The Possible Associations between Tauopathies and Atherosclerosis, Diabetes Mellitus, Dyslipidemias, Metabolic Syndrome and Niemann–Pick Disease
Abstract
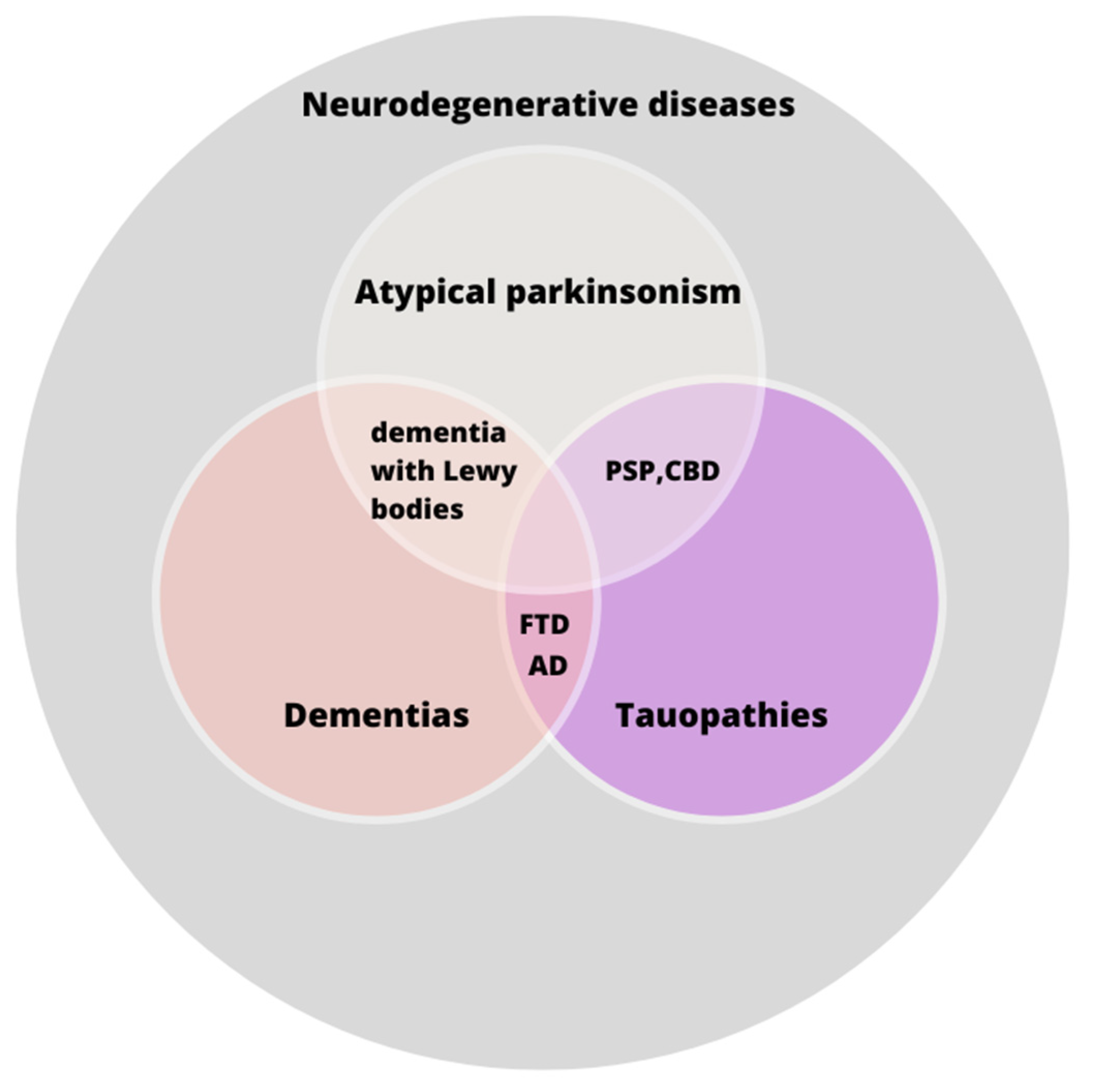
1. Introduction
2. Methods
3. State of the Art
3.1. Dyslipidemias and Tauopathies
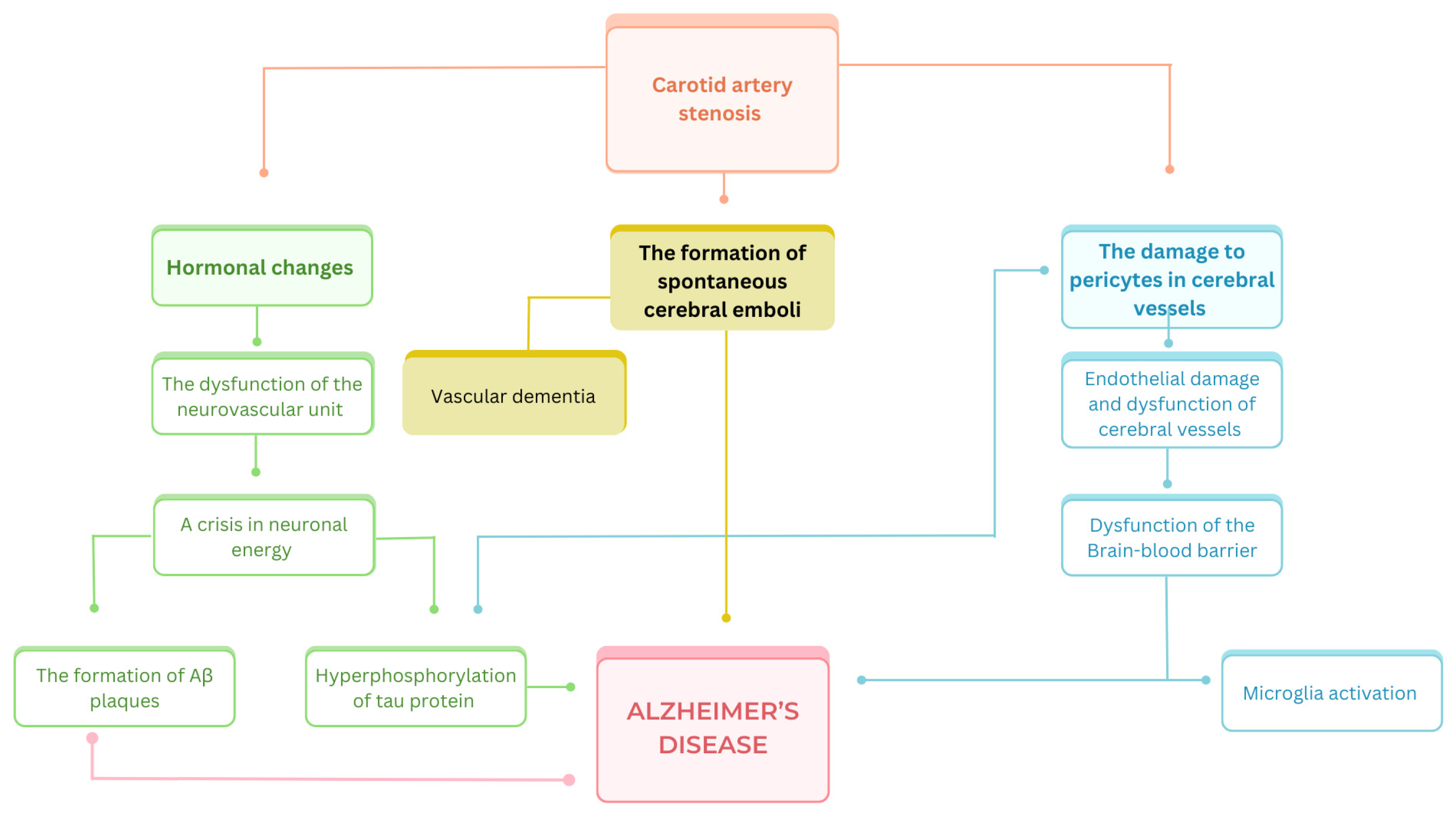
3.2. Atherosclerosis and Tauopathies
3.3. Diabetes Mellitus and Tauopathies
3.4. Metabolic Syndrome and Tauopathies
3.5. Niemann–Pick Disease and Tauopathies
4. Clinical Implications
4.1. Discussion—Common Mechanisms of Metabolic Diseases in Tauopathy Pathogenesis
4.2. The Most Recent Data from 2024
4.3. The Present and Future Medications for Metabolic Diseases and Their Impact on Tauopathies
4.4. Limitations
4.5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| BBB | Blood–brain barrier |
| BCAS | Bilateral carotid artery stenosis |
| CBD | Corticobasal degeneration |
| DM | Diabetes mellitus |
| FTD | Frontotemporal dementia |
| HDL | High-density lipoprotein |
| LDL | Low-density lipoprotein |
| MetS | Metabolic syndrome |
| NPD | Niemann–Pick disease |
| PSP | Progressive supranuclear palsy |
References
- Kovacs, G.G. Tauopathies. Handb. Clin. Neurol. 2017, 145, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, C.; Sorbi, S. The complexity of Alzheimer’s disease: An evolving puzzle. Physiol. Rev. 2021, 101, 1047–1081. [Google Scholar] [CrossRef] [PubMed]
- Rostagno, A.A. Pathogenesis of Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 24, 107. [Google Scholar] [CrossRef] [PubMed]
- Wegmann, S.; Biernat, J.; Mandelkow, E. A current view on Tau protein phosphorylation in Alzheimer’s disease. Curr. Opin. Neurobiol. 2021, 69, 131–138. [Google Scholar] [CrossRef]
- Liu, R.M. Aging, Cellular Senescence, and Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 1989. [Google Scholar] [CrossRef]
- Bang, J.; Spina, S.; Miller, B.L. Frontotemporal dementia. Lancet 2015, 386, 1672–1682. [Google Scholar] [CrossRef]
- Bäckman, L.; Jones, S.; Berger, A.-K.; Laukka, E.J.; Small, B.J. Multiple cognitive deficits during the transition to Alzheimer’s disease. J. Intern. Med. 2004, 256, 195–204. [Google Scholar] [CrossRef]
- Rowe, J.B.; Holland, N.; Rittman, T. Progressive supranuclear palsy: Diagnosis and management. Pract. Neurol. 2021, 21, 376–383. [Google Scholar] [CrossRef]
- Respondek, G.; Grimm, M.J.; Piot, I.; Arzberger, T.; Compta, Y.; Englund, E.; Ferguson, L.W.; Gelpi, E.; Roeber, S.; Giese, A.; et al. Validation of the movement disorder society criteria for the diagnosis of 4-repeat tauopathies. Mov. Disord. 2020, 35, 171–176. [Google Scholar] [CrossRef]
- Bianchi, V.E.; Herrera, P.F.; Laura, R. Effect of nutrition on neurodegenerative diseases. A systematic review. Nutr. Neurosci. 2021, 24, 810–834. [Google Scholar] [CrossRef]
- Sumien, N.; Cunningham, J.T.; Davis, D.L.; Engelland, R.; Fadeyibi, O.; Farmer, G.E.; Mabry, S.; Mensah-Kane, P.; Trinh, O.T.P.; Vann, P.H.; et al. Neurodegenerative Disease: Roles for Sex, Hormones, and Oxidative Stress. Endocrinology 2021, 162, bqab185. [Google Scholar] [CrossRef] [PubMed]
- Muriach, M.; Flores-Bellver, M.; Romero, F.J.; Barcia, J.M. Diabetes and the brain: Oxidative stress, inflammation, and autophagy. Oxid. Med. Cell. Longev. 2014, 2014, 102158. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Ruscica, M.; Tokgözoğlu, L.; Corsini, A.; Sirtori, C.R. PCSK9 inhibition and inflammation: A narrative review. Atherosclerosis 2019, 288, 146–155. [Google Scholar] [CrossRef]
- Thakur, S.; Dhapola, R.; Sarma, P.; Medhi, B.; Reddy, D.H. Neuroinflammation in Alzheimer’s Disease: Current Progress in Molecular Signaling and Therapeutics. Inflammation 2023, 46, 1–17. [Google Scholar] [CrossRef]
- Batista, C.R.A.; Gomes, G.F.; Candelario-Jalil, E.; Fiebich, B.L.; de Oliveira, A.C.P. Lipopolysaccharide-Induced Neuroinflammation as a Bridge to Understand Neurodegeneration. Int. J. Mol. Sci. 2019, 20, 2293. [Google Scholar] [CrossRef]
- Iadecola, C. The pathobiology of vascular dementia. Neuron 2013, 80, 844–866. [Google Scholar] [CrossRef]
- Koga, S.; Josephs, K.A.; Aiba, I.; Yoshida, M.; Dickson, D.W. Neuropathology and emerging biomarkers in corticobasal syndrome. J. Neurol. Neurosurg. Psychiatry 2022, 93, 919–929. [Google Scholar] [CrossRef]
- Berberich, A.J.; Hegele, R.A. A Modern Approach to Dyslipidemia. Endocr. Rev. 2022, 43, 611–653. [Google Scholar] [CrossRef]
- Janoska, M.; Chorążka, K.; Domitrz, I. Migraine frequency and its association with dyslipidemia in women. Neurol. Neurochir. Pol. 2015, 49, 95–98. [Google Scholar] [CrossRef]
- Kazamel, M.; Stino, A.M.; Smith, A.G. Metabolic syndrome and peripheral neuropathy. Muscle Nerve 2021, 63, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Strickland, M.R.; Soranno, A.; Holtzman, D.M. Apolipoprotein E: Structural Insights and Links to Alzheimer Disease Pathogenesis. Neuron 2021, 109, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Das, S.; Hyman, B.T. APOE and Alzheimer’s disease: Advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 2021, 20, 68–80. [Google Scholar] [CrossRef]
- Koutsodendris, N.; Nelson, M.R.; Rao, A.; Huang, Y. Apolipoprotein E and Alzheimer’s Disease: Findings, Hypotheses, and Potential Mechanisms. Annu. Rev. Pathol. 2022, 17, 73–99. [Google Scholar] [CrossRef]
- Agosta, F.; Vossel, K.A.; Miller, B.L.; Migliaccio, R.; Bonasera, S.J.; Filippi, M.; Boxer, A.L.; Karydas, A.; Possin, K.L.; Gorno-Tempini, M.L. Apolipoprotein E epsilon4 is associated with disease-specific effects on brain atrophy in Alzheimer’s disease and frontotemporal dementia. Proc. Natl. Acad. Sci. USA 2009, 106, 2018–2022. [Google Scholar] [CrossRef] [PubMed]
- Dilliott, A.A.; Sunderland, K.M.; McLaughlin, P.M.; Roberts, A.C.; Evans, E.C.; Abrahao, A.; Binns, M.A.; Black, S.E.; Borrie, M.; Casaubon, L.K.; et al. Association of apolipoprotein E variation with cognitive impairment across multiple neurodegenerative diagnoses. Neurobiol. Aging 2021, 105, 378.e1–378.e9. [Google Scholar] [CrossRef] [PubMed]
- Sabir, M.S.; Blauwendraat, C.; Ahmed, S.; Serrano, G.E.; Beach, T.G.; Perkins, M.; Rice, A.C.; Masliah, E.; Morris, C.M.; Pihlstrom, L.; et al. Assessment of APOE in atypical parkinsonism syndromes. Neurobiol. Dis. 2019, 127, 142–146. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Zheng, M.; Ji, L.; Wu, T.; Huang, G.; Ji, Y. Factors Associated with Frontotemporal Dementia in China: A Cross-Sectional Study. Arch. Med. Res. 2016, 47, 388–393. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, H.; Wang, Y.; Zhang, M.; Zhou, Y. Plasma cholesterol in Alzheimer’s disease and frontotemporal dementia. Transl. Neurosci. 2020, 11, 116–123. [Google Scholar] [CrossRef]
- Weng, R.; Wei, X.; Yu, B.; Zhu, S.; Yang, X.; Xie, F.; Zhang, M.; Jiang, Y.; Feng, Z.P.; Sun, H.S.; et al. Combined measurement of plasma cystatin C and low-density lipoprotein cholesterol: A valuable tool for evaluating progressive supranuclear palsy. Park. Relat. Disord. 2018, 52, 37–42. [Google Scholar] [CrossRef]
- Dugger, B.N.; Malek-Ahmadi, M.; Monsell, S.E.; Kukull, W.A.; Woodruff, B.K.; Reiman, E.M.; Beach, T.G.; Wilson, J. A Cross-Sectional Analysis of Late-Life Cardiovascular Factors and Their Relation to Clinically Defined Neurodegenerative Diseases. Alzheimer Dis. Assoc. Disord. 2016, 30, 223–229. [Google Scholar] [CrossRef]
- Kim, W.S.; Jary, E.; Pickford, R.; He, Y.; Ahmed, R.M.; Piguet, O.; Hodges, J.R.; Halliday, G.M. Lipidomics Analysis of Behavioral Variant Frontotemporal Dementia: A Scope for Biomarker Development. Front. Neurol. 2018, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.; He, Y.; Pickford, R.; Bhatia, S.; Katzeff, J.S.; Hodges, J.R.; Piguet, O.; Halliday, G.M.; Kim, W.S. Uncovering pathophysiological changes in frontotemporal dementia using serum lipids. Sci. Rep. 2020, 10, 3640. [Google Scholar] [CrossRef] [PubMed]
- Golimstok, A.; Campora, N.; Rojas, J.I.; Fernandez, M.C.; Elizondo, C.; Soriano, E.; Cristiano, E. Cardiovascular risk factors and frontotemporal dementia: A case-control study. Transl. Neurodegener. 2014, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Falk, E. Pathogenesis of Atherosclerosis. J. Am. Coll. Cardiol. 2006, 47, C7–C12. [Google Scholar] [CrossRef]
- Bonati, L.H.; Jansen, O.; de Borst, G.J.; Brown, M.M. Management of atherosclerotic extracranial carotid artery stenosis. Lancet Neurol. 2022, 21, 273–283. [Google Scholar] [CrossRef]
- Chang, R.W.; Tucker, L.Y.; Rothenberg, K.A.; Lancaster, E.; Faruqi, R.M.; Kuang, H.C.; Flint, A.C.; Avins, A.L.; Nguyen-Huynh, M.N. Incidence of Ischemic Stroke in Patients with Asymptomatic Severe Carotid Stenosis without Surgical Intervention. JAMA 2022, 327, 1974–1982. [Google Scholar] [CrossRef]
- Kopczak, A.; Schindler, A.; Sepp, D.; Bayer-Karpinska, A.; Malik, R.; Koch, M.L.; Zeller, J.; Strecker, C.; Janowitz, D.; Wollenweber, F.A.; et al. Complicated Carotid Artery Plaques and Risk of Recurrent Ischemic Stroke or TIA. J. Am. Coll. Cardiol. 2022, 79, 2189–2199. [Google Scholar] [CrossRef]
- Szarmach, A.; Halena, G.; Kaszubowski, M.; Piskunowicz, M.; Studniarek, M.; Lass, P.; Szurowska, E.; Winklewski, P.J. Carotid Artery Stenting and Blood-Brain Barrier Permeability in Subjects with Chronic Carotid Artery Stenosis. Int. J. Mol. Sci. 2017, 18, 1008. [Google Scholar] [CrossRef]
- Yang, L.; Song, J.; Nan, D.; Wan, Y.; Guo, H. Cognitive Impairments and blood-brain Barrier Damage in a Mouse Model of Chronic Cerebral Hypoperfusion. Neurochem. Res. 2022, 47, 3817–3828. [Google Scholar] [CrossRef]
- Liu, Q.; Radwanski, R.; Babadjouni, R.; Patel, A.; Hodis, D.M.; Baumbacher, P.; Zhao, Z.; Zlokovic, B.; Mack, W.J. Experimental chronic cerebral hypoperfusion results in decreased pericyte coverage and increased blood-brain barrier permeability in the corpus callosum. J. Cereb. Blood Flow Metab. 2019, 39, 240–250. [Google Scholar] [CrossRef]
- Fowler, J.H.; McQueen, J.; Holland, P.R.; Manso, Y.; Marangoni, M.; Scott, F.; Chisholm, E.; Scannevin, R.; Hardingham, G.; Horsburgh, K. [P4–119]: SEVERE CEREBRAL HYPOPERFUSION INDUCES A DEFICIT IN WHITE MATTER FUNCTION THAT IS ATTENUATED BY MODULATING MICROGLIA WITH DIMETHYL FUMARATE. Alzheimers Dement. 2017, 13, P1302–P1303. [Google Scholar] [CrossRef]
- Deng, S.; Shu, S.; Zhai, L.; Xia, S.; Cao, X.; Li, H.; Bao, X.; Liu, P.; Xu, Y. Optogenetic Stimulation of mPFC Alleviates White Matter Injury-Related Cognitive Decline after Chronic Ischemia through Adaptive Myelination. Adv. Sci. 2023, 10, 2202976. [Google Scholar] [CrossRef]
- Lamorie-Foote, K.; Liu, Q.; Shkirkova, K.; Ge, B.; He, S.; Morgan, T.E.; Mack, W.J.; Sioutas, C.; Finch, C.E.; Mack, W.J. Particulate matter exposure and chronic cerebral hypoperfusion promote oxidative stress and induce neuronal and oligodendrocyte apoptosis in male mice. J. Neurosci. Res. 2023, 101, 384–402. [Google Scholar] [CrossRef] [PubMed]
- Sutton, E.T.; Hellermann, G.R.; Thomas, T. beta-amyloid-induced endothelial necrosis and inhibition of nitric oxide production. Exp. Cell Res. 1997, 230, 368–376. [Google Scholar] [CrossRef]
- Tang, G.L. The tangled story of carotid disease, carotid revascularization, and Alzheimer’s disease: The plot thickens. J. Vasc. Surg. 2022, 75, 229. [Google Scholar] [CrossRef] [PubMed]
- Purandare, N.; Burns, A.; Daly, K.J.; Hardicre, J.; Morris, J.; Macfarlane, G.; McCollum, C. Cerebral emboli as a potential cause of Alzheimer’s disease and vascular dementia: Case-control study. BMJ 2006, 332, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.; Byrne, J.; Hardicre, J.; McCollum, C.; Purandare, N.; Voshaar, R.C.O. Cerebral emboli and depressive symptoms in dementia. Br. J. Psychiatry 2006, 189, 260–263. [Google Scholar] [CrossRef]
- Li, H.; Tian, J.; Yin, Y.; Diao, S.; Zhang, X.; Zuo, T.; Miao, Z.; Yang, Y. Interleukin-18 mediated inflammatory brain injury after intracerebral hemorrhage in male mice. J. Neurosci. Res. 2022, 100, 1359–1369. [Google Scholar] [CrossRef]
- Arias, J.C.; Edwards, M.; Vitali, F.; Beach, T.G.; Serrano, G.E.; Weinkauf, C.C. Extracranial carotid atherosclerosis is associated with increased neurofibrillary tangle accumulation. J. Vasc. Surg. 2022, 75, 223–228. [Google Scholar] [CrossRef]
- Silvestrini, M.; Viticchi, G.; Falsetti, L.; Balucani, C.; Vernieri, F.; Cerqua, R.; Luzzi, S.; Bartolini, M.; Provinciali, L. The role of carotid atherosclerosis in Alzheimer’s disease progression. J. Alzheimers Dis. 2011, 25, 719–726. [Google Scholar] [CrossRef]
- de la Torre, J.C. Carotid Artery Ultrasound and Echocardiography Testing to Lower the Prevalence of Alzheimer’s Disease. J. Stroke Cerebrovasc. Dis. 2009, 18, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, J.C.; Castellano, J.M.; Fuster, V. The links between complex coronary disease, cerebrovascular disease, and degenerative brain disease. Ann. N. Y. Acad. Sci. 2012, 1254, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Whooley, J.L.; David, B.C.; Woo, H.H.; Hoh, B.L.; Raftery, K.B.; Hussain Siddiqui, A.; Westerveld, M.; Amin-Hanjani, S.; Ghogawala, Z. Carotid Revascularization and Its Effect on Cognitive Function: A Prospective Nonrandomized Multicenter Clinical Study. J. Stroke Cerebrovasc. Dis. 2020, 29, 104702. [Google Scholar] [CrossRef] [PubMed]
- Mas, J.L. [Internal carotid artery revascularization]. Rev. Esp. Cardiol. 2007, 60, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Schröder, J.; Heinze, M.; Günther, M.; Cheng, B.; Nickel, A.; Schröder, T.; Fischer, F.; Kessner, S.S.; Magnus, T.; Fiehler, J.; et al. Dynamics of brain perfusion and cognitive performance in revascularization of carotid artery stenosis. NeuroImage Clin. 2019, 22, 101779. [Google Scholar] [CrossRef]
- Mayfield, J. Diagnosis and classification of diabetes mellitus: New criteria. Am. Fam. Physician 1998, 58, 1355–1362, 1369–1370. [Google Scholar]
- Araszkiewicz, A.; Bandurska-Stankiewicz, E.; Borys, S.; Budzyński, A.; Cyganek, K.; Cypryk, K.; Czech, A.; Czupryniak, L.; Drzewoski, J.; Dzida, G. Zalecenia kliniczne dotyczące postępowania u osób z cukrzycą 2023-Stanowisko Polskiego Towarzystwa Diabetologicznego. Curr. Top. Diabet. 2023, 3, 1–140. [Google Scholar] [CrossRef]
- Biessels, G.J.; Despa, F. Cognitive decline and dementia in diabetes mellitus: Mechanisms and clinical implications. Nat. Rev. Endocrinol. 2018, 14, 591–604. [Google Scholar] [CrossRef]
- Burillo, J.; Marqués, P.; Jiménez, B.; González-Blanco, C.; Benito, M.; Guillén, C. Insulin Resistance and Diabetes Mellitus in Alzheimer’s Disease. Cells 2021, 10, 1236. [Google Scholar] [CrossRef]
- Devi, L.; Alldred, M.J.; Ginsberg, S.D.; Ohno, M. Mechanisms underlying insulin deficiency-induced acceleration of β-amyloidosis in a mouse model of Alzheimer’s disease. PLoS ONE 2012, 7, e32792. [Google Scholar] [CrossRef] [PubMed]
- Kertesz, A. Corticobasal degeneration. J. Neurol. Neurosurg. Psychiatry 2000, 68, 275. [Google Scholar] [CrossRef]
- Wallin, A.; Öhrfelt, A.; Bjerke, M. Characteristic clinical presentation and CSF biomarker pattern in cerebral small vessel disease. J. Neurol. Sci. 2012, 322, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Janelidze, S.; Hertze, J.; Nägga, K.; Nilsson, K.; Nilsson, C.; Wennström, M.; van Westen, D.; Blennow, K.; Zetterberg, H.; Hansson, O. Increased blood-brain barrier permeability is associated with dementia and diabetes but not amyloid pathology or APOE genotype. Neurobiol. Aging 2017, 51, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Varatharaj, A.; Galea, I. The blood-brain barrier in systemic inflammation. Brain Behav. Immun. 2017, 60, 1–12. [Google Scholar] [CrossRef]
- Evans, L.E.; Taylor, J.L.; Smith, C.J.; Pritchard, H.A.T.; Greenstein, A.S.; Allan, S.M. Cardiovascular comorbidities, inflammation, and cerebral small vessel disease. Cardiovasc. Res. 2021, 117, 2575–2588. [Google Scholar] [CrossRef]
- Fraga, V.G.; Magalhães, C.A.; Loures, C.d.M.G.; de Souza, L.C.; Guimarães, H.C.; Zauli, D.A.G.; Carvalho, M.d.G.; Ferreira, C.N.; Caramelli, P.; de Sousa, L.P.; et al. Inflammatory and Pro-resolving Mediators in Frontotemporal Dementia and Alzheimer’s Disease. Neuroscience 2019, 421, 123–135. [Google Scholar] [CrossRef]
- Platt, T.L.; Beckett, T.L.; Kohler, K.; Niedowicz, D.M.; Murphy, M.P. Obesity, diabetes, and leptin resistance promote tau pathology in a mouse model of disease. Neuroscience 2016, 315, 162–174. [Google Scholar] [CrossRef]
- Leroy, K.; Yilmaz, Z.; Brion, J.-P. Increased level of active GSK-3β in Alzheimer’s disease and accumulation in argyrophilic grains and in neurones at different stages of neurofibrillary degeneration. Neuropathol. Appl. Neurobiol. 2007, 33, 43–55. [Google Scholar] [CrossRef]
- Lauretti, E.; Dincer, O.; Praticò, D. Glycogen synthase kinase-3 signaling in Alzheimer’s disease. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118664. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Toledo, J.B.; Lee, E.B.; Arvanitakis, Z.; Kazi, H.; Han, L.Y.; Louneva, N.; Lee, V.M.; Kim, S.F.; Trojanowski, J.Q.; et al. Abnormal serine phosphorylation of insulin receptor substrate 1 is associated with tau pathology in Alzheimer’s disease and tauopathies. Acta Neuropathol. 2014, 128, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Hull, C.; Dekeryte, R.; Buchanan, H.; Kamli-Salino, S.; Robertson, A.; Delibegovic, M.; Platt, B. NLRP3 inflammasome inhibition with MCC950 improves insulin sensitivity and inflammation in a mouse model of frontotemporal dementia. Neuropharmacology 2020, 180, 108305. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D.; Papageorgiou, N.; Androulakis, E.; Siasos, G.; Latsios, G.; Tentolouris, K.; Stefanadis, C. Diabetes mellitus-associated vascular impairment: Novel circulating biomarkers and therapeutic approaches. J. Am. Coll. Cardiol. 2013, 62, 667–676. [Google Scholar] [CrossRef]
- Joshi, R.D.; Dhakal, C.K. Predicting Type 2 Diabetes Using Logistic Regression and Machine Learning Approaches. Int. J. Environ. Res. Public Health 2021, 18, 7346. [Google Scholar] [CrossRef]
- Morgan, J.C.; Ye, X.; Mellor, J.A.; Golden, K.J.; Zamudio, J.; Chiodo, L.A.; Bao, Y.; Xie, T. Disease course and treatment patterns in progressive supranuclear palsy: A real-world study. J. Neurol. Sci. 2021, 421, 117293. [Google Scholar] [CrossRef]
- Rabadia, S.V.; Litvan, I.; Juncos, J.; Bordelon, Y.; Riley, D.E.; Standaert, D.; Reich, S.G.; Hall, D.A.; Kluger, B.; Shprecher, D.; et al. Hypertension and progressive supranuclear palsy. Park. Relat. Disord. 2019, 66, 166–170. [Google Scholar] [CrossRef]
- Greten, S.; Wegner, F.; Jensen, I.; Krey, L.; Rogozinski, S.; Fehring, M.; Heine, J.; Doll-Lee, J.; Pötter-Nerger, M.; Zeitzschel, M.; et al. The comorbidity and co-medication profile of patients with progressive supranuclear palsy. J. Neurol. 2023, 271, 782–793. [Google Scholar] [CrossRef]
- Kwasny, M.J.; Oleske, D.M.; Zamudio, J.; Diegidio, R.; Höglinger, G.U. Clinical Features Observed in General Practice Associated with the Subsequent Diagnosis of Progressive Supranuclear Palsy. Front. Neurol. 2021, 12, 637176. [Google Scholar] [CrossRef]
- Kalkonde, Y.V.; Jawaid, A.; Qureshi, S.U.; Shirani, P.; Wheaton, M.; Pinto-Patarroyo, G.P.; Schulz, P.E. Medical and environmental risk factors associated with frontotemporal dementia: A case-control study in a veteran population. Alzheimers Dement. 2012, 8, 204–210. [Google Scholar] [CrossRef]
- Rus, M.; Crisan, S.; Andronie-Cioara, F.L.; Indries, M.; Marian, P.; Pobirci, O.L.; Ardelean, A.I. Prevalence and Risk Factors of Metabolic Syndrome: A Prospective Study on Cardiovascular Health. Medicina 2023, 59, 1711. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Magnuson, A.M.; Fouts, J.K.; Regan, D.P.; Booth, A.D.; Dow, S.W.; Foster, M.T. Adipose tissue extrinsic factor: Obesity-induced inflammation and the role of the visceral lymph node. Physiol. Behav. 2018, 190, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Y.; Choi, W.J.; Lee, H.S.; Lee, J.W. Relationship between inflammatory markers and visceral obesity in obese and overweight Korean adults: An observational study. Medicine 2019, 98, e14740. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alsayegh, A.A.; Hakami, Z.H.; Khamjan, N.A.; Saad, H.M.; Batiha, G.E.; De Waard, M. A Potential Link Between Visceral Obesity and Risk of Alzheimer’s Disease. Neurochem. Res. 2023, 48, 745–766. [Google Scholar] [CrossRef]
- Zuin, M.; Roncon, L.; Passaro, A.; Cervellati, C.; Zuliani, G. Metabolic syndrome and the risk of late onset Alzheimer’s disease: An updated review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2244–2252. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, D.; Collister, J.; Allen, N.E.; Kuźma, E.; Littlejohns, T. Association between metabolic syndrome and risk of incident dementia in UK Biobank. Alzheimers Dement. 2024, 20, 447–458. [Google Scholar] [CrossRef]
- Atti, A.R.; Valente, S.; Iodice, A.; Caramella, I.; Ferrari, B.; Albert, U.; Mandelli, L.; De Ronchi, D. Metabolic Syndrome, Mild Cognitive Impairment, and Dementia: A Meta-Analysis of Longitudinal Studies. Am. J. Geriatr. Psychiatry 2019, 27, 625–637. [Google Scholar] [CrossRef]
- Rodriguez-Santiago, M.A.; Jiménez-Velazquez, I.Z.; Maldonado, A.; Valentin, E.M.; Rivera, H.S. Metabolic syndrome and its effect in dementia: Not your typical memory loss symptoms. Alzheimers Dement. 2021, 17, e052604. [Google Scholar] [CrossRef]
- He, Q.; Wang, W.; Li, H.; Xiong, Y.; Tao, C.; Ma, L.; You, C. Genetic Insights into the Risk of Metabolic Syndrome and Its Components on Dementia: A Mendelian Randomization. J. Alzheimers Dis. 2023, 96, 725–743. [Google Scholar] [CrossRef]
- Więckowska-Gacek, A.; Mietelska-Porowska, A.; Wydrych, M.; Wojda, U. Western diet as a trigger of Alzheimer’s disease: From metabolic syndrome and systemic inflammation to neuroinflammation and neurodegeneration. Ageing Res. Rev. 2021, 70, 101397. [Google Scholar] [CrossRef] [PubMed]
- Pugazhenthi, S. Chapter Eight—Metabolic Syndrome and the Cellular Phase of Alzheimer’s Disease. In Progress in Molecular Biology and Translational Science; Reddy, P.H., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 146, pp. 243–258. [Google Scholar]
- Sitarska, D.; Tylki-Szymańska, A.; Ługowska, A. Treatment trials in Niemann-Pick type C disease. Metab. Brain Dis. 2021, 36, 2215–2221. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, H.; Azhar, W. Niemann-Pick Disease. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2023. [Google Scholar]
- Vanier, M.T. Niemann-Pick diseases. Handb. Clin. Neurol. 2013, 113, 1717–1721. [Google Scholar] [CrossRef] [PubMed]
- Rintz, E.; Higuchi, T.; Kobayashi, H.; Galileo, D.S.; Wegrzyn, G.; Tomatsu, S. Promoter considerations in the design of lentiviral vectors for use in treating lysosomal storage diseases. Mol. Ther.-Methods Clin. Dev. 2022, 24, 71–87. [Google Scholar] [CrossRef]
- Boenzi, S.; Dardis, A.; Russo, P.; Bellofatto, M.; Imbriglio, T.; Fico, T.; De Michele, G.; De Rosa, A. Screening for Niemann-Pick type C disease in neurodegenerative diseases. J. Clin. Neurosci. 2019, 68, 266–267. [Google Scholar] [CrossRef]
- Zech, M.; Nübling, G.; Castrop, F.; Jochim, A.; Schulte, E.C.; Mollenhauer, B.; Lichtner, P.; Peters, A.; Gieger, C.; Marquardt, T.; et al. Niemann-Pick C disease gene mutations and age-related neurodegenerative disorders. PLoS ONE 2013, 8, e82879. [Google Scholar] [CrossRef]
- Balázs, N.; Milanovich, D.; Hornyák, C.; Bereczki, D.; Kovács, T. Late-onset Niemann-Pick disease type C overlapping with frontotemporal dementia syndromes: A case report. J. Neural Transm. 2019, 126, 1501–1504. [Google Scholar] [CrossRef]
- Kresojević, N.; Mandić-Stojmenović, G.; Dobričić, V.; Petrović, I.; Brajković, L.; Stefanova, E.; Svetel, M.; Kostić, V. Very Late-Onset Niemann Pick Type C Disease: Example of Progressive Supranuclear Palsy Look-Alike Disorder. Mov. Disord. Clin. Pract. 2020, 7, 211–214. [Google Scholar] [CrossRef]
- Godeiro-Júnior, C.; Jun Inaoka, R.; Rocha Barbosa, M.; Regis Silva, M.R.; de Carvalho Aguiar, P.; Barsottini, O. Mutations in NPC1 in two Brazilian patients with Niemann-Pick disease type C and progressive supranuclear palsy-like presentation. Mov. Disord. 2006, 21, 2270–2272. [Google Scholar] [CrossRef]
- Cupidi, C.; Frangipane, F.; Gallo, M.; Clodomiro, A.; Colao, R.; Bernardi, L.; Anfossi, M.; Conidi, M.E.; Vasso, F.; Curcio, S.A.; et al. Role of Niemann-Pick Type C Disease Mutations in Dementia. J. Alzheimers Dis. 2017, 55, 1249–1259. [Google Scholar] [CrossRef]
- Voinea, C.; Gonzalez Rodriguez, E.; Beigelman-Aubry, C.; Leroy, V.; Aubry-Rozier, B.; Campos-Xavier, B.; Ballhausen, D.; Lazor, R.; Barbey, F.; Bonafé, L.; et al. Hepatosplenomegaly, pneumopathy, bone changes and fronto-temporal dementia: Niemann-Pick type B and SQSTM1-associated Paget’s disease in the same individual. J. Bone Miner. Metab. 2019, 37, 378–383. [Google Scholar] [CrossRef]
- De Feo, D.; D’Anzi, A.; Pestrichella, V.; Scicchitano, P.; Lafranceschina, C.; Caragnano, V.; Tiecco, F.; Scialpi, A.; Laronga, G.; Ciccone, M.M.; et al. TAVI in Patient Suffering from Niemann-Pick Disease (Acid Sphingomyelinase Deficiency) with Concomitant Situs Inversus and Dextrocardia. Cardiol. Ther. 2023, 12, 409–414. [Google Scholar] [CrossRef]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Soleymani, Y.; Batouli, S.A.H.; Ahangar, A.A.; Pourabbasi, A. Association of glycosylated hemoglobin concentrations with structural and functional brain changes in the normoglycemic population: A systematic review. J. Neuroendocrinol. 2024, e13437. [Google Scholar] [CrossRef]
- Tominaga, M. [Diabetes mellitus and HbA1c]. Rinsho Byori 2003, 51, 586–591. [Google Scholar] [PubMed]
- Repple, J.; Karliczek, G.; Meinert, S.; Förster, K.; Grotegerd, D.; Goltermann, J.; Redlich, R.; Arolt, V.; Baune, B.T.; Dannlowski, U.; et al. Variation of HbA1c affects cognition and white matter microstructure in healthy, young adults. Mol. Psychiatry 2021, 26, 1399–1408. [Google Scholar] [CrossRef]
- Guo, B.; Li, Q.-y.; Liu, X.-j.; Luo, G.-h.; Wu, Y.-j.; Nie, J. Diabetes mellitus and Alzheimer’s disease: Vacuolar adenosine triphosphatase as a potential link. Eur. J. Neurosci. 2024, 59, 2577–2595. [Google Scholar] [CrossRef]
- Abosharaf, H.A.; Elsonbaty, Y.; Tousson, E.; Mohamed, T.M. Alzheimer’s disease-related brain insulin resistance and the prospective therapeutic impact of metformin. J. Neuroendocrinol. 2024, 36, e13356. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kang, Y.T.; Mendelson, F.E.; Hayes, J.M.; Savelieff, M.G.; Nagrath, S.; Feldman, E.L. Palmitate and glucose increase amyloid precursor protein in extracellular vesicles: Missing link between metabolic syndrome and Alzheimer’s disease. J. Extracell. Vesicles 2023, 12, e12340. [Google Scholar] [CrossRef]
- Eisenbaum, M.; Pearson, A.; Ortiz, C.; Mullan, M.; Crawford, F.; Ojo, J.; Bachmeier, C. ApoE4 expression disrupts tau uptake, trafficking, and clearance in astrocytes. Glia 2024, 72, 184–205. [Google Scholar] [CrossRef] [PubMed]
- Youn, C.; Caillaud, M.L.; Li, Y.; Gallagher, I.; Strasser, B.; Tanaka, H.; Haley, A.P. Interplay of Large Neutral Amino Acids, Metabolic Syndrome, and ApoE ε4 on Brain Integrity at Midlife. Lifestyle Genom. 2024. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhou, X.; Liu, Y.; Li, D.; Li, Y.; Zhang, T.; Fu, C.; Li, L.; Hu, Y.; Jiang, L. Serum amyloid beta 42 levels correlated with metabolic syndrome and its components. Front. Endocrinol. 2024, 15, 1278477. [Google Scholar] [CrossRef]
- Azizan, Z.; Zali, H.; Mirmotalebisohi, S.A.; Bazrgar, M.; Ahmadiani, A. Deciphering molecular bridges: Unveiling the interplay between metabolic syndrome and Alzheimer’s disease through a systems biology approach and drug repurposing. PLoS ONE 2024, 19, e0304410. [Google Scholar] [CrossRef]
- Okoshi, A.; Shinagawa, S.; Takasaki, E.; Susa, Y.; Inamura, K.; Shigeta, M. Risk factors of frontotemporal dementia compared with Alzheimer disease: Single psychiatric hospital–based research in Japan. Geriatr. Gerontol. Int. 2023, 23, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Zeraatkar, D.; Glymour, M.; Ellis, R.J.; Estiri, H.; Patel, C.J. Specification curve analysis to identify heterogeneity in risk factors for dementia: Findings from the UK Biobank. BMC Med. 2024, 22, 216. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, Y.; Ding, R.; Zheng, Z.; Wang, J.; Fu, Q.; Deng, J.; Zhou, H. Association between metabolically healthy obesity and risk of dementia: A systematic review and meta-analysis. Med. Adv. 2024, 2, 176–185. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Gan, W.; Babici, D.; Hagan, V.; Wald, R.; Swerdloff, M. Supranuclear Palsy as an Initial Presentation of the Adult-Onset Niemann-Pick Type C. Neurol. Int. 2024, 16, 561–566. [Google Scholar] [CrossRef]
- Xu, L.; Ota, T. Emerging roles of SGLT2 inhibitors in obesity and insulin resistance: Focus on fat browning and macrophage polarization. Adipocyte 2018, 7, 121–128. [Google Scholar] [CrossRef]
- Lebovitz, H.E. Thiazolidinediones: The Forgotten Diabetes Medications. Curr. Diabetes Rep. 2019, 19, 151. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, L.; Xing, M.; Xu, X.; Wei, J.; Wang, J.; Kang, W. Trelagliptin succinate: DPP-4 inhibitor to improve insulin resistance in adipocytes. Biomed. Pharmacother. 2020, 125, 109952. [Google Scholar] [CrossRef]
- Ray, S. Role of statins in the management of dyslipidaemia. Indian. Heart J. 2024, 76 (Suppl. S1), S33–S37. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S. PCSK9 inhibitors: Clinical evidence and implementation. Nat. Rev. Cardiol. 2019, 16, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Macyczko, J.R.; Liu, C.C.; Bu, G. ApoE4 reduction: An emerging and promising therapeutic strategy for Alzheimer’s disease. Neurobiol. Aging 2022, 115, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, V.C.; Paraskevas, G.P.; Paraskevas, P.G.; Stefanis, L.; Kapaki, E. Corticobasal degeneration and corticobasal syndrome: A review. Clin. Park. Relat. Disord. 2019, 1, 66–71. [Google Scholar] [CrossRef] [PubMed]
| Discussed Metabolic Disorder | Associations with Tauopathies |
|---|---|
| Dyslipidemia |
|
| Atherosclerosis | |
| Diabetes mellitus (DM) |
|
| Niemann-Pick disease (NPD) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fryncel, A.; Madetko-Alster, N.; Krępa, Z.; Kuch, M.; Alster, P. The Possible Associations between Tauopathies and Atherosclerosis, Diabetes Mellitus, Dyslipidemias, Metabolic Syndrome and Niemann–Pick Disease. Diagnostics 2024, 14, 1831. https://doi.org/10.3390/diagnostics14161831
Fryncel A, Madetko-Alster N, Krępa Z, Kuch M, Alster P. The Possible Associations between Tauopathies and Atherosclerosis, Diabetes Mellitus, Dyslipidemias, Metabolic Syndrome and Niemann–Pick Disease. Diagnostics. 2024; 14(16):1831. https://doi.org/10.3390/diagnostics14161831
Chicago/Turabian StyleFryncel, Aleksandra, Natalia Madetko-Alster, Zuzanna Krępa, Marek Kuch, and Piotr Alster. 2024. "The Possible Associations between Tauopathies and Atherosclerosis, Diabetes Mellitus, Dyslipidemias, Metabolic Syndrome and Niemann–Pick Disease" Diagnostics 14, no. 16: 1831. https://doi.org/10.3390/diagnostics14161831
APA StyleFryncel, A., Madetko-Alster, N., Krępa, Z., Kuch, M., & Alster, P. (2024). The Possible Associations between Tauopathies and Atherosclerosis, Diabetes Mellitus, Dyslipidemias, Metabolic Syndrome and Niemann–Pick Disease. Diagnostics, 14(16), 1831. https://doi.org/10.3390/diagnostics14161831






