Asthma and Hyperglycemia: Exploring the Interconnected Pathways
Abstract
1. Introduction
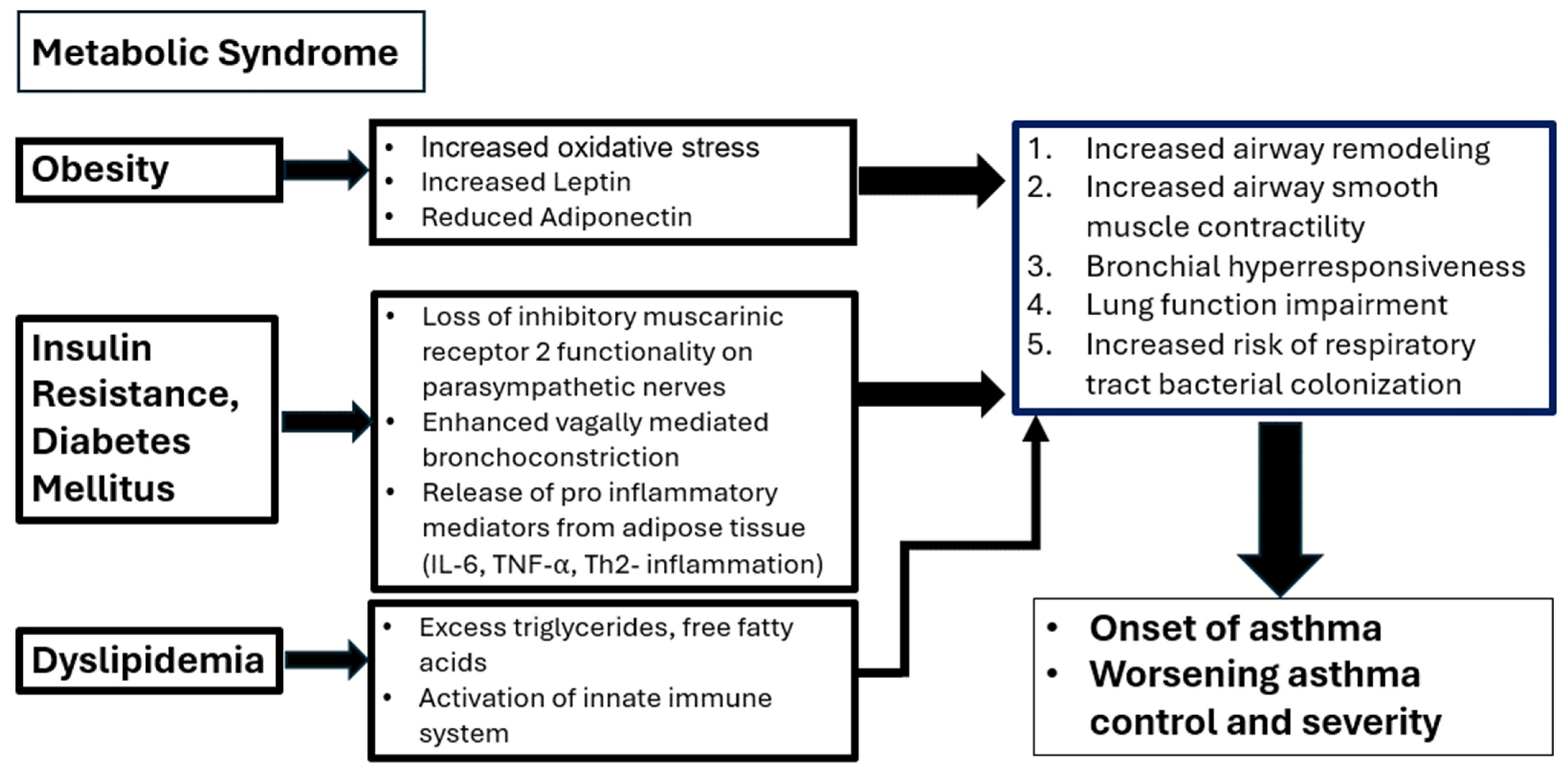
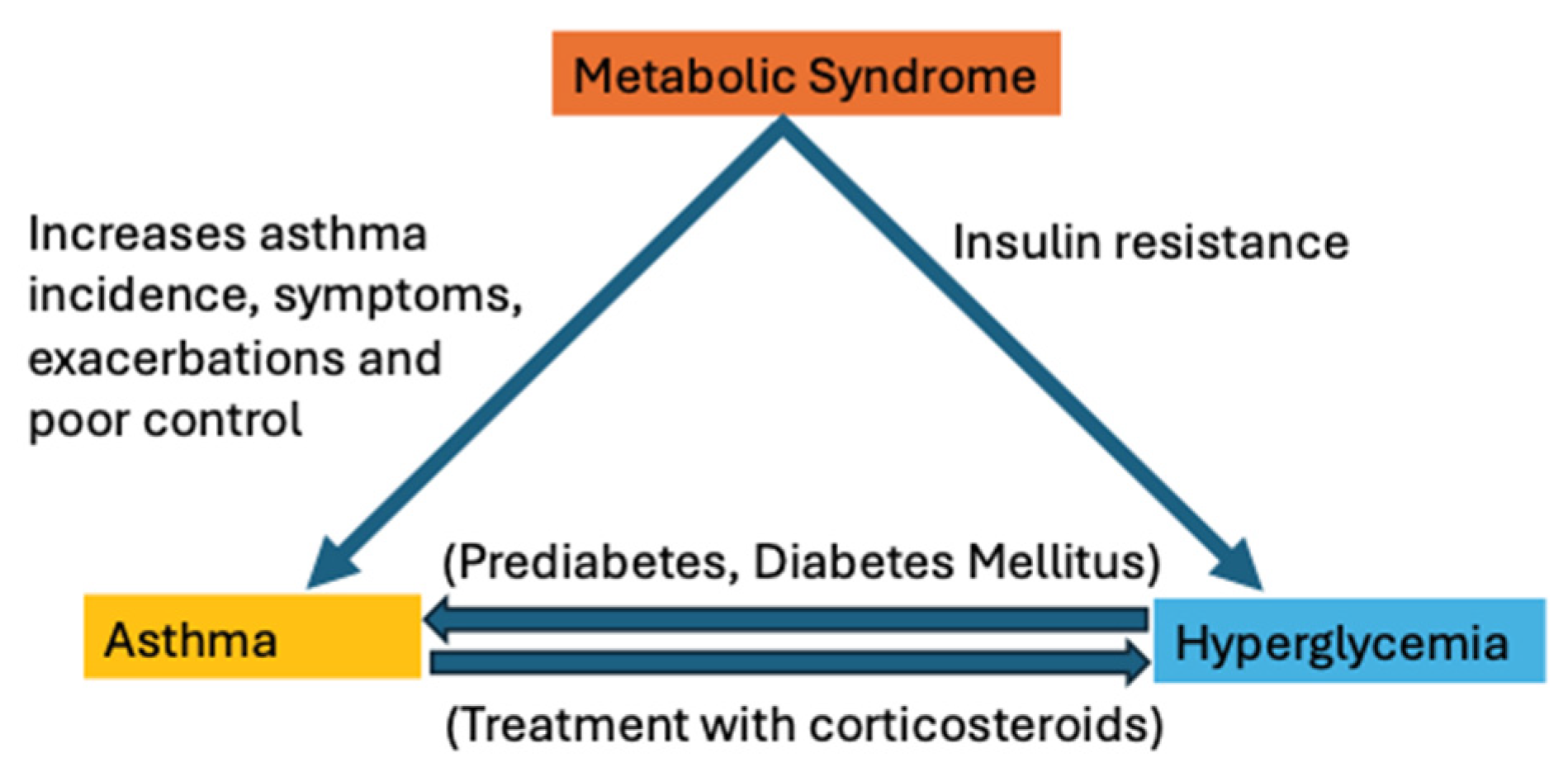
2. Pathophysiological Link between Asthma and Hyperglycemia
3. Effect of Asthma Medications on Glucose
4. Treatment Implications
5. Effect of Antidiabetic Medications on Asthma
6. Future Directions and Research Needs
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- GINA Committee. Global Strategy for Asthma Management and Prevention 2023 Update; Global Initiative for Asthma: Fontana, WI, USA, 2023. [Google Scholar]
- Bentur, L.; Gur, M.; Hakim, F. Better understanding of childhood asthma, towards primary prevention—Are we there yet? Consideration of pertinent literature. F1000Research 2017, 6, 2152. [Google Scholar]
- Momtazmanesh, S.; Moghaddam, S.S.; Ghamari, S.H.; Rad, E.M.; Rezaei, N.; Shobeiri, P.; Aali, A.; Abbasi-Kangevari, M.; Abbasi-Kangevari, Z.; Abdelmasseh, M. Global burden of chronic respiratory diseases and risk factors, 1990–2019: An update from the Global Burden of Disease Study 2019. EClinicalMedicine 2023, 59, 101936. [Google Scholar] [CrossRef] [PubMed]
- Sgrazzutti, L.; Sansone, F.; Attanasi, M.; Di Pillo, S.; Chiarelli, F. Coaggregation of asthma and type 1 diabetes in children: A narrative review. Int. J. Mol. Sci. 2021, 22, 5757. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.D. Diabetes, insulin resistance, and asthma: A review of potential links. Curr. Opin. Pulm. Med. 2021, 27, 29–36. [Google Scholar] [CrossRef]
- Dungan, K.M.; Braithwaite, S.S.; Preiser, J.C. Stress hyperglycaemia. Lancet 2009, 373, 1798–1807. [Google Scholar] [CrossRef] [PubMed]
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [CrossRef]
- Huang, P.L. A comprehensive definition for metabolic syndrome. DMM Dis. Models Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef]
- Beltrán-Sánchez, H.; Harhay, M.O.; Harhay, M.M.; McElligott, S. Prevalence and Trends of Metabolic Syndrome in the Adult U.S. Population, 1999–2010. J. Am. Coll. Cardiol. 2013, 62, 697–703. [Google Scholar] [CrossRef]
- Mohamed, S.M.; Shalaby, M.A.; El-Shiekh, R.A.; El-Banna, H.A.; Emam, S.R.; Bakr, A.F. Metabolic syndrome: Risk factors, diagnosis, pathogenesis, and management with natural approaches. Food Chem. Adv. 2023, 3, 100335. [Google Scholar] [CrossRef]
- Molina-Luque, R.; Molina-Recio, G.; de-Pedro-Jiménez, D.; Fernández, C.Á.; García-Rodríguez, M.; Romero-Saldaña, M. The Impact of Metabolic Syndrome Risk Factors on Lung Function Impairment: Cross-Sectional Study. JMIR Public Health Surveill. 2023, 9, e43737. [Google Scholar] [CrossRef]
- Leone, N.; Courbon, D.; Thomas, F.; Bean, K.; Jégo, B.; Leynaert, B.; Guize, L.; Zureik, M. Lung function impairment and metabolic syndrome the critical role of abdominal obesity. Am. J. Respir. Crit. Care Med. 2009, 179, 509–516. [Google Scholar] [CrossRef]
- Forno, E.; Han, Y.Y.; Muzumdar, R.H.; Celedón, J.C. Insulin resistance, metabolic syndrome, and lung function in US adolescents with and without asthma. J. Allergy Clin. Immunol. 2015, 136, 304–311. [Google Scholar] [CrossRef]
- Forno, E.; Han, Y.Y.; Mullen, J.; Celedón, J.C. Overweight, Obesity, and Lung Function in Children and Adults—A Meta-analysis. J. Allergy Clin. Immunol. Pract. 2018, 6, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Carpaij, O.A.; Van Den Berge, M. The asthma-obesity relationship: Underlying mechanisms and treatment implications. Curr. Opin. Pulm. Med. 2018, 24, 42–49. [Google Scholar] [CrossRef]
- Cardet, J.C.; Ash, S.; Kusa, T.; Camargo, C.A.; Israel, E. Insulin resistance modifies the association between obesity and current asthma in adults. Eur. Respir. J. 2016, 48, 403–410. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, B.; Wang, Y.; Wang, K.; Zhang, Z.; Niu, W. Pre-pregnancy maternal weight and gestational weight gain increase the risk for childhood asthma and wheeze: An updated meta-analysis. Front. Pediatr. 2020, 8, 134. [Google Scholar] [CrossRef]
- Muc, M.; Mota-Pinto, A.; Padez, C. Association between obesity and asthma—Epidemiology, pathophysiology and clinical profile. Nutr. Res. Rev. 2016, 29, 194–201. [Google Scholar] [CrossRef]
- Brumpton, B.M.; Camargo, C.A.; Romundstad, P.R.; Langhammer, A.; Chen, Y.; Mai, X.M. Metabolic syndrome and incidence of asthma in adults: The HUNT study. Eur. Respir. J. 2013, 42, 1495–1502. [Google Scholar] [CrossRef]
- Khateeb, J.; Fuchs, E.; Khamaisi, M. Diabetes and lung disease: An underestimated relationship. Rev. Diabet. Stud. 2019, 15, 1–15. [Google Scholar] [CrossRef]
- Sivapalan, P.; Diamant, Z.; Ulrik, C.S. Obesity and asthma: Current knowledge and future needs. Curr. Opin. Pulm. Med. 2015, 21, 80–85. [Google Scholar] [CrossRef]
- Cordova-Rivera, L.; Gibson, P.G.; Gardiner, P.A.; McDonald, V.M. A systematic review of associations of physical activity and sedentary time with asthma outcomes. J. Allergy Clin. Immunol. Pract. 2018, 6, 1968–1981. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Suzukawa, M.; Arakawa, S.; Kobayashi, K.; Igarashi, S.; Tashimo, H.; Nagai, H.; Tohma, S.; Nagase, T.; Ohta, K. Leptin enhances cytokine/chemokine production by normal lung fibroblasts by binding to leptin receptor. Allergol. Int. 2019, 68, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Mather, K.J.; Goldberg, R.B. Clinical use of adiponectin as a marker of metabolic dysregulation. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 107–117. [Google Scholar] [CrossRef]
- Santos Coelho, R.; Paula Castro Melo, A.; dos Santos Silva, H.; De Cassia Ribeiro Silva, R.; Maria Alvim Matos, S.; Lima Barreto, M.; Alcantara-Neves, N.M.; Viana de Figueiredo, C.A.; do Santos Costa, R. ADIPOQ and LEP variants on asthma and atopy: Genetic association modified by overweight. Gene 2021, 781, 145540. [Google Scholar] [CrossRef]
- Yung, J.A.; Fuseini, H.; Newcomb, D.C. Hormones, sex, and asthma. Ann. Allergy Asthma Immunol. 2018, 120, 488–494. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petersen, M.C.; Shulman, G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Bartziokas, K.; Papaioannou, A.I.; Drakopanagiotakis, F.; Gouveri, E.; Papanas, N.; Steiropoulos, P. Unraveling the Link between Ιnsulin Resistance and Bronchial Asthma. Biomedicines 2024, 12, 437. [Google Scholar] [CrossRef] [PubMed]
- Kauppi, P.; Linna, M.; Jantunen, J.; Martikainen, J.E.; Haahtela, T.; Pelkonen, A.; Makela, M. Chronic co-morbidities increase burden and costs of persistent asthma. Allergy Eur. J. Allergy Clin. Immunol. 2016, 71 (Suppl. 102), 537–538. [Google Scholar]
- Kauppi, P.; Linna, M.; Jantunen, J.; Martikainen, J.E.; Haahtela, T.; Pelkonen, A.; Mäkelä, M. Chronic comorbidities contribute to the burden and costs of persistent asthma. Mediat. Inflamm. 2015, 2015, 819194. [Google Scholar] [CrossRef]
- Ehrlich, S.F.; Quesenberry, C.P.; Van Den Eeden, S.K.; Shan, J.; Ferrara, A. Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care 2010, 33, 55–60. [Google Scholar] [CrossRef]
- Uppal, P.; Mohammed, S.A.; Rajashekar, S.; Giri Ravindran, S.; Kakarla, M.; Gambo, M.A.; Salama, M.Y.; Ismail, N.H.; Tavalla, P.; Hamid, P. Type 2 Diabetes Mellitus and Asthma: Pathomechanisms of Their Association and Clinical Implications. Cureus 2023, 15, e36047. [Google Scholar] [CrossRef]
- Wu, T.D.; Brigham, E.P.; Keet, C.A.; Brown, T.T.; Hansel, N.N.; McCormack, M.C. Association Between Prediabetes/Diabetes and Asthma Exacerbations in a Claims-Based Obese Asthma Cohort. J. Allergy Clin. Immunol. Pract. 2019, 7, 1868–1873. [Google Scholar] [CrossRef]
- Milutinovic, P.S.; Alcorn, J.F.; Englert, J.M.; Crum, L.T.; Oury, T.D. The receptor for advanced glycation end products is a central mediator of asthma pathogenesis. Am. J. Pathol. 2012, 181, 1215–1225. [Google Scholar] [CrossRef]
- Ramasamy, R.; Yan, S.F.; Schmidt, A.M. RAGE: Therapeutic target and biomarker of the inflammatory response—The evidence mounts. J. Leukoc. Biol. 2009, 86, 505–512. [Google Scholar] [CrossRef]
- Peters, M.C.; McGrath, K.W.; Hawkins, G.A.; Hastie, A.T.; Levy, B.D.; Israel, E.; Phillips, B.R.; Mauger, D.T.; A Comhair, S.; Erzurum, S.C.; et al. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: A cross-sectional analysis of two cohorts. Lancet Respir. Med. 2016, 4, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yang, J.; Xiao, Q.; Lü, Y.; Zhou, X.; Xia, L.; Nie, D. Regulation of high glucose-mediated mucin expression by matrix metalloproteinase-9 in human airway epithelial cells. Exp. Cell Res. 2015, 333, 127–135. [Google Scholar] [CrossRef]
- See, K.C. Impact of inhaled and intranasal corticosteroids on glucose metabolism and diabetes mellitus: A mini review. World J. Diabetes 2023, 14, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Volmer, T.; Effenberger, T.; Trautner, C.; Buhl, R. Consequences of long-term oral corticosteroid therapy and its side-effects in severe asthma in adults: A focused review of the impact data in the literature. Eur. Respir. J. 2018, 52, 1800703. [Google Scholar] [CrossRef] [PubMed]
- Alwarith, J.; Kahleova, H.; Crosby, L.; Brooks, A.; Brandon, L.; Levin, S.M.; Barnard, N.D. The role of nutrition in asthma prevention and treatment. Nutr. Rev. 2020, 78, 928–938. [Google Scholar] [CrossRef]
- Hakala, K.; Stenius-Aarniala, B.; Sovijärvi, A. Effects of weight loss on peak flow variability, airways obstruction, and lung volumes in obese patients with asthma. Chest 2000, 118, 1315–1321. [Google Scholar] [CrossRef]
- Stenius-Aarniala, B.; Poussa, T.; Kvarnström, J.; Grönlund, E.L.; Ylikahri, M.; Mustajoki, P. Immediate and long term effects of weight reduction in obese people with asthma: Randomised controlled study. Br. Med. J. 2000, 320, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Juel, C.T.B.; Ali, Z.; Nilas, L.; Ulrik, C.S. Asthma and obesity: Does weight loss improve asthma control? A systematic review. J. Asthma Allergy 2012, 5, 21–26. [Google Scholar]
- Novosad, S.; Khan, S.; Wolfe, B.; Khan, A. Role of Obesity in Asthma Control, the Obesity-Asthma Phenotype. J. Allergy 2013, 2013, 538642. [Google Scholar] [CrossRef] [PubMed]
- Özbey, Ü.; Balaban, S.; Sözener, Z.Ç.; Uçar, A.; Mungan, D.; Mısırlıgil, Z. The effects of diet-induced weight loss on asthma control and quality of life in obese adults with asthma: A randomized controlled trial. J. Asthma 2020, 57, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Johnson, O.; Gerald, L.B.; Harvey, J.; Roy, G.; Hazucha, H.; Large, C.; Burke, A.; McCormack, M.; Wise, R.A.; Holbrook, J.T.; et al. An Online Weight Loss Intervention for People With Obesity and Poorly Controlled Asthma. J. Allergy Clin. Immunol. Pract. 2022, 10, 1577–1586. [Google Scholar] [CrossRef]
- Park, C.S.; Bang, B.R.; Kwon, H.S.; Moon, K.A.; Kim, T.B.; Lee, K.Y.; Moon, H.B.; Cho, Y.S. Metformin reduces airway inflammation and remodeling via activation of AMP-activated protein kinase. Biochem. Pharmacol. 2012, 84, 1660–1670. [Google Scholar] [CrossRef]
- Wu, T.D.; Keet, C.A.; Fawzy, A.; Segal, J.B.; Brigham, E.P.; McCormack, M.C. Association of Metformin Initiation and Risk of Asthma Exacerbation A Claims-based Cohort Study. Ann. Am. Thorac. Soc. 2019, 16, 1527–1533. [Google Scholar] [CrossRef]
- Calixto, M.C.; Lintomen, L.; André, D.M.; Leiria, L.O.; Ferreira, D.; Lellis-Santos, C.; Anhê, G.F.; Bordin, S.; Landgraf, R.G.; Antunes, E. Metformin Attenuates the Exacerbation of the Allergic Eosinophilic Inflammation in High Fat-Diet-Induced Obesity in Mice. PLoS ONE 2013, 8, e76786. [Google Scholar] [CrossRef]
- Rayner, L.H.; Mcgovern, A.; Sherlock, J.; Gatenby, P.; Correa, A.; Creagh-Brown, B.; Delusignan, S. The impact of therapy on the risk of asthma in type 2 diabetes. Clin. Respir. J. 2019, 13, 299–305. [Google Scholar] [CrossRef]
- Ge, D.; Foer, D.; Cahill, K.N. Utility of Hypoglycemic Agents to Treat Asthma with Comorbid Obesity. Pulm. Ther. 2023, 9, 71–89. [Google Scholar] [CrossRef]
- Kaler, M.; Barochia, A.V.; Weir, N.A.; Cuento, R.A.; Stylianou, M.; Roth, M.J.; Filie, A.C.; Vaughey, E.C.; Nathan, S.D.; Levine, S.J. A randomized, placebo-controlled, double-blinded, crossover trial of pioglitazone for severe asthma. J. Allergy Clin. Immunol. 2017, 140, 1716–1718. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.R.; Mortimer, K.; Pang, L.; Smith, K.M.; Bailey, H.; Hodgson, D.B.; Shaw, D.E.; Knox, A.J.; Harrison, T.W. Evaluation of the PPAR-γ agonist pioglitazone in mild asthma: A double-blind randomized controlled trial. PLoS ONE 2016, 11, e0160257. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.E.; Subramanian, M.; DeSarno, M.; Black, K.; Lane, L.; Holguin, F. A pilot randomized controlled trial of pioglitazone for the treatment of poorly controlled asthma in obesity. Respir. Res. 2015, 16, 143. [Google Scholar] [CrossRef]
- Wang, A.; Tang, H.; Zhang, N.; Feng, X. Association between novel Glucose-Lowering drugs and risk of Asthma: A network Meta-Analysis of cardiorenal outcome trials. Diabetes Res. Clin. Pr. 2022, 183, 109080. [Google Scholar] [CrossRef]
- Zhu, T.; Wu, X.L.; Zhang, W.; Xiao, M. Glucagon like peptide-1 (GLP-1) modulates OVA-induced airway inflammation and mucus secretion involving a protein kinase A (PKA)-dependent nuclear factor-κB (NF-κB) signaling pathway in mice. Int. J. Mol. Sci. 2015, 16, 20195–20211. [Google Scholar] [CrossRef]
- Kaplan, A.G.; Kim, J.W. Asthma Exacerbations and Glucagon-Like Peptide-1 Receptor Agonists: A Review of the Current Evidence. Pulm. Therapy. 2022, 8, 343–358. [Google Scholar] [CrossRef]
- Rogliani, P.; Matera, M.G.; Calzetta, L.; Hanania, N.A.; Page, C.; Rossi, I.; Andreadi, A.; Galli, A.; Coppola, A.; Cazzola, M.; et al. Long-term observational study on the impact of GLP-1R agonists on lung function in diabetic patients. Respir. Med. 2019, 154, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Z.; Hsu, C.H.; Li, C.Y.; Hsiue, T.R. Insulin use increases risk of asthma but metformin use reduces the risk in patients with diabetes in a Taiwanese population cohort. J. Asthma 2017, 54, 1019–1025. [Google Scholar] [CrossRef]
- Terzano, C.; Morano, S.; Ceccarelli, D.; Conti, V.; Paone, G.; Petroianni, A.; Graziani, E.; Carnovale, A.; Fallarino, M.; Gatti, A.; et al. Effect of insulin on airway responsiveness in patients with type 2 diabetes mellitus: A cohort study. J. Asthma 2009, 46, 703–707. [Google Scholar] [CrossRef]
- Singh, S.; Bodas, M.; Bhatraju, N.K.; Pattnaik, B.; Gheware, A.; Parameswaran, P.K.; Thompson, M.; Freeman, M.; Mabalirajan, U.; Gosens, R.; et al. Hyperinsulinemia adversely affects lung structure and function. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2016, 310, L837–L845. [Google Scholar] [CrossRef]
|
|
|
|
|
|
|
|
|
|
|
| Antidiabetic Drugs | Mechanism of Action | Effects on Asthma |
|---|---|---|
| Metformin |
|
|
| Sulfonylureas |
|
|
| Thiazolidines (TZD) |
|
|
| Sodium-glucose cotransporter-2 inhibitors (SGLT-2) |
|
|
| Glucagon like peptide 1 agonists (GLP-1A) |
|
|
| Dipeptidyl peptodase-4 inhibitors (DPP4is) |
|
|
| Insulin |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narendra, D.K.; Khurana, S. Asthma and Hyperglycemia: Exploring the Interconnected Pathways. Diagnostics 2024, 14, 1869. https://doi.org/10.3390/diagnostics14171869
Narendra DK, Khurana S. Asthma and Hyperglycemia: Exploring the Interconnected Pathways. Diagnostics. 2024; 14(17):1869. https://doi.org/10.3390/diagnostics14171869
Chicago/Turabian StyleNarendra, Dharani K., and Sandhya Khurana. 2024. "Asthma and Hyperglycemia: Exploring the Interconnected Pathways" Diagnostics 14, no. 17: 1869. https://doi.org/10.3390/diagnostics14171869
APA StyleNarendra, D. K., & Khurana, S. (2024). Asthma and Hyperglycemia: Exploring the Interconnected Pathways. Diagnostics, 14(17), 1869. https://doi.org/10.3390/diagnostics14171869






