Polygenic Risk Score (PRS) Combined with NGS Panel Testing Increases Accuracy in Hereditary Breast Cancer Risk Estimation
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Information
2.2. PRS Calculation
2.3. Statistical Analysis
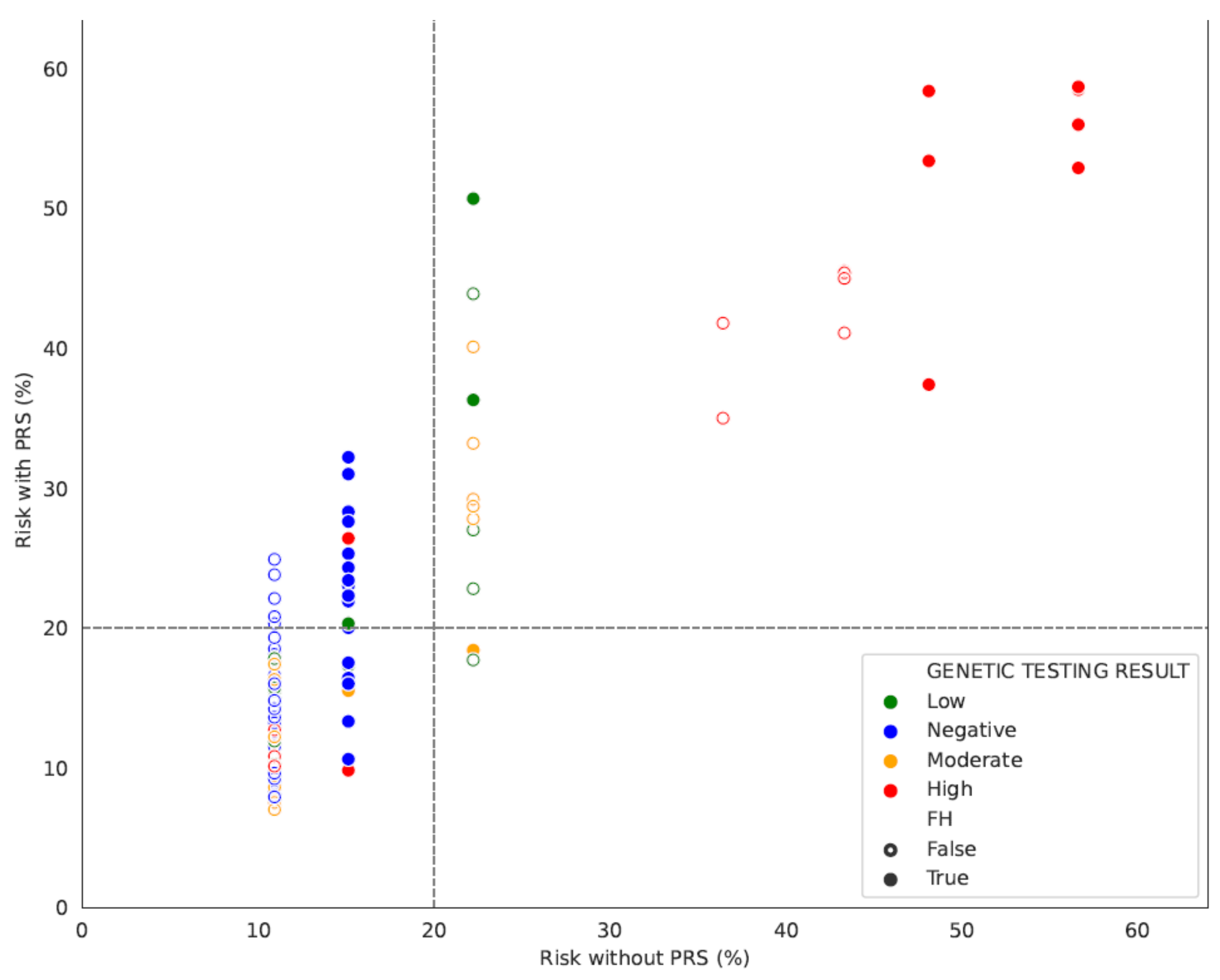
3. Results
3.1. PRS Results
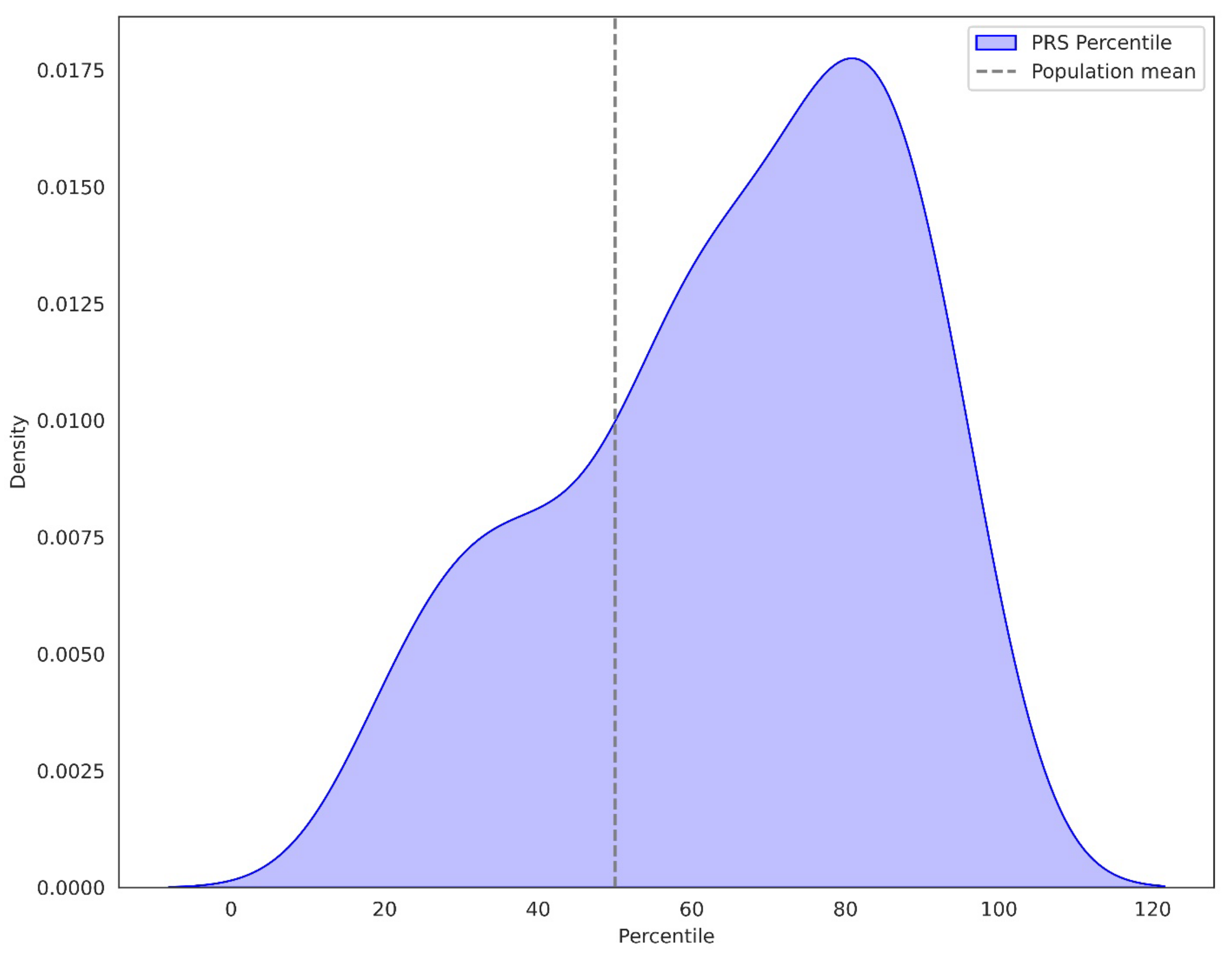
3.1.1. Percentile of PRS in BC Patients
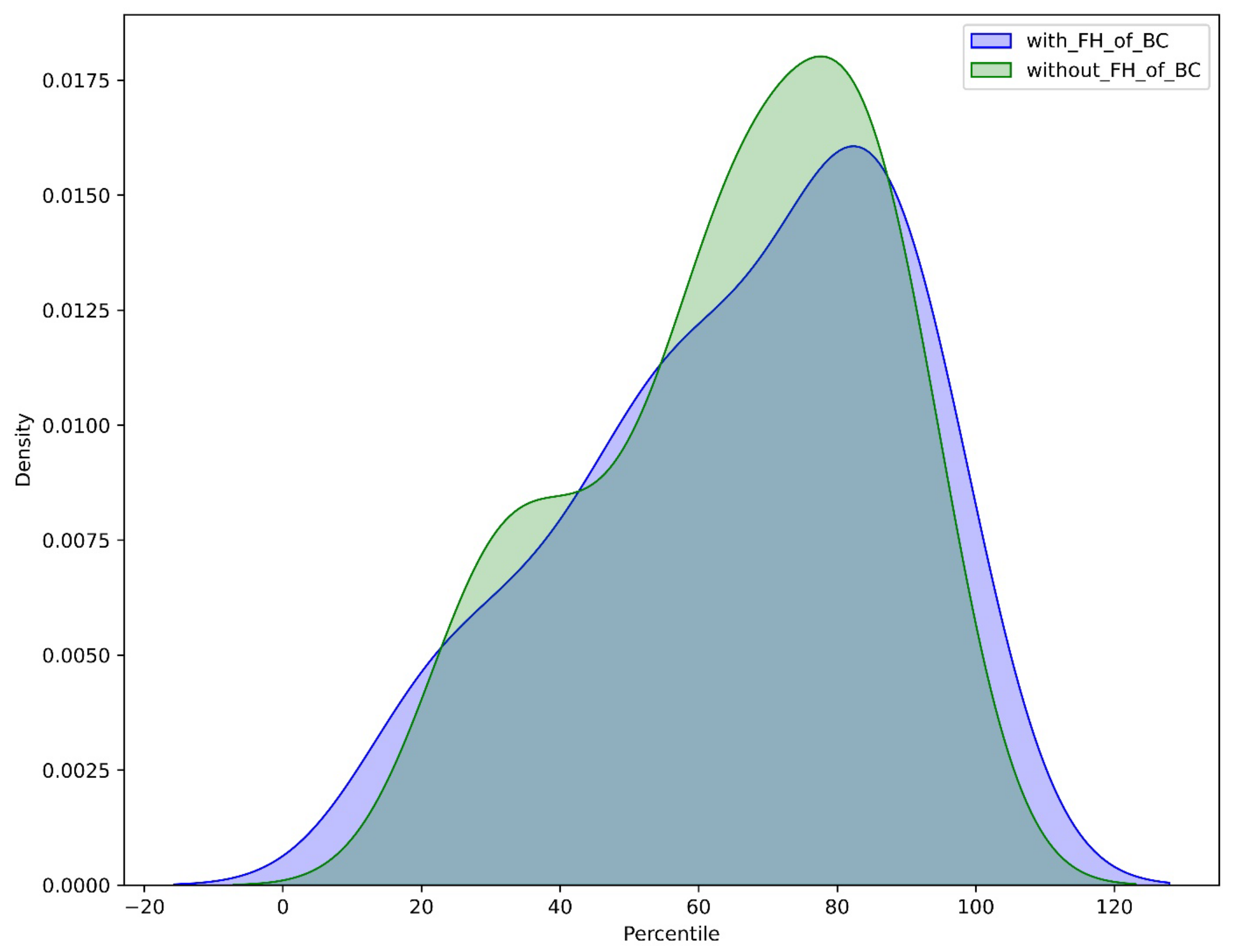
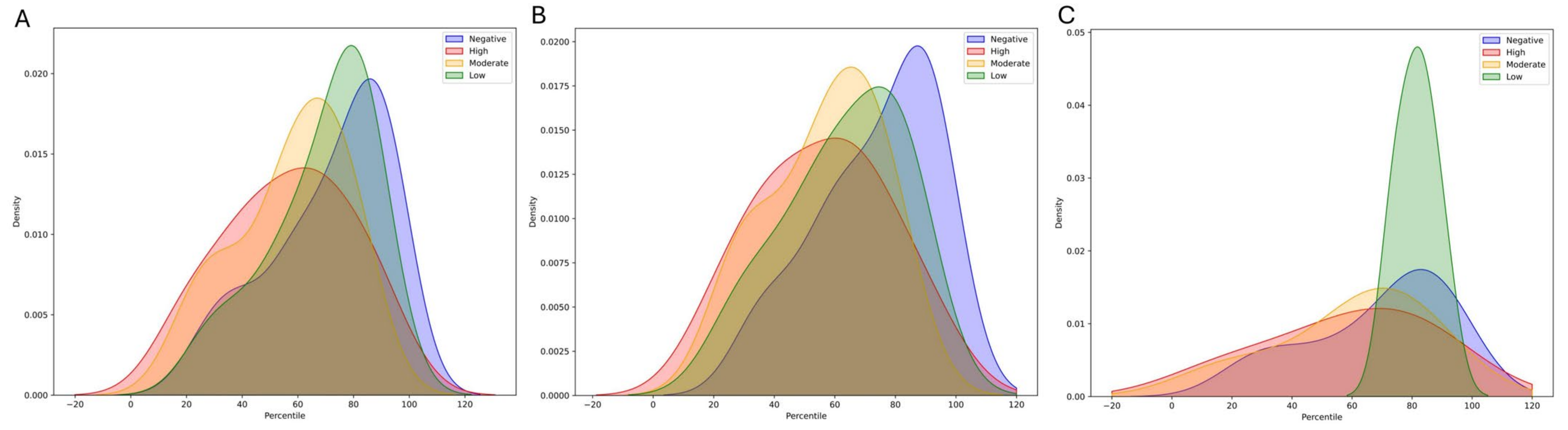
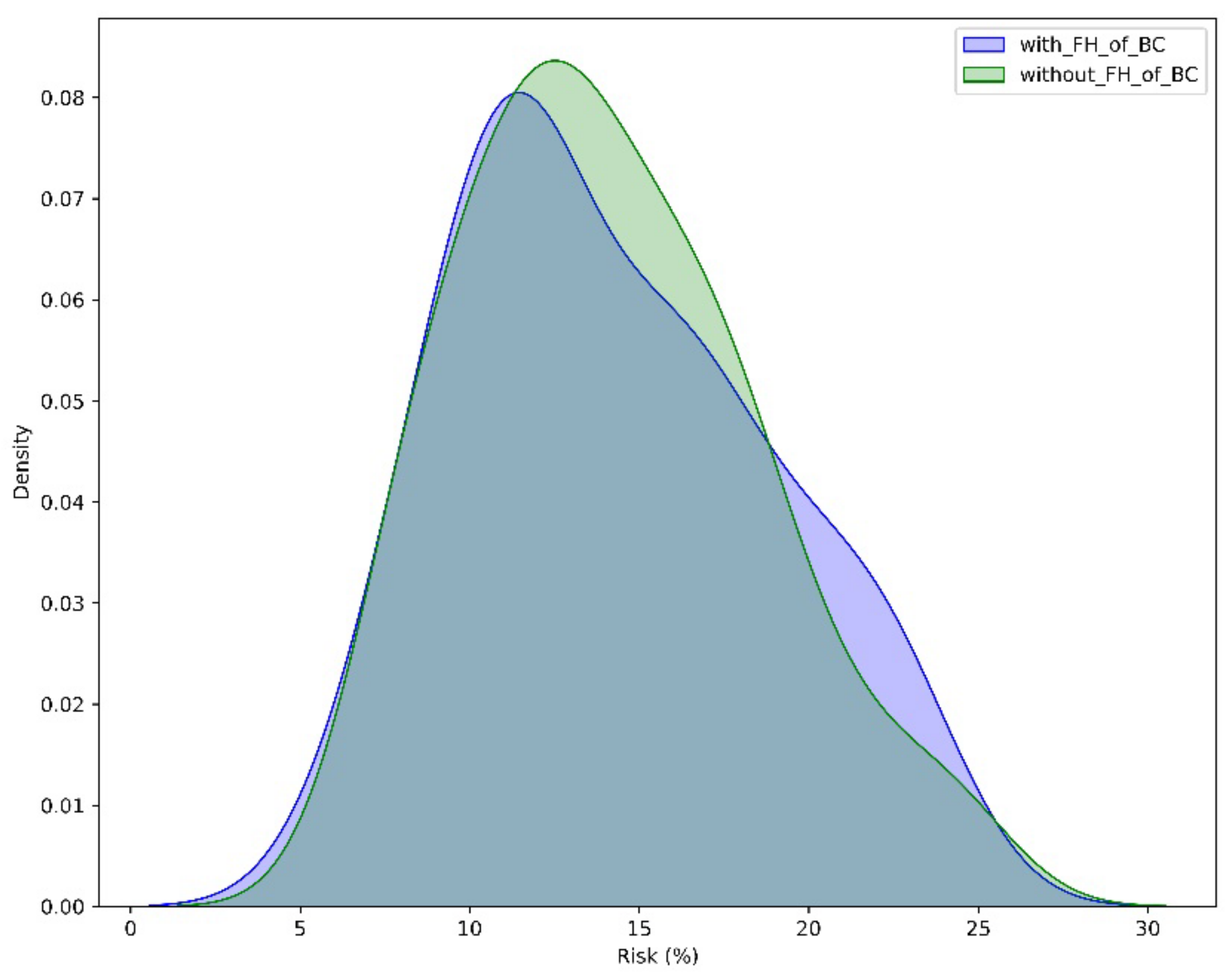
3.1.2. The Contribution of FH and Gene Status in Accurate Risk Estimation
4. Discussion
4.1. PRS-Based BC Risk Estimations
4.2. Clinical Applicability of PRS-Guided BC Risk Assessment
4.3. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar]
- Breast Cancer. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 2 May 2024).
- Løyland, B.; Sandbekken, I.H.; Grov, E.K.; Utne, I. Causes and Risk Factors of Breast Cancer, What Do We Know for Sure? An Evidence Synthesis of Systematic Reviews and Meta-Analyses. Cancers 2024, 16, 1583. [Google Scholar] [CrossRef]
- Roberts, E.; Howell, S.; Evans, D.G. Polygenic risk scores and breast cancer risk prediction. Breast 2023, 67, 71–77. [Google Scholar]
- Wang, Y.; Zhu, M.; Ma, H.; Shen, H. Polygenic risk scores: The future of cancer risk prediction, screening, and precision prevention. Med. Rev. 2021, 1, 129–149. [Google Scholar]
- Lambert, S.A.; Abraham, G.; Inouye, M. Towards clinical utility of polygenic risk scores. Hum. Mol. Genet. 2019, 28, 133–142. [Google Scholar]
- Gallagher, S.; Hughes, E.; Wagner, S.; Tshiaba, P.; Rosenthal, E.; Roa, B.B.; Kurian, A.W.; Domchek, S.M.; Garber, J.; Lancaster, J.; et al. Association of a Polygenic Risk Score with Breast Cancer among Women Carriers of High- and Moderate-Risk Breast Cancer Genes. JAMA Netw. Open 2020, 3, e208501. [Google Scholar]
- Gao, C.; Polley, E.C.; Hart, S.N.; Huang, H.; Hu, C.; Gnanaolivu, R.; Lilyquist, J.; Boddicker, N.J.; Na, J.; Ambrosone, C.B.; et al. Risk of Breast Cancer among Carriers of Pathogenic Variants in Breast Cancer Predisposition Genes Varies by Polygenic Risk Score. J. Clin. Oncol. 2021, 39, 2564–2573. [Google Scholar] [CrossRef]
- Lakeman, I.M.M.; Rodríguez-Girondo, M.D.M.; Lee, A.; Celosse, N.; Braspenning, M.E.; van Engelen, K.; van de Beek, I.; van der Hout, A.H.; Gómez García, E.B.; Mensenkamp, A.R.; et al. Clinical Applicability of the Polygenic Risk Score for Breast Cancer Risk Prediction in Familial Cases. J. Med. Genet. 2023, 60, 327–336. [Google Scholar] [CrossRef]
- Kamps, R.; Brandão, R.D.; van den Bosch, B.J.; Paulussen, A.D.; Xanthoulea, S.; Blok, M.J.; Romano, A. Next-Generation Sequencing in Oncology: Genetic Diagnosis, Risk Prediction and Cancer Classification. Int. J. Mol. Sci. 2017, 18, 308. [Google Scholar] [CrossRef]
- Tsaousis, G.N.; Papadopoulou, E.; Apessos, A.; Agiannitopoulos, K.; Pepe, G.; Kampouri, S.; Diamantopoulos, N.; Floros, T.; Iosifidou, R.; Katopodi, O.; et al. Analysis of hereditary cancer syndromes by using a panel of genes: Novel and multiple pathogenic mutations. BMC Cancer 2019, 19, 535. [Google Scholar]
- Henkel, J.; Laner, A.; Locher, M.; Wohlfrom, T.; Neitzel, B.; Becker, K.; Neuhann, T.; Abicht, A.; Steinke-Lange, V.; Holinski-Feder, E. Diagnostic yield and clinical relevance of expanded germline genetic testing for nearly 7000 suspected HBOC patients. Eur. J. Hum. Genet. 2023, 31, 925–930. [Google Scholar] [PubMed]
- Michailidou, K.; The Breast and Ovarian Cancer Susceptibility Collaboration; Hall, P.; Gonzalez-Neira, A.; Ghoussaini, M.; Dennis, J.; Milne, R.L.; Schmidt, M.K.; Chang-Claude, J.; Bojesen, E.S.; et al. Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat. Genet. 2013, 45, 353–361. [Google Scholar] [PubMed]
- Bolli, A.; Di Domenico, P.; Bottà, G. Software as a Service for the Genomic Prediction of Complex Diseases. bioRxiv 2019, 763722. Available online: http://biorxiv.org/content/early/2019/09/27/763722.abstract (accessed on 2 May 2024).
- Busby, G.B.; Craig, P.; Yousfi, N.; Hebbalkar, S.; Di Domenico, P.; Bottà, G. Genetic Assessments of Breast Cancer Risk That Do not Account for Polygenic Background Are Incomplete and Lead to Incorrect Preventative Strategies. medRxiv. 2021. Available online: http://medrxiv.org/content/early/2021/08/23/2021.08.13.21262050.abstract (accessed on 2 May 2024).
- Bycroft, C.; Freeman, C.; Petkova, D.; Band, G.; Elliott, L.T.; Sharp, K.; Motyer, A.; Vukcevic, D.; Delaneau, O.; O’connell, J.; et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018, 562, 203–209. [Google Scholar] [PubMed]
- Busby, G.; Kulm, S.; Bolli, A.; Kintzle, J.; Di Domenico, P.; Neary, J.; Morganstern, D.; Bottà, G. Ancestry-Speciic Polygenic Risk Scores Improve Clinical Assessments of Breast Cancer Risk in Diverse Populations Ancestry-Specific Polygenic Risk Scores Improve Clinical 1 Assessments of Breast Cancer Risk in Diverse Populations. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Onishi, N.; Kataoka, M. Breast cancer screening for women at high risk: Review of current guidelines from leading specialty societies. Breast Cancer 2021, 2, 1195–1211. [Google Scholar]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [PubMed]
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar]
- Waskom, M. seaborn: Statistical data visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar]
- Riddle, L.; Joseph, G.; Caruncho, M.; Koenig, B.A.; James, J.E. The role of polygenic risk scores in breast cancer risk perception and decision-making. J. Community Genet. 2023, 14, 489–501. [Google Scholar] [PubMed]
- Agiannitopoulos, K.; Pepe, G.; Tsaousis, G.N.; Potska, K.; Bouzarelou, D.; Katseli, A.; Ntogka, C.; Meintani, A.; Tsoulos, N.; Giassas, S.; et al. Copy Number Variations (CNVs) Account for 10.8% of Pathogenic Variants in Patients Referred for Hereditary Cancer Testing. Cancer Genom. Proteom. 2023, 20, 448–455. [Google Scholar]
- Hassanin, E.; Spier, I.; Bobbili, D.R.; Aldisi, R.; Klinkhammer, H.; David, F.; Dueñas, N.; Hüneburg, R.; Perne, C.; Brunet, J.; et al. Clinically relevant combined effect of polygenic background, rare pathogenic germline variants, and family history on colorectal cancer incidence. BMC Med. Genom. 2023, 16, 42. [Google Scholar]
- Collister, J.A.; Liu, X.; Clifton, L. Calculating Polygenic Risk Scores (PRS) in UK Biobank: A Practical Guide for Epidemiologists. Front. Genet. 2022, 13, 818574. [Google Scholar]
- Dwyer, M. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. 2024. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=2&id=1503 (accessed on 2 May 2024).
- Zirpoli, G.R.; Pfeiffer, R.M.; Bertrand, K.A.; Huo, D.; Lunetta, K.L.; Palmer, J.R. Addition of polygenic risk score to a risk calculator for prediction of breast cancer in US Black women. Breast Cancer Res. 2024, 26, 2. [Google Scholar] [PubMed]
- Tamlander, M.; Jermy, B.; Seppälä, T.T.; Färkkilä, M.; FinnGen Widén, E.; Ripatti, S.; Mars, N. Genome-wide polygenic risk scores for colorectal cancer have implications for risk-based screening. Br. J. Cancer 2024, 130, 651–659. [Google Scholar]
- Mars, N.; Kerminen, S.; Tamlander, M.; Pirinen, M.; Jakkula, E.; Aaltonen, K.; Meretoja, T.; Heinävaara, S.; Widén, E.; Ripatti, S. Comprehensive Inherited Risk Estimation for Risk-Based Breast Cancer Screening in Women. J. Clin. Oncol. 2024, 42, 1477–1487. [Google Scholar] [PubMed]
- Bolze, A.; Cirulli, E.T.; Hajek, C.; Schnell Blitstein, J.M.; Grzymski, J.J. The Potential of Genetics in Identifying Women at Lower Risk of Breast Cancer. JAMA Oncol. 2024, 10, 236. [Google Scholar] [PubMed]
- Koch, S.; Schmidtke, J.; Krawczak, M.; Caliebe, A. Clinical utility of polygenic risk scores: A critical 2023 appraisal. J. Community Genet. 2023, 14, 471–487. [Google Scholar] [PubMed]
- Lewis, A.C.F.; Green, R.C. Polygenic risk scores in the clinic: New perspectives needed on familiar ethical issues. Genome Med. 2021, 13, 14. [Google Scholar]
- Mars, N.; Lindbohm, J.V.; Parolo, P.d.B.; Widén, E.; Kaprio, J.; Palotie, A.; Ripatti, S. Systematic comparison of family history and polygenic risk across 24 common diseases. Am. J. Human. Genet. 2022, 109, 2152–2162. [Google Scholar]
- Michailidou, K.; Lindstrom, S.; Dennis, J.; Beesley, J.; Hui, S.; Kar, S.; Lemacon, A.; Soucy, P.; Glubb, D.; Rostamianfar, A.; et al. Association analysis identifies 65 new breast cancer risk loci. Nature 2017, 551, 92–94. [Google Scholar]
- Carver, T.; Hartley, S.; Lee, A.; Cunningham, A.P.; Archer, S.; Babb de Villiers, C.; Roberts, J.; Ruston, R.; Walter, F.M.; Tischkowitz, M.; et al. CanRisk Tool-A Web Interface for the Prediction of Breast and Ovarian Cancer Risk and the Likelihood of Carrying Genetic Pathogenic Variants. Cancer Epidemiol. Biomark. Prev. 2021, 30, 469–473. [Google Scholar] [CrossRef]
- Yiangou, K.; Mavaddat, N.; Dennis, J.; Zanti, M.; Wang, Q.; Bolla, M.K.; Abubakar, M.; Ahearn, T.U.; Andrulis, I.L.; Anton-Culver, H.; et al. Differences in Polygenic Score Distributions in European Ancestry Populations: Implications for Breast Cancer Risk Prediction. medRxiv 2024. [Google Scholar] [CrossRef]
- Wolfson, M.; Gribble, S.; Pashayan, N.; Easton, D.F.; Antoniou, A.C.; Lee, A.; van Katwyk, S.; Simard, J. Potential of Polygenic Risk Scores for Improving Population Estimates of Women’s Breast Cancer Genetic Risks. Genet. Med. 2021, 23, 2114–2121. [Google Scholar] [CrossRef]
- Ho, P.J.; Lim, E.H.; Hartman, M.; Wong, F.Y.; Li, J. Breast Cancer Risk Stratification Using Genetic and Non-Genetic Risk Assessment Tools for 246,142 Women in the UK Biobank. Genet. Med. 2023, 25, 100917. [Google Scholar] [CrossRef]
- Dixon, P.; Keeney, E.; Taylor, J.C.; Wordsworth, S.; Martin, R.M. Can Polygenic Risk Scores Contribute to Cost-Effective Cancer Screening? A Systematic Review. Genet. Med. 2022, 24, 1604–1617. [Google Scholar] [CrossRef]
- Yanes, T.; Tiller, J.; Haining, C.M.; Wallingford, C.; Otlowski, M.; Keogh, L.; McInerney-Leo, A.; Lacaze, P. Future Implications of Polygenic Risk Scores for Life Insurance Underwriting. NPJ Genom. Med. 2024, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.C.F.; Perez, E.F.; Prince, A.E.R.; Flaxman, H.R.; Gomez, L.; Brockman, D.G.; Chandler, P.D.; Kerman, B.J.; Lebo, M.S.; Smoller, J.W.; et al. Patient and Provider Perspectives on Polygenic Risk Scores: Implications for Clinical Reporting and Utilization. Genome Med. 2022, 14, 114. [Google Scholar] [CrossRef] [PubMed]

| All | With FH of BC | Without FH of BC | |
|---|---|---|---|
| Age at Diagnosis | |||
| Number of Women < 45 yo | 67 | 20 | 47 |
| Number of Women > 45 yo | 38 | 20 | 18 |
| Average age | 47 | 47.6 | 42.7 |
| Genetic testing | |||
| Number of Negative cases | 47 | 22 | 25 |
| Number of Pathogenic in high risk gene | 20 | 11 | 9 |
| Number of Pathogenic in moderate risk gene | 20 | 3 | 17 |
| Number of Pathogenic in low/unspesified risk gene | 18 | 4 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsoulos, N.; Papadopoulou, E.; Agiannitopoulos, K.; Grigoriadis, D.; Tsaousis, G.N.; Bouzarelou, D.; Gogas, H.; Troupis, T.; Venizelos, V.; Fountzilas, E.; et al. Polygenic Risk Score (PRS) Combined with NGS Panel Testing Increases Accuracy in Hereditary Breast Cancer Risk Estimation. Diagnostics 2024, 14, 1826. https://doi.org/10.3390/diagnostics14161826
Tsoulos N, Papadopoulou E, Agiannitopoulos K, Grigoriadis D, Tsaousis GN, Bouzarelou D, Gogas H, Troupis T, Venizelos V, Fountzilas E, et al. Polygenic Risk Score (PRS) Combined with NGS Panel Testing Increases Accuracy in Hereditary Breast Cancer Risk Estimation. Diagnostics. 2024; 14(16):1826. https://doi.org/10.3390/diagnostics14161826
Chicago/Turabian StyleTsoulos, Nikolaos, Eirini Papadopoulou, Konstantinos Agiannitopoulos, Dimitrios Grigoriadis, Georgios N. Tsaousis, Dimitra Bouzarelou, Helen Gogas, Theodore Troupis, Vassileios Venizelos, Elena Fountzilas, and et al. 2024. "Polygenic Risk Score (PRS) Combined with NGS Panel Testing Increases Accuracy in Hereditary Breast Cancer Risk Estimation" Diagnostics 14, no. 16: 1826. https://doi.org/10.3390/diagnostics14161826
APA StyleTsoulos, N., Papadopoulou, E., Agiannitopoulos, K., Grigoriadis, D., Tsaousis, G. N., Bouzarelou, D., Gogas, H., Troupis, T., Venizelos, V., Fountzilas, E., Theochari, M., Ziogas, D. C., Giassas, S., Koumarianou, A., Christopoulou, A., Busby, G., Nasioulas, G., & Markopoulos, C. (2024). Polygenic Risk Score (PRS) Combined with NGS Panel Testing Increases Accuracy in Hereditary Breast Cancer Risk Estimation. Diagnostics, 14(16), 1826. https://doi.org/10.3390/diagnostics14161826






