No Correlation between PD-L1 and NIS Expression in Lymph Node Metastatic Papillary Thyroid Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’Andréa, G.; Lassalle, S.; Guevara, N.; Mograbi, B.; Hofman, P. From biomarkers to therapeutic targets: The promise of PD-L1 in thyroid autoimmunity and cancer. Theranostics 2021, 11, 1310–1325. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Fagin, J.A.; Wells, S.A. Biologic and Clinical Perspectives on Thyroid Cancer. N. Engl. J. Med. 2016, 75, 1054–1067. [Google Scholar] [CrossRef] [PubMed]
- Weitzman, S.P.; Sherman, S.I. Novel Drug Treatments of Progressive Radioiodine-Refractory Differentiated Thyroid Cancer. Endocrinol. Metab. Clin. N. Am. 2019, 48, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Dohán, O.; De la Vieja, A.; Paroder, V.; Riedel, C.; Artani, M.; Reed, M.; Ginter, C.S.; Carrasco, N. The sodium/iodide Symporter (NIS): Characterization, regulation, and medical significance. Endocr. Rev. 2003, 24, 48–77. [Google Scholar] [CrossRef]
- Shao, C.; Li, Z.; Zhang, C.; Zhang, W.; He, R.; Xu, J.; Cai, Y. Optical diagnostic imaging and therapy for thyroid cancer. Mater. Today Bio 2022, 26, 100441. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dohán, O.; Baloch, Z.; Bánrévi, Z.; Livolsi, V.; Carrasco, N. Rapid communication: Predominant intracellular overexpression of the Na+/I− symporter (NIS) in a large sampling of thyroid cancer cases. J. Clin. Endocrinol. Metab. 2001, 86, 2697–2700. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.; Gaweł, D.; Godlewska, M. Novel Inhibitor-Based Therapies for Thyroid Cancer—An Update. Int. J. Mol. Sci. 2021, 22, 11829. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhang, X.; Li, L.; Zhang, C.; Huang, J.; Huang, W. Molecular basis and targeted therapies for radioiodine refractory thyroid cancer. Asia Pac. J. Clin. Oncol. 2023, 19, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Chen, Y.; Li, X.; Long, S.; Shi, Y.; Yu, Y.; Wu, W.; Han, L.; Wang, S. The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Front. Immunol. 2022, 13, 964442. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kornepati, A.V.R.; Vadlamudi, R.K.; Curiel, T.J. Programmed death ligand 1 signals in cancer cells. Nat. Rev. Cancer 2022, 22, 174–189. [Google Scholar] [CrossRef] [PubMed]
- Levy, O.; Dai, G.; Riedel, C.; Ginter, C.S.; Paul, E.M.; Lebowitz, A.N.; Carrasco, N. Characterization of the thyroid Na+/I− symporter with an anti-COOH terminus antibody. Proc. Natl. Acad. Sci. USA 1997, 27, 5568–5573. [Google Scholar] [CrossRef] [PubMed]
- Fadia, M.; Fookeerah, P.; Ali, S.; Shadbolt, B.; Greenaway, T.; Perampalam, S. PD-L1 expression in papillary thyroid cancer with and without lymphocytic thyroiditis: A cross sectional study. Pathology 2020, 52, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Veyhl, J.; Jessa, F.; Polyakova, O.; Alenzi, A.; MacMillan, C.; Ralhan, R.; Walfish, P.G. Programmed death-ligand 1 overexpression is a prognostic marker for aggressive papillary thyroid cancer and its variants. Oncotarget 2016, 31, 32318–32328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wapnir, I.L.; Van De Rijn, M.; Nowels, K.; Amenta, P.S.; Walton, K.; Montgomery, K.; Greco, R.S.; Dohán, O.; Carrasco, N. Immunohistochemical profile of the sodium/iodide symporter in thyroid, breast, and other carcinomas using high density tissue microarrays and conventional sections. J. Clin. Endocrinol. Metab. 2003, 88, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Wan, B.; Deng, P.; Dai, W.; Wang, P.; Dong, Z.; Yang, C.; Tian, J.; Hu, T.; Yan, K. Association between programmed cell death ligand 1 expression and thyroid cancer: A meta-analysis. Medicine 2021, 100, 25315. [Google Scholar] [CrossRef] [PubMed]
- Lubin, D.; Baraban, E.; Lisby, A.; Jalali-Farahani, S.; Zhang, P.; Livolsi, V. Papillary Thyroid Carcinoma Emerging from Hashimoto Thyroiditis Demonstrates Increased PD-L1 Expression, Which Persists with Metastasis. Endocr. Pathol. 2018, 29, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.L.; Marcello, M.A.; Ward, L.S. The role of the inflammatory microenvironment in thyroid carcinogenesis. Endocr. Relat. Cancer 2014, 21, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Niu, D.; Huang, X.; Jia, L.; Kang, Q.; Dou, F.; Ji, X.; Xue, W.; Liu, Y.; Li, Z.; et al. PD-L1 and PD-1 expression are correlated with distinctive clinicopathological features in papillary thyroid carcinoma. Diagn Pathol. 2017, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Karapanou, O.; Simeakis, G.; Vlassopoulou, B.; Alevizaki, M.; Saltiki, K. Advanced RAI-refractory thyroid cancer: An update on treatment perspectives. Endocr. Relat. Cancer 2022, 22, 57–66. [Google Scholar] [CrossRef] [PubMed]
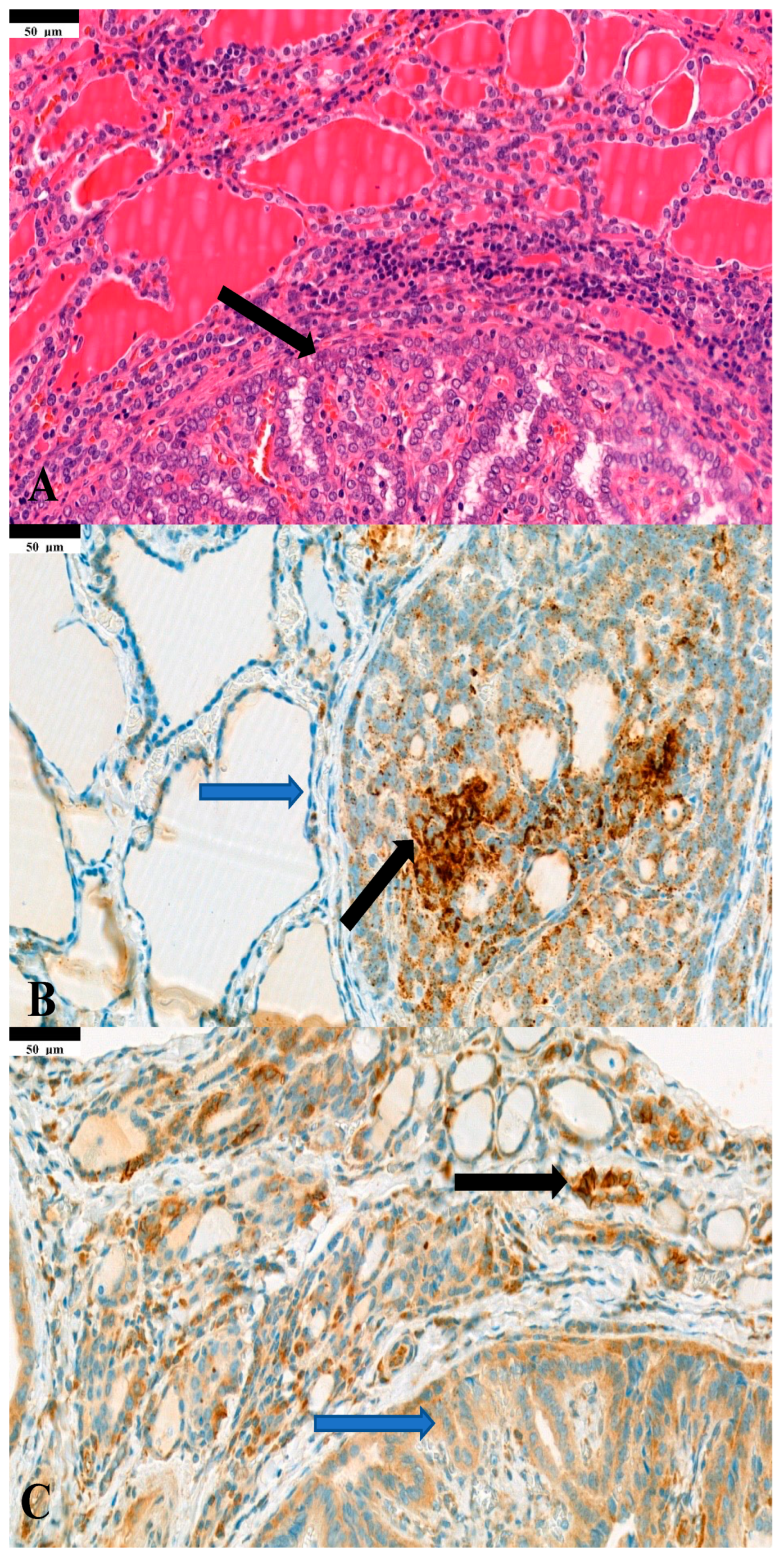
| Parameters | Mean (Min–Max) |
|---|---|
| PD-L1 (%) | 17.7 (0.0–90.0) |
| Range | 1.8 (1.0–3.0) |
| NIS total (%) | 18.7 (0.0–100.0) |
| NIS only citoplasmatic (%) | 17.2 (0.0–100.0) |
| NIS plasmamembrane (%) also present | 1.5 (0.0–30.0) |
| Clinicopathological Parameter | PD-L1 | NIS |
|---|---|---|
| Gender | NS | NS |
| Age | 0.0183 | NS |
| TNM stage | NS | NS |
| Tumor size | 0.0237 | NS |
| Multifocality | NS | NS |
| (Nr.) Number of metastatic lymph nodes | NS | NS |
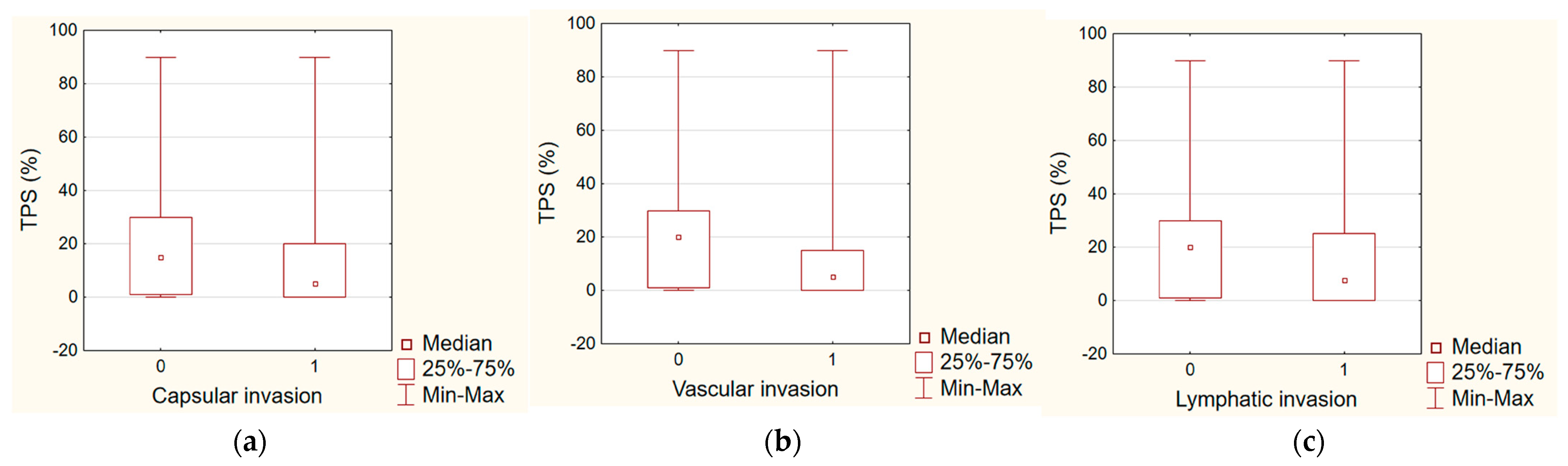
| Capsular invasion | NS | NS |
| Vascular invasion | 0.0165 | NS |
| Lymphatic invasion | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernadett, L.; Alexandra, K.; Georgina, F.; Erika, T.; András, S.; Ilona, P.; Ferenc, O.; Orsolya, D. No Correlation between PD-L1 and NIS Expression in Lymph Node Metastatic Papillary Thyroid Carcinoma. Diagnostics 2024, 14, 1858. https://doi.org/10.3390/diagnostics14171858
Bernadett L, Alexandra K, Georgina F, Erika T, András S, Ilona P, Ferenc O, Orsolya D. No Correlation between PD-L1 and NIS Expression in Lymph Node Metastatic Papillary Thyroid Carcinoma. Diagnostics. 2024; 14(17):1858. https://doi.org/10.3390/diagnostics14171858
Chicago/Turabian StyleBernadett, Lévay, Kiss Alexandra, Fröhlich Georgina, Tóth Erika, Slezák András, Péter Ilona, Oberna Ferenc, and Dohán Orsolya. 2024. "No Correlation between PD-L1 and NIS Expression in Lymph Node Metastatic Papillary Thyroid Carcinoma" Diagnostics 14, no. 17: 1858. https://doi.org/10.3390/diagnostics14171858





