, G.A.; et al. Serum high-density lipoprotein cholesterol levels as a prognostic indicator in patients with idiopathic pulmonary arterial hypertension. Am. J. Cardiol. 2012, 110, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Kopeć, G.; Waligóra, M.; Tyrka, A.; Jonas, K.; Pencina, M.J.; Zdrojewski, T.; Moertl, D.; Stokwiszewski, J.; Zagożdżon, P.; Podolec, P. Low-density lipoprotein cholesterol and survival in pulmonary arterial hypertension. Sci. Rep. 2017, 7, 41650. [Google Scholar] [CrossRef]
- West, J.; Niswender, K.D.; Johnson, J.A.; Pugh, M.E.; Gleaves, L.; Fessel, J.P.; Hemnes, A.R. A potential role for insulin resistance in experimental pulmonary hypertension. Eur. Respir. J. 2013, 41, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Hemnes, A.R.; Brittain, E.L.; Trammell, A.W.; Fessel, J.P.; Austin, E.D.; Penner, N.; Maynard, K.B.; Gleaves, L.; Talati, M.; Absi, T.; et al. Evidence for right ventricular lipotoxicity in heritable pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2014, 189, 325–334. [Google Scholar] [CrossRef]
- Gopal, D.M.; Santhanakrishnan, R.; Wang, Y.C.; Ayalon, N.; Donohue, C.; Rahban, Y.; Perez, A.J.; Downing, J.; Liang, C.S.; Gokce, N.; et al. Impaired right ventricular hemodynamics indicate preclinical pulmonary hypertension in patients with metabolic syndrome. J. Am. Heart Assoc. 2015, 4, e001597. [Google Scholar] [CrossRef]
- Ussavarungsi, K.; Thomas, C.S.; Burger, C.D. Prevalence of metabolic syndrome in patients with pulmonary hypertension. Clin. Respir. J. 2017, 11, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Robbins, I.M.; Newman, J.H.; Johnson, R.F.; Hemnes, A.R.; Fremont, R.D.; Piana, R.N.; Zhao, D.X.; Byrne, D.W. Association of the metabolic syndrome with pulmonary venous hypertension. Chest 2009, 136, 31–36. [Google Scholar] [CrossRef]
- Khirfan, G.; Tejwani, V.; Wang, X.; Li, M.; DiDonato, J.; Dweik, R.A.; Smedira, N.; Heresi, G.A. Plasma levels of high density lipoprotein cholesterol and outcomes in chronic thromboembolic pulmonary hypertension. PLoS ONE 2018, 13, e0197700. [Google Scholar]
- Feingold, K.R.; Grunfeld, C. The Effect of Inflammation and Infection on Lipids and Lipoproteins. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; Endotext [Internet]: South Dartmouth, MA, USA, 2022. [Google Scholar]
- Price, L.C.; Wort, S.J.; Perros, F.; Dorfmüller, P.; Huertas, A.; Montani, D.; Cohen-Kaminsky, S.; Humbert, M. Inflammation in pulmonary arterial hypertension. Chest 2012, 141, 210–221. [Google Scholar] [CrossRef]
- Gajecki, D.; Gawrys, J.; Szahidewicz-Krupska, E.; Doroszko, A. Novel Molecular Mechanisms of Pulmonary Hypertension: A Search for Biomarkers and Novel Drug Targets-From Bench to Bed Site. Oxid. Med. Cell Longev. 2020, 2020, 7265487. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, J.M.; Assad, T.R.; Xu, M.; Birdwell, K.A.; Farber-Eger, E.; Wells, Q.S.; Hemnes, A.R.; Brittain, E.L. Pulmonary hypertension in patients with chronic kidney disease: Invasive hemodynamic etiology and outcomes. Pulm. Circ. 2017, 7, 674–683. [Google Scholar] [CrossRef]
- Shah, S.J.; Thenappan, T.; Rich, S.; Tian, L.; Archer, S.L.; Gomberg-Maitland, M. Association of serum creatinine with abnormal hemodynamics and mortality in pulmonary arterial hypertension. Circulation 2008, 117, 2475–2483. [Google Scholar] [CrossRef]
- Forfia, P.R.; Mathai, S.C.; Fisher, M.R.; Housten-Harris, T.; Hemnes, A.R.; Champion, H.C.; Girgis, R.E.; Hassoun, P.M. Hyponatremia predicts right heart failure and poor survival in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2008, 177, 1364–1369. [Google Scholar] [CrossRef] [PubMed]
- Benza, R.L.; Gomberg-Maitland, M.; Miller, D.P.; Frost, A.; Frantz, R.P.; Foreman, A.J.; Badesch, D.B.; McGoon, M.D. The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest 2012, 141, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013, 128, e240–e327. [Google Scholar] [CrossRef] [PubMed]
- Testani, J.M.; Khera, A.V.; St John Sutton, M.G.; Keane, M.G.; Wiegers, S.E.; Shannon, R.P.; Kirkpatrick, J.N. Effect of right ventricular function and venous congestion on cardiorenal interactions during the treatment of decompensated heart failure. Am. J. Cardiol. 2010, 105, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, P.M. Pulmonary Arterial Hypertension. N. Engl. J. Med. 2021, 385, 2361–2376. [Google Scholar] [CrossRef]
- Butler, J.; Hernandez, A.F.; Anstrom, K.J.; Kalogeropoulos, A.; Redfield, M.M.; Konstam, M.A.; Tang, W.H.; Felker, G.M.; Shah, M.R.; Braunwald, E. Rationale and Design of the ATHENA-HF Trial: Aldosterone Targeted Neurohormonal Combined with Natriuresis Therapy in Heart Failure. JACC Heart Fail. 2016, 4, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, B.; Peterson, L.; Comhair, S.A.A.; Sharp, J.; Park, M.M.; Tang, W.H.W.; Neumann, D.R.; DiFilippo, F.P.; Farha, S.; Erzurum, S.C.; et al. Blood Cholesterol and Triglycerides Associate with Right Ventricular Function in Pulmonary Hypertension. medRxiv 2024, preprint. [Google Scholar] [CrossRef]
- Horwich, T.B.; Hamilton, M.A.; Maclellan, W.R.; Fonarow, G.C. Low serum total cholesterol is associated with marked increase in mortality in advanced heart failure. J. Card. Fail. 2002, 8, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.F.; Guan, L.H.; Zhou, D.X.; Chen, D.D.; Zhang, X.C.; Ge, J.B. Serum High-Density Lipoprotein Cholesterol is Significantly Associated with the Presence and Severity of Pulmonary Arterial Hypertension: A Retrospective Cross-Sectional Study. Adv. Ther. 2020, 37, 2199–2209. [Google Scholar] [CrossRef] [PubMed]
- Hemnes, A.R.; Luther, J.M.; Rhodes, C.J.; Burgess, J.P.; Carlson, J.; Fan, R.; Fessel, J.P.; Fortune, N.; Gerszten, R.E.; Halliday, S.J.; et al. Human PAH is characterized by a pattern of lipid-related insulin resistance. JCI Insight 2019, 4, e123611. [Google Scholar] [CrossRef] [PubMed]
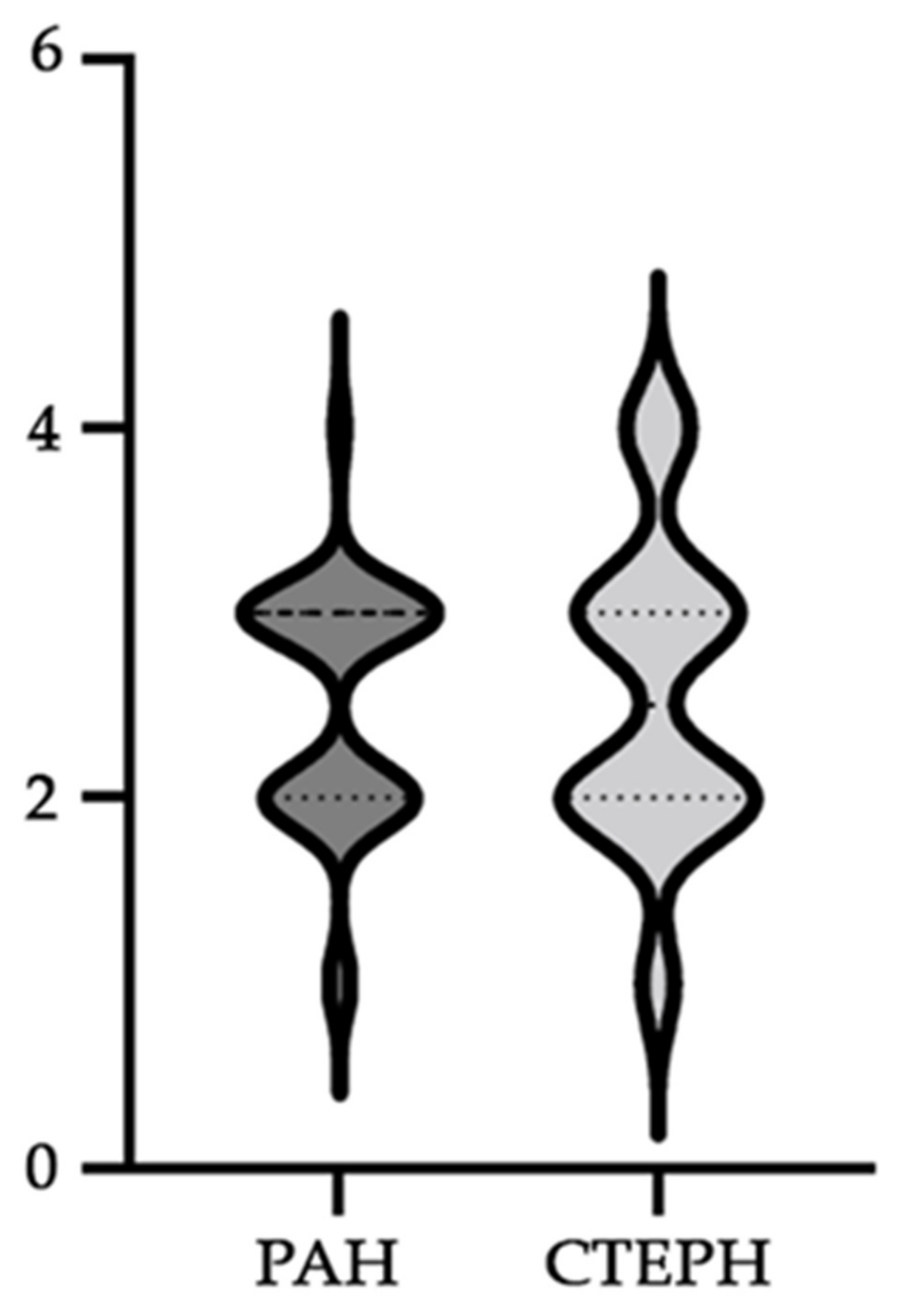


| General Characteristics | PAH Patients | CTEPH Patients | p-Value |
|---|---|---|---|
| (n = 35) | (n = 14) | ||
| Sex (female) | 22 (62.85) | 8 (57.1) | ns |
| Age (years) | 47 (27–63) | 63.5 (53.75–69) | 0.0142 |
| BMI (kg/m2) | 24.68 (21.09–29.68) | 31.07 (26.31–32.52) | 0.0149 |
| Heart rate (bpm) | 85 (70–92) | 76 (69.75–93.75) | ns |
| Systolic BP (mmHg) | 120 (110–130) | 117.5 (110–131.3) | ns |
| Diastolic BP (mmHg) | 70 (60–85) | 80 (70–82.5) | ns |
| Mean BP (mmHg, IQR) | 86.67 (78.33–100) | 91.67 (83.33–97.92) | ns |
| Time from the onset of symptoms to diagnosis (months, median, IQR) | 12.00 (6.00–24.00) | 12.00 (5.25–17.25) | ns |
| 6-MWD (m) | |||
| At inclusion | 400 (255–455) | 300 (95–387) | 0.0345 |
| At 6 months | 430 (283–465) | 367 (195–422) | ns |
| At 12 months | 439 (293–473.5) | 386.5 (253.8–492.5) | ns |
| NT-proBNP (pg/mL, median, IQR) | |||
| Baseline | 400 (145–2619) | 1243 (340.5–5843) | ns |
| At 6 months | 1106 (263.5–2128) | 1666 (528–3997) | ns |
| At 12 months | 485.5 (144.3–1402) | 1465 (388–4626) | 0.045 |
| Right heart catheterization data | |||
| mPAP (mmHg) | 51.22 ± 17.14 | 42.50 ± 10.67 | ns |
| PAWP (mmHg) | 11.00 (6.50–13.00) | 11.50 (8.75–14.50) | ns |
| PVR (WU) | 10.00 (4.01–14.00) | 9.50 (5.50–12.00) | ns |
| CO (l/min) | 5.14 ± 2.07 | 4.57 ± 2.04 | ns |
| CI (L/min/m2) | 2.95 (2.19–3.70) | 2.38 (1.90–2.65) | ns |
| Proximal vessel disease (n, %) | N/A | 7 (50) |
| PAH Patients | CTEPH Patients | p-Value | |||
|---|---|---|---|---|---|
| Median WHO functional class (IQR) | |||||
| Baseline | 3.00 (2.00–3.00) | 2.50 (2.00–3.00) | ns | ||
| At 12 months | 2.00 (2.00–3.00) | 2.00 (2.00–3.00) | ns | ||
| WHO functional class | Baseline | At 12 months | Baseline | At 12 months | |
| I (n, %) | 2 (5.72) | 2 (5.72) | 1 (7.15) | 2 (14.28) | |
| II (n, %) | 14 (40) | 17 (48.57) | 6 (42.85) | 6 (42.85) | |
| III (n, %) | 18 (51.43) | 16 (45.71) | 5 (35.72) | 5 (35.72) | |
| IV (n, %) | 1 (2.85) | 0 | 2 (14.28) | 1 (7.15) | |
| Median severity of right heart failure (IQR) | |||||
| Baseline | 2.00 (1.00–2.00) | 2.00 (1.00–2.00) | ns | ||
| At 12 months | 2.00 (1.00–2.00) | 2.00 (1.25–2.00) | ns | ||
| Severity of right heart failure | Baseline | At 12 months | Baseline | At 12 months | |
| Normal (n, %) | 19 (54.28) | 25 (71.42) | 6 (42.85) | 9 (64.28) | |
| Mild/Moderate (n, %) | 13 (37.14) | 10 (28.58) | 5 (35.72) | 4 (28.57) | |
| Severe (n, %) | 3 (8.58) | 0 | 3 (21.43) | 1 (7.15) | |
| Duration of the PH (months, IQR) | 30.00 (13.00–113.50) | 20.00 (14.50–34.00) | ns | ||
| PAH Patients | CTEPH Patients | p | |
|---|---|---|---|
| T2DM (n, %) | 7 (20.00) | 5 (35.71) | ns |
| HTN (n, %) | 11 (31.42) | 7 (50.00) | ns |
| CHF (n, %) | 20 (57.1) | 10 (71.42) | ns |
| Coronary heart disease (n, %) | 1 (2.85) | 2 (14.28) | ns |
| Deep vein thrombosis (n, %) | 0 | 14 (100.0) | <0.0001 |
| Atrial fibrillation (n, %) | 11 (31.42) | 6 (42.85) | ns |
| Thyroid disease (n, %) | 11 (31.42) | 3 (21.42) | ns |
| Obstructive sleep apnea (n, %) | 2 (5.71) | 3 (21.42) | ns |
| Lung disease—other than COPD and asthma (n, %) | 12 (34.28) | 4 (28.57) | ns |
| COPD (n, %) | 4 (11.42) | 1 (7.14) | ns |
| Asthma (n, %) | 4 (11.42) | 2 (14.28) | ns |
| SARS-CoV-2 (n, %) | 12 (34.28) | 4 (28.57) | ns |
| Parameters | PAH Patients (n = 35) | CTEPH Patients (n = 14) | p |
|---|---|---|---|
| Kidney dysfunction (n, %) | |||
| Baseline | 1 (2.86) | 5 (35.71) | 0.0052 |
| At 6 months | 3 (8.57) | 5 (35.71) | 0.0333 |
| At 12 months | 1 (2.86) | 3 (21.43) | 0.0052 |
| Creatinine (mg/dL) | |||
| Baseline | 0.83 (0.75–0.96) | 1.08 (0.96–1.35) | 0.0011 |
| At 6 months | 0.83 (0.72–0.96) | 0.94 (0.82–1.37) | 0.031 |
| At 12 months | 0.84 (0.73–0.96) | 0.91 (0.86–1.23) | ns |
| eGFR (mL/min/1.73 m2) | |||
| Baseline | 94.66 ± 22.88 | 67.84 ± 19.7 | 0.0004 |
| At 6 months | 96.6 ± 22.91 | 69.57 ± 26.37 | 0.0008 |
| At 12 months | 96.98 ± 21.12 | 74.48 ± 24.66 | 0.0024 |
| Hepatic impairment (n, %) | |||
| Baseline | 17 (48.57) | 11(78.57) | ns |
| At 6 months | 16 (45.71) | 10(71.43) | ns |
| At 12 months | 10 (28.57) | 11(78.57) | 0.0031 |
| Total bilirubin (mg/dL) | |||
| Baseline | 0.9 (0.6–1.4) | 1.2 (0.77–1.72) | ns |
| At 6 months | 0.8 (0.5–1.3) | 0.9 (0.67–1.55) | ns |
| At 12 months | 0.7 (0.5–1.1) | 0.9 (0.77–1.52) | 0.0384 |
| GGT (U/L) | |||
| Baseline | 30 (22–57) | 56 (35.5–79.5) | 0.013 |
| At 6 months | 29 (23–66) | 59.5 (29.75–86) | 0.0424 |
| At 12 months | 26 (21–43) | 59.5 (31.75–180) | 0.0031 |
| AST (U/L) | |||
| Baseline | 22 (17–28) | 21 (16.75–27) | ns |
| At 6 months | 21 (15–25) | 18 (17.5–25.5) | ns |
| At 12 months | 20 (16–27) | 22 (19–25.25) | ns |
| ALT (U/L) | |||
| Baseline | 15 (11–23) | 18 (13.75–27.5) | ns |
| At 6 months | 16 (12–23) | 17.5 (12.5–26.75) | ns |
| At 12 months | 16 (10–23) | 19 (16.5–26.75) | ns |
| ALP (U/L) | |||
| Baseline | 196 (173–236) | 247.5 (151.8–351.3) | ns |
| At 6 months | 186 (149–288) | 266 (195.8–400.5) | 0.05 |
| At 12 months | 187 (156–257) | 240.5 (202.3–375) | 0.0169 |
| Lipid metabolism abnormalities | |||
| Baseline | 6 (17.14) | 5 (35.71) | ns |
| At 6 months | 3 (8.57) | 3 (21.43) | ns |
| At 12 months | 8 (22.86) | 4 (28.57) | ns |
| Total cholesterol (mmol/L) | |||
| Baseline | 4.29 ± 0.95 | 4.59 ± 1.75 | ns |
| At 6 months | 4.08 ± 0.65 | 4.31 ± 1.44 | ns |
| At 12 months | 4.15 ± 0.94 | 4.23 ± 1.16 | ns |
| Triglycerides (mmol/L) | |||
| Baseline | 1.07 (0.78–1.35) | 1.15 (0.91–1.84) | ns |
| At 6 months | 0.97 (0.71–1.16) | 1.23 (0.87–1.62) | ns |
| At 12 months | 1.01 (0.63–1.43) | 1.39 (0.91–1.89) | ns |
| Medication | PAH (n = 35) | CTEPH (n = 14) | p |
|---|---|---|---|
| ERA | |||
| Baseline | 5 (14.29) | 2 (14.29) | ns |
| At 6 months | 3 (8.57) | 3 (21.43) | ns |
| At 12 months | 2 (5.71) | 2 (14.29) | ns |
| PDE5i | |||
| Baseline | 14 (40) | 5 (35.71) | ns |
| At 6 months | 0 (0) | 6 (17.14) | |
| At 12 months | 0 (0) | 6 (17.14) | |
| ERA + PDE5i | |||
| Baseline | 15 (42.86) | 5 (35.71) | ns |
| At 6 months | 25 (71.43) | 8 (57.14) | ns |
| At 12 months | 26 (74.29) | 8 (57.14) | ns |
| SGCSs | |||
| Baseline | 1 (2.86) | 3 (21.43) | ns |
| At 6 months | 1 (2.86) | 4 (28.57) | 0.0194 |
| At 12 months | 1 (2.86) | 5 (35.71) | 0.0052 |
| ACEI/ARB | |||
| Baseline | 5 (14.71) | 0 (0) | |
| At 6 months | 2 (5.71) | 0 (0) | |
| At 12 months | 3 (8.57) | 0 (0) | |
| Loop diuretics | |||
| Baseline | 29 (82.86) | 14 (100) | ns |
| At 6 months | 32 (91.42) | 14 (100 | ns |
| At 12 months | 32 (91.42) | 14 (100 | ns |
| Spironolactone | |||
| Baseline | 29 (82.86) | 14 (100) | ns |
| At 6 months | 33 (94.29) | 14 (100 | ns |
| At 12 months | 33 (94.29) | 14 (100 | ns |
| Statins | |||
| Baseline | 7 (20) | 4 (28.57) | ns |
| At 6 months | 5 (14.29) | 5 (35.71) | ns |
| At 12 months | 5 (14.29) | 6 (42.86) | ns |
| Beta-blockers | |||
| Baseline | 11 (31.43) | 10 (71.43) | 0.0233 |
| At 6 months | 14 (40) | 8 (57.14) | ns |
| At 12 months | 16 (45.71) | 8 (57.14) | ns |
| If channel blocker | |||
| Baseline | 9 (25.71) | 3 (21.43) | ns |
| At 6 months | 8 (22.86) | 3 (21.43) | ns |
| At 12 months | 7 (20) | 2 (14.29) | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iancu, D.G.; Varga, A.; Cristescu, L.; Dumbrava, R.A.; Stoica, F.; Moldovan, D.A.; Suteu, R.A.; Tilea, I. Kidney Dysfunction, Hepatic Impairment, and Lipid Metabolism Abnormalities in Patients with Precapillary Pulmonary Hypertension. Diagnostics 2024, 14, 1824. https://doi.org/10.3390/diagnostics14161824
Iancu DG, Varga A, Cristescu L, Dumbrava RA, Stoica F, Moldovan DA, Suteu RA, Tilea I. Kidney Dysfunction, Hepatic Impairment, and Lipid Metabolism Abnormalities in Patients with Precapillary Pulmonary Hypertension. Diagnostics. 2024; 14(16):1824. https://doi.org/10.3390/diagnostics14161824
Chicago/Turabian StyleIancu, Dragos Gabriel, Andreea Varga, Liviu Cristescu, Robert Adrian Dumbrava, Florin Stoica, Diana Andreea Moldovan, Radu Adrian Suteu, and Ioan Tilea. 2024. "Kidney Dysfunction, Hepatic Impairment, and Lipid Metabolism Abnormalities in Patients with Precapillary Pulmonary Hypertension" Diagnostics 14, no. 16: 1824. https://doi.org/10.3390/diagnostics14161824





