Cardiovascular Disease Risk Stratification Using Hybrid Deep Learning Paradigm: First of Its Kind on Canadian Trial Data
Abstract
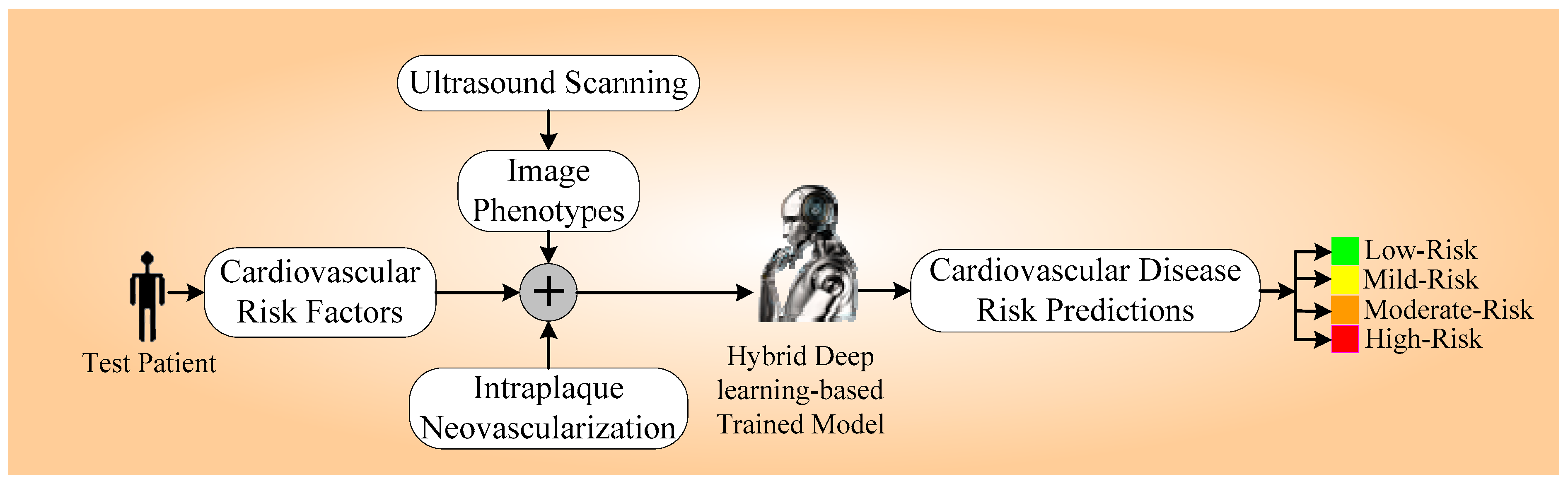
1. Introduction
2. The Background Literature
3. Methodology
3.1. Patient Demographics and Baseline Characteristics
3.2. Acquisition of Ultrasound Scans and Intraplaque Neovascularization
3.3. Cardiovascular Disease Endpoint: Angiographic Score
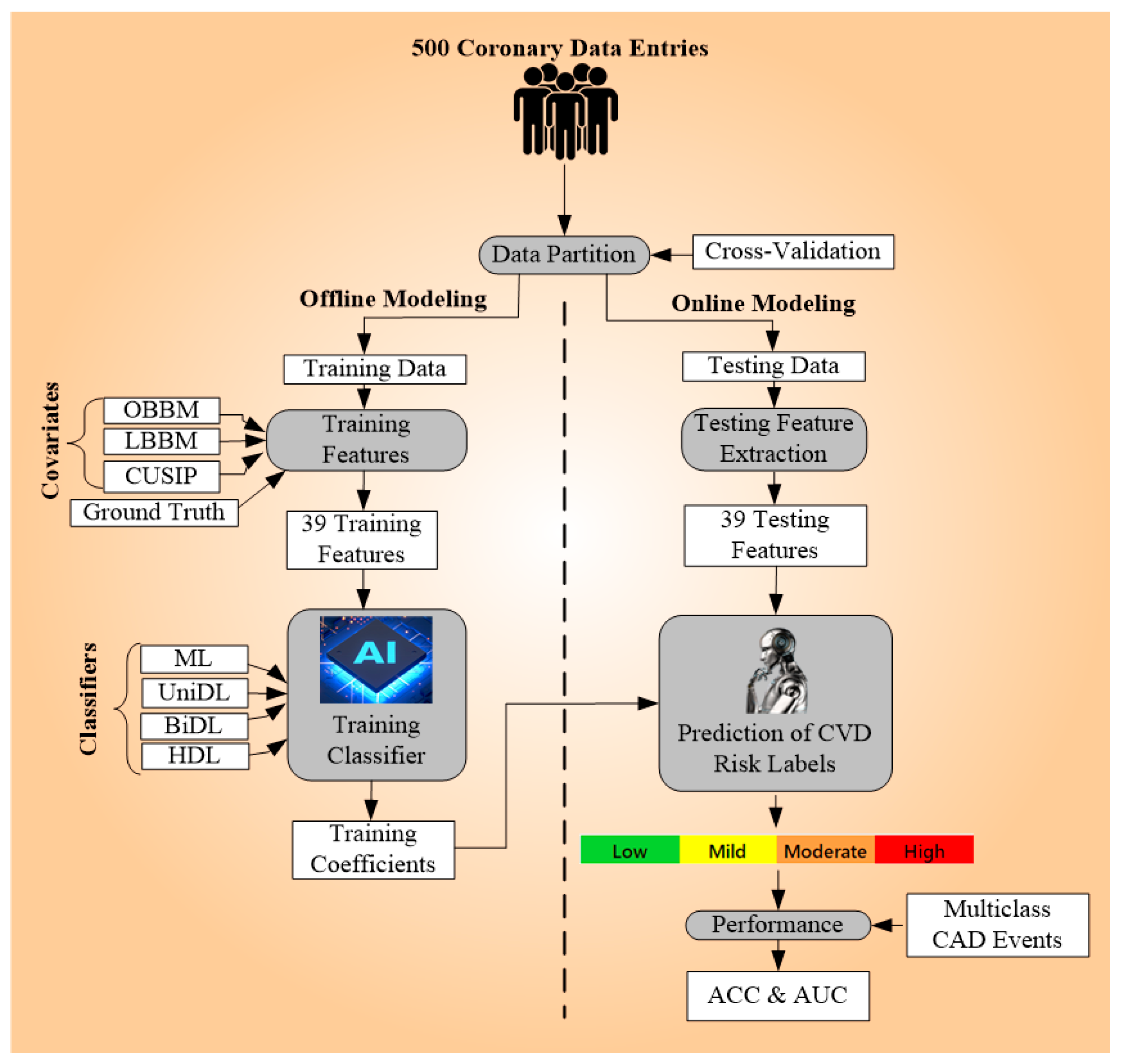
3.4. Overall Architecture
3.4.1. Data Preparation and Pre-Processing
3.4.2. Model Building
3.4.3. The Rationale behind Using RNN, GRU, and LSTM
3.5. Experimental Protocols
3.5.1. Experimental Protocol 1: Three Unidirectional Models
3.5.2. Experimental Protocol 2: Three Bidirectional Models
BiRNN
BiLSTM
BiGRU
3.5.3. Experimental Protocol 3: Six Hybrid Models
3.6. Loss Function and Training Parameters
3.7. Performance Metrics
4. Results
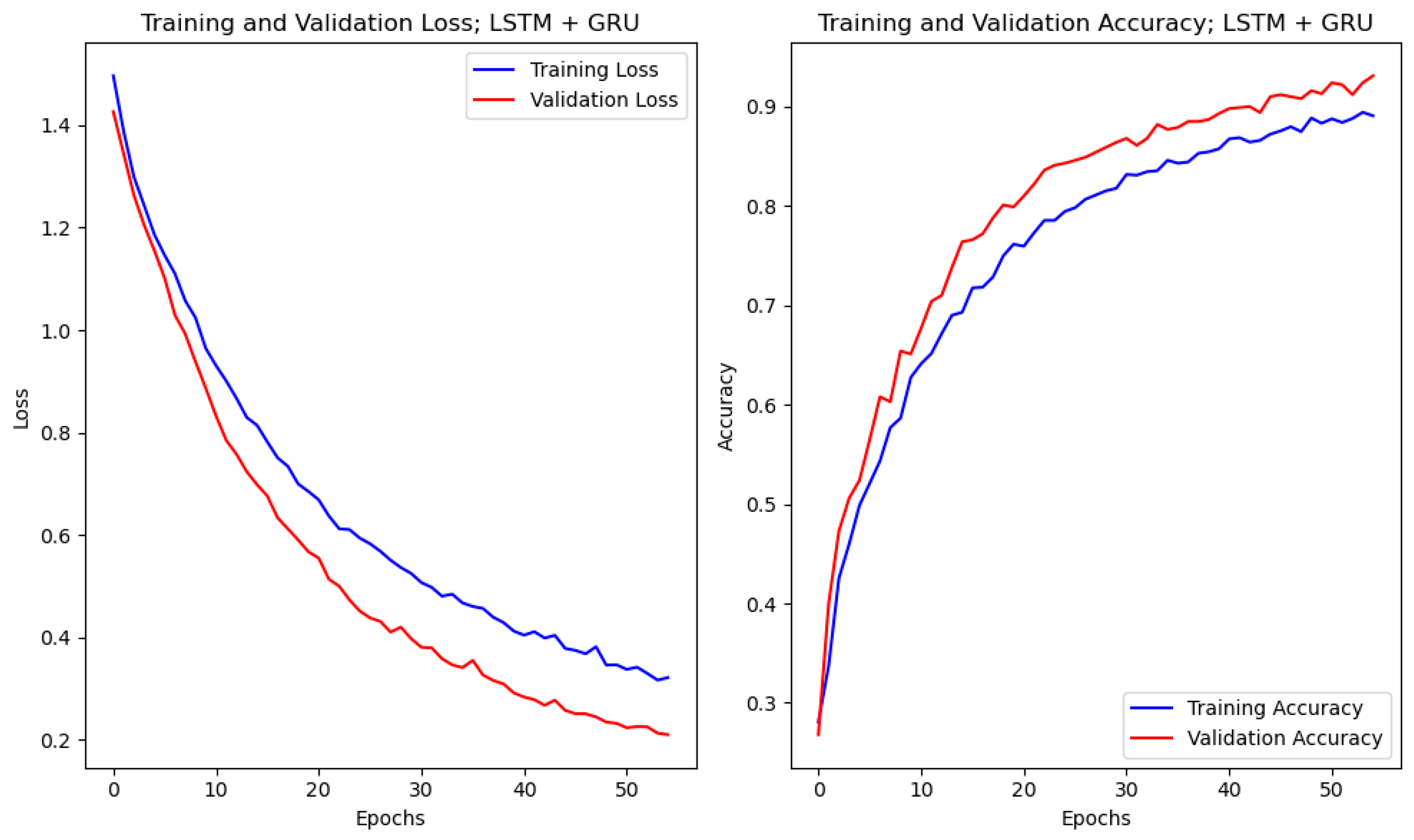
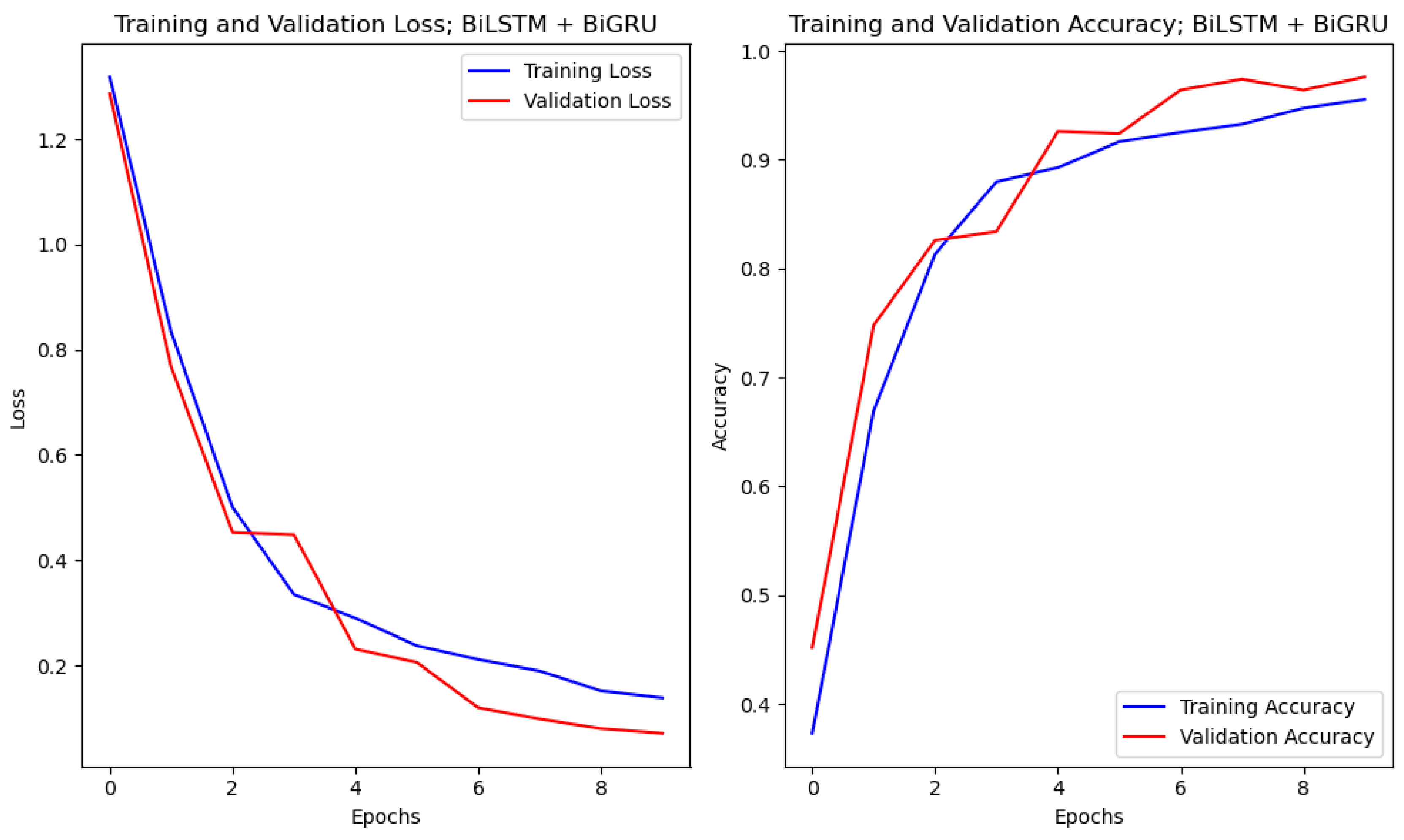
4.1. Accuracy and Loss Curves
4.2. Results of Experimental Protocol 1
4.3. Results of Experimental Protocol 2
4.4. Results of Experimental Protocol 3
4.5. Comparison of Three Feature Selection Methods for Three Machine Learning Models
5. Performance Evaluation
5.1. Receiver Operating Characteristics of All Four Kinds of AI Models
5.2. Effect of Sample Sizes on the Training System
5.3. Hybrid Deep Learning Performance against Other AI Models
6. Scientific Validation
7. Reliability and Stability of Hybrid Deep Learning System
8. Discussion
8.1. Benchmarking Table
8.2. A Special Note on Hybrid Deep Learning
8.3. Strengths, Weaknesses, and Extensions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Unidirectional Architecture
| SN | Risk Factors or Target Variable | Samples (N) or Mean | Pr (%) |
|---|---|---|---|
| 1 | Age | 64.49 years | - |
| 2 | Gender | 349 | 69.8 |
| 3 | Obesity | 215 | 43.0 |
| 4 | Ethnicity | 486 | 97.2 |
| 5 | BMI | 31.12 kg/m2 | - |
| 6 | Hypertension | 338 | 67.6 |
| 7 | Angina | 124 | 24.8 |
| 8 | Diastolic blood pressure | 76.7 mmHg | - |
| 9 | Systolic blood pressure | 135.35 mmHg | - |
| 10 | Smoking history | 330 | 66.0 |
| 11 | Casual smoker | 15 | 3.0 |
| 12 | Current smoker | 100 | 20.0 |
| 13 | Previous smoker | 218 | 43.6 |
| 14 | Drinks | 4.94 ± 10.4 per week | - |
| 15 | Family history of diabetics | 195 | 39.0 |
| 16 | Premature CVD in the family | 146 | 29.2 |
| 17 | CVD in the family | 321 | 64.2 |
| 18 | Pre-diabetic | 20 | 40.0 |
| 19 | Hyperlipidemia | 288 | 57.6 |
| 20 | Type II diabetes | 114 | 22.8 |
| 21 | Type I diabetes | 5 | 1.0 |
| 22 | Creatinine | 83.99 ± 22.6 μmol/L | - |
| 23 | Estimated glomerular filtration rate | 78.96 mL/min/1.73 m2 | - |
| 24 | Diabetes of any type | 118 | 23.6 |
| 25 | Total plaque area (TPA) | 47.68 mm2 | - |
| 26 | Maximum plaque height (MPH) | 2.64 mm | - |
| 27 | Intra-plaque neovascularization (IPN) | 1.16 | - |
| 28 | Angiotensin-converting enzyme (ACE) inhibitors | 191 | 38.2 |
| 29 | HMG-Co reductase inhibitors | 272 | 54.4 |
| 30 | Angiotensin receptor blockers (ARBs) | 45 | 9.0 |
| 31 | Other antilipemic agents | 9 | 1.80 |
| 32 | Alpha-blockers | 30 | 6.0 |
| 33 | Calcium channel-blockers | 93 | 18.6 |
| 34 | Beta-blockers | 236 | 47.2 |
| 35 | Diuretics | 99 | 19.8 |
| 36 | Anti-platelet | 368 | 73.6 |
| 37 | Insulin | 38 | 7.6 |
| 38 | Anti-anginals and NSAIDS | 81 | 16.20 |
| 39 | Non-insulin diabetes medications | 72 | 14.4 |
Appendix B. Unidirectional Architecture


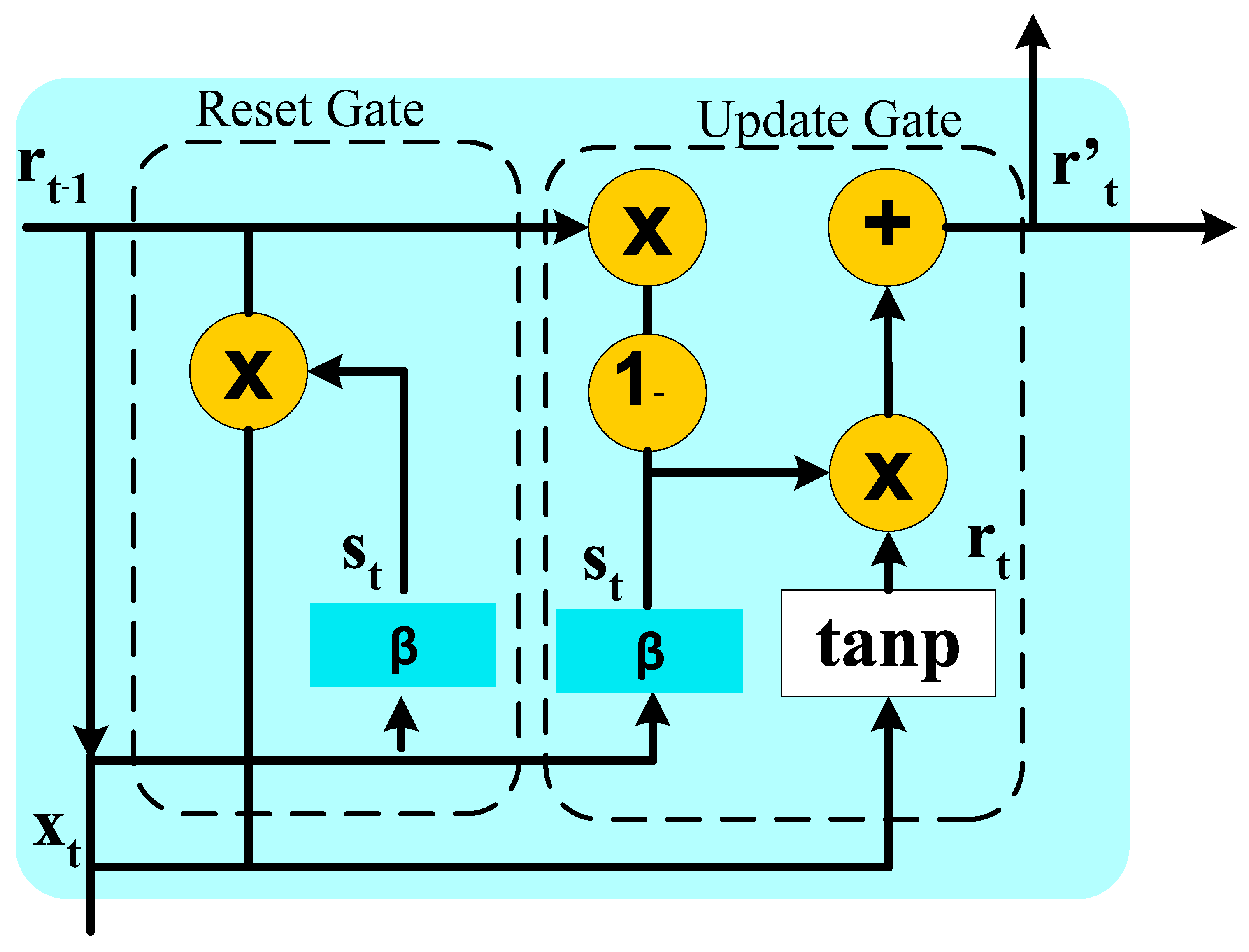
Appendix C. Bidirectional Architecture
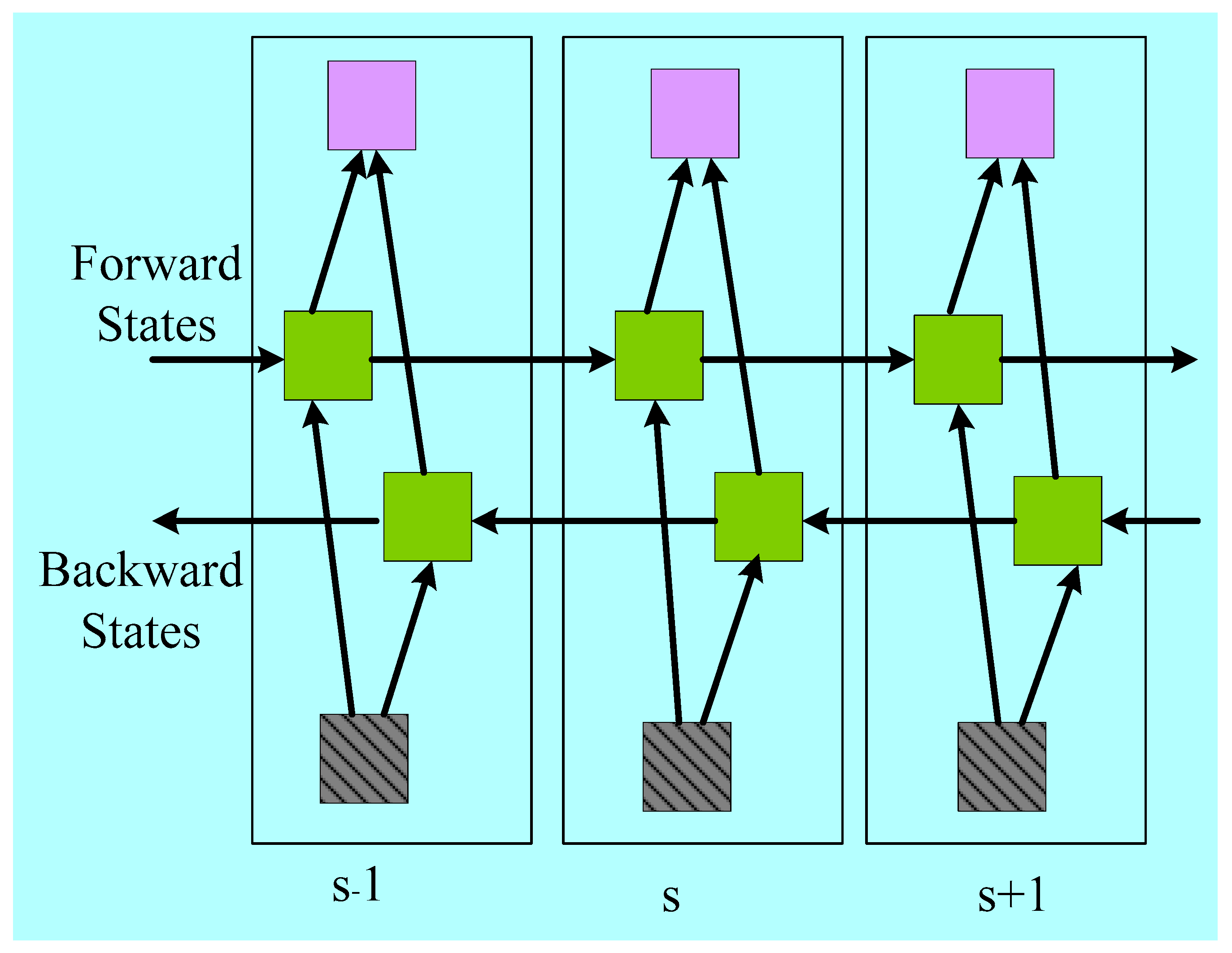


Appendix D. Hybrid Deep Learning Architecture

Appendix E. Scientific Validation with the Unseen Data
| Unseen Data (Train A-Test B) | Unseen Data (Train B-Test A) | ||||
|---|---|---|---|---|---|
| SN | Models | Mean AUC | p-Value | Mean AUC | p-Value |
| 1 | ML | 0.683 | <0.005 | 0.683 | <0.005 |
| 2 | UniDL | 0.884 | <0.001 | 0.880 | <0.001 |
| 3 | BiDL | 0.905 | <0.001 | 0.900 | <0.001 |
| 4 | HDL | 0.947 | <0.001 | 0.940 | <0.001 |
Appendix F. Confusion Matrix for Unidirectional HDL and Bidirectional HDL Model
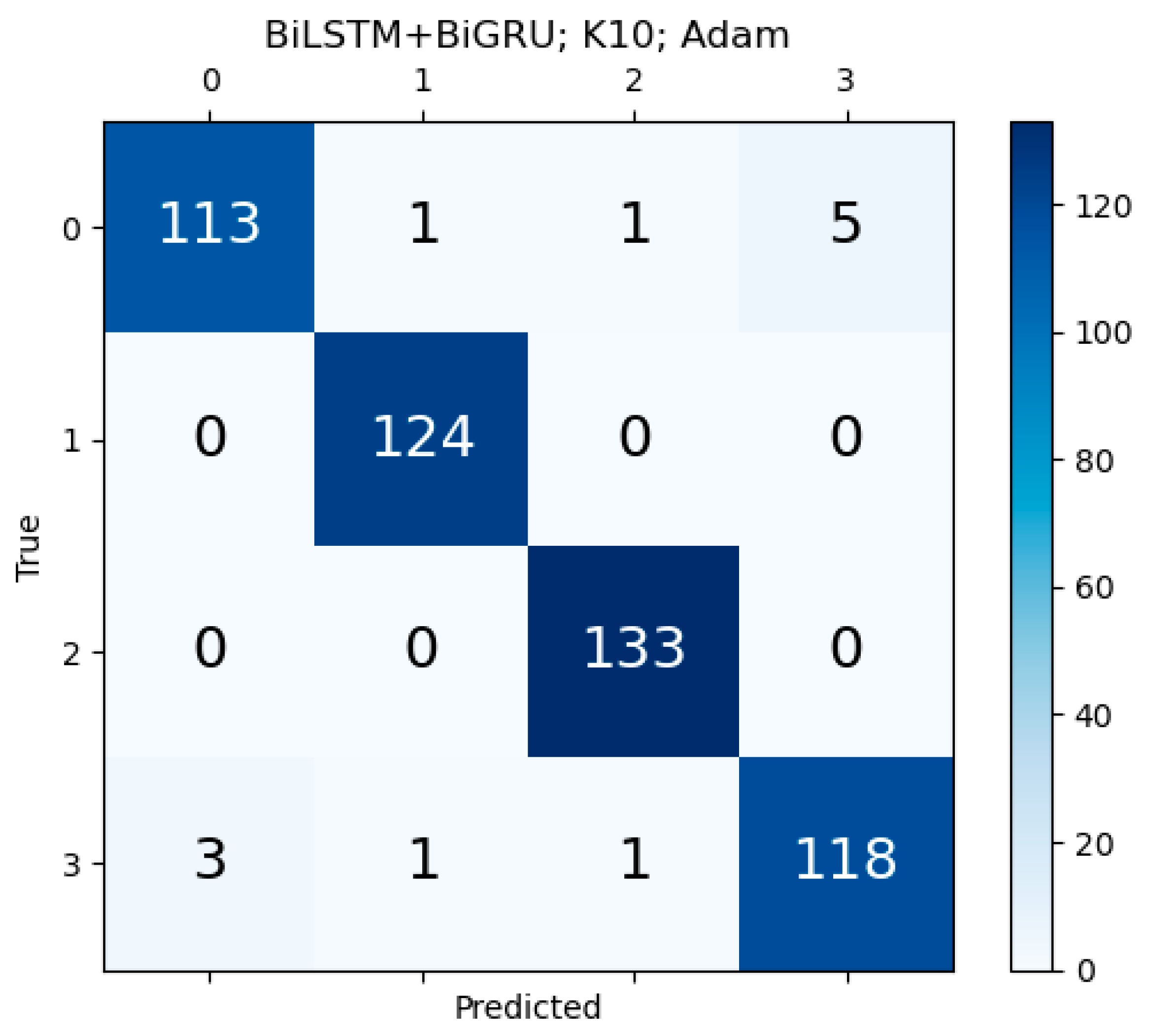
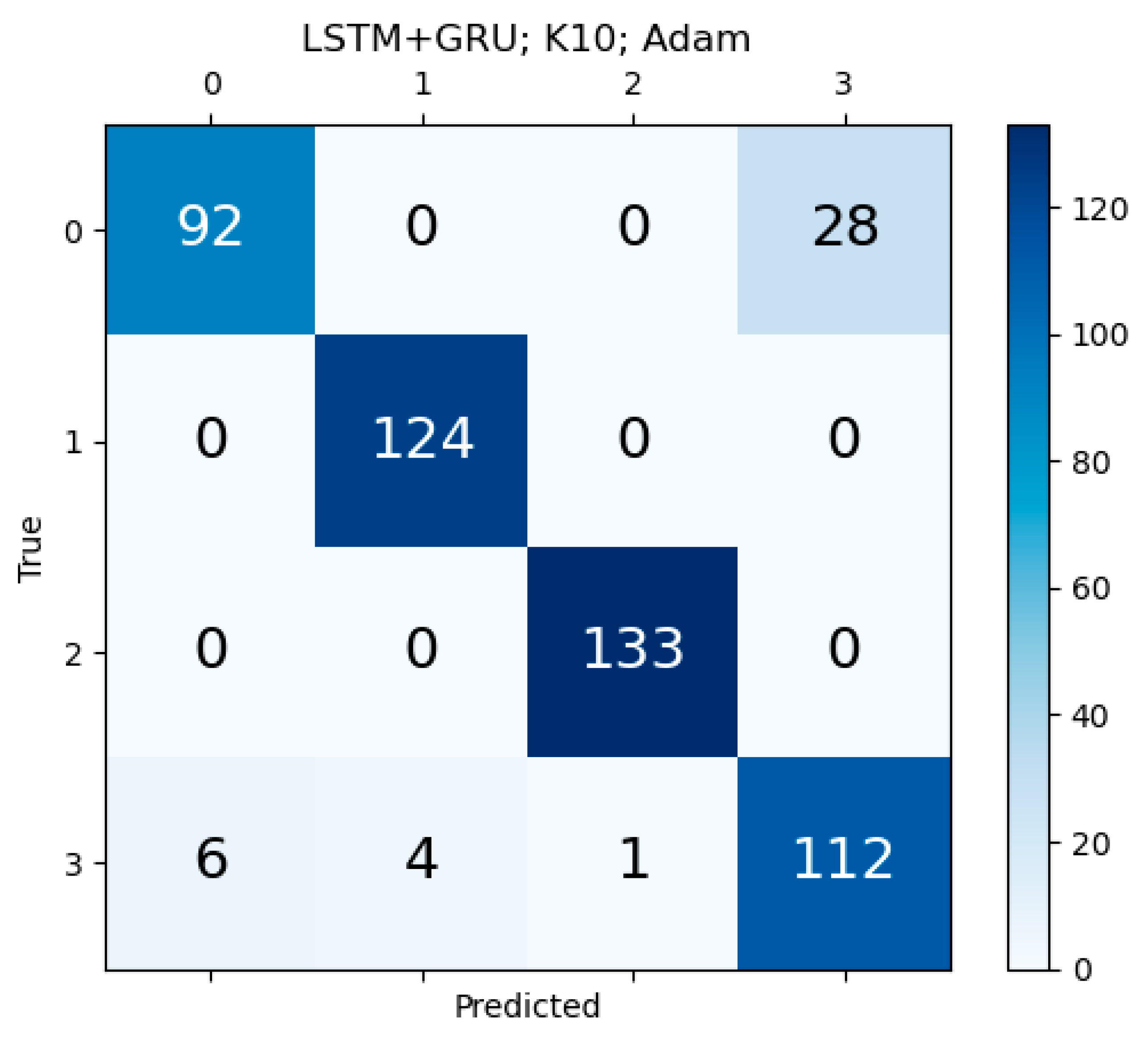
References
- Kaptoge, S.; Pennells, L.; De Bacquer, D.; Cooney, M.T.; Kavousi, M.; Stevens, G.; Riley, L.M.; Savin, S.; Khan, T.; Altay, S.; et al. World Health Organization cardiovascular disease risk charts: Revised models to estimate risk in 21 global regions. Lancet Glob. Health 2019, 7, e1332–e1345. [Google Scholar] [CrossRef] [PubMed]
- Suri, J.S.; Agarwal, S.; Gupta, S.K.; Puvvula, A.; Biswas, M.; Saba, L.; Bit, A.; Tandel, G.S.; Agarwal, M.; Patrick, A.; et al. A narrative review on characterization of acute respiratory distress syndrome in COVID-19-infected lungs using artificial intelligence. Comput. Biol. Med. 2021, 130, 104210. [Google Scholar] [CrossRef] [PubMed]
- Jamthikar, A.D.; Puvvula, A.; Gupta, D.; Johri, A.M.; Nambi, V.; Khanna, N.N.; Saba, L.; Mavrogeni, S.; Laird, J.R.; Pareek, G. Cardiovascular disease and stroke risk assessment in patients with chronic kidney disease using integration of estimated glomerular filtration rate, ultrasonic image phenotypes, and artificial intelligence: A narrative review. Int. Angiol. A J. Int. Union Angiol. 2020, 40, 150–164. [Google Scholar] [CrossRef]
- Saba, L.; Anzidei, M.; Sanfilippo, R.; Montisci, R.; Lucatelli, P.; Catalano, C.; Passariello, R.; Mallarini, G. Imaging of the carotid artery. Atherosclerosis 2012, 220, 294–309. [Google Scholar] [CrossRef]
- Griffin, M.; Nicolaides, A.N.; Belcaro, G.; Shah, E. Cardiovascular risk assessment using ultrasound: The value of arterial wall changes including the presence, severity and character of plaques. Pathophysiol. Haemost. Thromb. 2002, 32, 367–370. [Google Scholar] [CrossRef]
- Suri, J.S.; Kathuria, C.; Molinari, F. Atherosclerosis Disease Management; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Saba, L.; Jamthikar, A.; Gupta, D.; Khanna, N.N.; Viskovic, K.; Suri, H.S.; Gupta, A.; Mavrogeni, S.; Turk, M.; Laird, J.R. Global perspective on carotid intima-media thickness and plaque: Should the current measurement guidelines be revisited? Int. Angiol. 2019, 38, 451–465. [Google Scholar] [CrossRef]
- Giannopoulos, A.A.; Kyriacou, E.; Griffin, M.; Pattichis, C.S.; Michael, J.; Richards, T.; Geroulakos, G.; Nicolaides, A.N. Dynamic carotid plaque imaging using ultrasonography. J. Vasc. Surg. 2021, 73, 1630–1638. [Google Scholar] [CrossRef]
- Amato, M.; Montorsi, P.; Ravani, A.; Oldani, E.; Galli, S.; Ravagnani, P.M.; Tremoli, E.; Baldassarre, D. Carotid intima-media thickness by B-mode ultrasound as surrogate of coronary atherosclerosis: Correlation with quantitative coronary angiography and coronary intravascular ultrasound findings. Eur. Heart J. 2007, 28, 2094–2101. [Google Scholar] [CrossRef]
- Bots, M.L. Carotid intima-media thickness as a surrogate marker for cardiovascular disease in intervention studies. Curr. Med. Res. Opin. 2006, 22, 2181–2190. [Google Scholar] [CrossRef]
- Spence, J.D. Ultrasound measurement of carotid plaque as a surrogate outcome for coronary artery disease. Am. J. Cardiol. 2002, 89, 10–15. [Google Scholar] [CrossRef]
- Puvvula, A.; Jamthikar, A.D.; Gupta, D.; Khanna, N.N.; Porcu, M.; Saba, L.; Viskovic, K.; Ajuluchukwu, J.N.; Gupta, A.; Mavrogeni, S. Morphological carotid plaque area is associated with glomerular filtration rate: A study of south asian indian patients with diabetes and chronic kidney disease. Angiology 2020, 71, 520–535. [Google Scholar] [CrossRef]
- Biswas, M.; Saba, L.; Chakrabartty, S.; Khanna, N.N.; Song, H.; Suri, H.S.; Sfikakis, P.P.; Mavrogeni, S.; Viskovic, K.; Laird, J.R. Two-stage artificial intelligence model for jointly measurement of atherosclerotic wall thickness and plaque burden in carotid ultrasound: A screening tool for cardiovascular/stroke risk assessment. Comput. Biol. Med. 2020, 123, 103847. [Google Scholar] [CrossRef]
- Landry, A.; Spence, J.D.; Fenster, A. Measurement of carotid plaque volume by 3-dimensional ultrasound. Stroke 2004, 35, 864–869. [Google Scholar] [CrossRef]
- Johri, A.M.; Lajkosz, K.A.; Grubic, N.; Islam, S.; Li, T.Y.; Simpson, C.S.; Ewart, P.; Suri, J.S.; Hétu, M.-F. Maximum plaque height in carotid ultrasound predicts cardiovascular disease outcomes: A population-based validation study of the American society of echocardiography’s grade II–III plaque characterization and protocol. Int. J. Cardiovasc. Imaging 2021, 37, 1601–1610. [Google Scholar] [CrossRef]
- Mantella, L.E.; Colledanchise, K.N.; Hetu, M.-F.; Feinstein, S.B.; Abunassar, J.; Johri, A.M. Carotid intraplaque neovascularization predicts coronary artery disease and cardiovascular events. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1239–1247. [Google Scholar] [CrossRef]
- Goldstein, B.A.; Navar, A.M.; Carter, R.E. Moving beyond regression techniques in cardiovascular risk prediction: Applying machine learning to address analytic challenges. Eur. Heart J. 2017, 38, 1805–1814. [Google Scholar] [CrossRef]
- Jamthikar, A.D.; Gupta, D.; Saba, L.; Khanna, N.N.; Viskovic, K.; Mavrogeni, S.; Laird, J.R.; Sattar, N.; Johri, A.M.; Pareek, G. Artificial intelligence framework for predictive cardiovascular and stroke risk assessment models: A narrative review of integrated approaches using carotid ultrasound. Comput. Biol. Med. 2020, 126, 104043. [Google Scholar] [CrossRef]
- Alaa, A.M.; Bolton, T.; Di Angelantonio, E.; Rudd, J.H.; Van der Schaar, M. Cardiovascular disease risk prediction using automated machine learning: A prospective study of 423,604 UK Biobank participants. PLoS ONE 2019, 14, e0213653. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.F.; Reps, J.; Kai, J.; Garibaldi, J.M.; Qureshi, N. Can machine-learning improve cardiovascular risk prediction using routine clinical data? PLoS ONE 2017, 12, e0174944. [Google Scholar] [CrossRef]
- Biswas, M.; Kuppili, V.; Saba, L.; Edla, D.R.; Suri, H.S.; Cuadrado-Godia, E.; Laird, J.R.; Marinhoe, R.T.; Sanches, J.M.; Nicolaides, A. State-of-the-art review on deep learning in medical imaging. Front. Biosci. Landmark 2019, 24, 380–406. [Google Scholar]
- Saba, L.; Biswas, M.; Kuppili, V.; Godia, E.C.; Suri, H.S.; Edla, D.R.; Omerzu, T.; Laird, J.R.; Khanna, N.N.; Mavrogeni, S. The present and future of deep learning in radiology. Eur. J. Radiol. 2019, 114, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Hosny, A.; Parmar, C.; Quackenbush, J.; Schwartz, L.H.; Aerts, H.J. Artificial intelligence in radiology. Nat. Rev. Cancer 2018, 18, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Pianykh, O.S.; Langs, G.; Dewey, M.; Enzmann, D.R.; Herold, C.J.; Schoenberg, S.O.; Brink, J.A. Continuous learning AI in radiology: Implementation principles and early applications. Radiology 2020, 297, 6–14. [Google Scholar] [CrossRef]
- Thrall, J.H.; Li, X.; Li, Q.; Cruz, C.; Do, S.; Dreyer, K.; Brink, J. Artificial intelligence and machine learning in radiology: Opportunities, challenges, pitfalls, and criteria for success. J. Am. Coll. Radiol. 2018, 15, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, V.K.; Londhe, N.D.; Sonawane, R.S.; Suri, J.S. Reliable and accurate psoriasis disease classification in dermatology images using comprehensive feature space in machine learning paradigm. Expert Syst. Appl. 2015, 42, 6184–6195. [Google Scholar] [CrossRef]
- Shrivastava, V.K.; Londhe, N.D.; Sonawane, R.S.; Suri, J.S. A novel and robust Bayesian approach for segmentation of psoriasis lesions and its risk stratification. Comput. Methods Programs Biomed. 2017, 150, 9–22. [Google Scholar] [CrossRef]
- Du-Harpur, X.; Watt, F.; Luscombe, N.; Lynch, M. What is AI? Applications of artificial intelligence to dermatology. Br. J. Dermatol. 2020, 183, 423–430. [Google Scholar] [CrossRef]
- Li, C.-X.; Shen, C.-B.; Xue, K.; Shen, X.; Jing, Y.; Wang, Z.-Y.; Xu, F.; Meng, R.-S.; Yu, J.-B.; Cui, Y. Artificial intelligence in dermatology: Past, present, and future. Chin. Med. J. 2019, 132, 2017–2020. [Google Scholar] [CrossRef]
- Fritzsche, K.; Can, A.; Shen, H.; Tsai, C.; Turner, J.; Tanenbuam, H.; Stewart, C.; Roysam, B.; Suri, J.; Laxminarayan, S. Automated model based segmentation, tracing and analysis of retinal vasculature from digital fundus images. In State-of-The-Art Angiography, Applications and Plaque Imaging Using MR, CT, Ultrasound and X-rays; CRC Press: Boca Raton, FL, USA, 2003; Volume 29, pp. 225–298. [Google Scholar]
- Hogarty, D.T.; Mackey, D.A.; Hewitt, A.W. Current state and future prospects of artificial intelligence in ophthalmology: A review. Clin. Exp. Ophthalmol. 2019, 47, 128–139. [Google Scholar] [CrossRef]
- Tong, Y.; Yu, Y.; Xing, Y.; Chen, C.; Shen, Y. Applications of artificial intelligence in ophthalmology: General overview. J. Ophthalmol. 2018, 2018, 5278196. [Google Scholar]
- Ting, D.S.W.; Pasquale, L.R.; Peng, L.; Campbell, J.P.; Lee, A.Y.; Raman, R.; Tan, G.S.W.; Schmetterer, L.; Keane, P.A.; Wong, T.Y. Artificial intelligence and deep learning in ophthalmology. Br. J. Ophthalmol. 2019, 103, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Reiter, G.S.; Riedl, S.; Seeböck, P.; Vogl, W.-D.; Blodi, B.A.; Domalpally, A.; Fawzi, A.; Jia, Y.; Sarraf, D. AI-based monitoring of retinal fluid in disease activity and under therapy. Prog. Retin. Eye Res. 2022, 86, 100972. [Google Scholar] [CrossRef]
- Sorrentino, F.S.; Jurman, G.; De Nadai, K.; Campa, C.; Furlanello, C.; Parmeggiani, F. Application of artificial intelligence in targeting retinal diseases. Curr. Drug Targets 2020, 21, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Saba, L.; Sanfilippo, R.; Sannia, S.; Anzidei, M.; Montisci, R.; Mallarini, G.; Suri, J.S. Association between carotid artery plaque volume, composition, and ulceration: A retrospective assessment with MDCT. Am. J. Roentgenol. 2012, 199, 151–156. [Google Scholar] [CrossRef]
- Acharya, U.R.; Sree, S.V.; Krishnan, M.M.R.; Krishnananda, N.; Ranjan, S.; Umesh, P.; Suri, J.S. Automated classification of patients with coronary artery disease using grayscale features from left ventricle echocardiographic images. Comput. Methods Programs Biomed. 2013, 112, 624–632. [Google Scholar] [CrossRef]
- Johnson, K.W.; Soto, J.T.; Glicksberg, B.S.; Shameer, K.; Miotto, R.; Ali, M.; Ashley, E.; Dudley, J.T. Artificial intelligence in cardiology. J. Am. Coll. Cardiol. 2018, 71, 2668–2679. [Google Scholar] [CrossRef]
- Lopez-Jimenez, F.; Attia, Z.; Arruda-Olson, A.M.; Carter, R.; Chareonthaitawee, P.; Jouni, H.; Kapa, S.; Lerman, A.; Luong, C.; Medina-Inojosa, J.R. Artificial intelligence in cardiology: Present and future. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Molinari, F.; Mantovani, A.; Deandrea, M.; Limone, P.; Garberoglio, R.; Suri, J.S. Characterization of single thyroid nodules by contrast-enhanced 3-D ultrasound. Ultrasound Med. Biol. 2010, 36, 1616–1625. [Google Scholar] [CrossRef]
- Gubbi, S.; Hamet, P.; Tremblay, J.; Koch, C.A.; Hannah-Shmouni, F. Artificial intelligence and machine learning in endocrinology and metabolism: The dawn of a new era. Front. Endocrinol. 2019, 10, 185. [Google Scholar] [CrossRef]
- Giorgini, F.; Di Dalmazi, G.; Diciotti, S. Artificial intelligence in endocrinology: A comprehensive review. J. Endocrinol. Investig. 2024, 47, 1067–1082. [Google Scholar] [CrossRef]
- Thomasian, N.M.; Kamel, I.R.; Bai, H.X. Machine intelligence in non-invasive endocrine cancer diagnostics. Nat. Rev. Endocrinol. 2022, 18, 81–95. [Google Scholar] [CrossRef]
- Jamthikar, A.; Gupta, D.; Khanna, N.N.; Saba, L.; Araki, T.; Viskovic, K.; Suri, H.S.; Gupta, A.; Mavrogeni, S.; Turk, M. A low-cost machine learning-based cardiovascular/stroke risk assessment system: Integration of conventional factors with image phenotypes. Cardiovasc. Diagn. Ther. 2019, 9, 420. [Google Scholar] [CrossRef]
- Kakadiaris, I.A.; Vrigkas, M.; Yen, A.A.; Kuznetsova, T.; Budoff, M.; Naghavi, M. Machine learning outperforms ACC/AHA CVD risk calculator in MESA. J. Am. Heart Assoc. 2018, 7, e009476. [Google Scholar] [CrossRef]
- Arsenescu, T.; Chifor, R.; Marita, T.; Santoma, A.; Lebovici, A.; Duma, D.; Vacaras, V.; Badea, A.F. 3D ultrasound reconstructions of the carotid artery and thyroid gland using artificial-intelligence-based automatic segmentation—Qualitative and quantitative evaluation of the segmentation results via comparison with CT angiography. Sensors 2023, 23, 2806. [Google Scholar] [CrossRef] [PubMed]
- Acharya, U.R.; Mookiah, M.R.K.; Sree, S.V.; Yanti, R.; Martis, R.; Saba, L.; Molinari, F.; Guerriero, S.; Suri, J.S. Evolutionary algorithm-based classifier parameter tuning for automatic ovarian cancer tissue characterization and classification. Ultraschall Med. Eur. J. Ultrasound 2014, 35, 237–245. [Google Scholar]
- Araki, T.; Ikeda, N.; Shukla, D.; Jain, P.K.; Londhe, N.D.; Shrivastava, V.K.; Banchhor, S.K.; Saba, L.; Nicolaides, A.; Shafique, S. PCA-based polling strategy in machine learning framework for coronary artery disease risk assessment in intravascular ultrasound: A link between carotid and coronary grayscale plaque morphology. Comput. Methods Programs Biomed. 2016, 128, 137–158. [Google Scholar] [CrossRef] [PubMed]
- Anaya-Isaza, A.; Mera-Jiménez, L.; Zequera-Diaz, M. An overview of deep learning in medical imaging. Inform. Med. Unlocked 2021, 26, 100723. [Google Scholar] [CrossRef]
- Lee, J.-G.; Jun, S.; Cho, Y.-W.; Lee, H.; Kim, G.B.; Seo, J.B.; Kim, N. Deep learning in medical imaging: General overview. Korean J. Radiol. 2017, 18, 570–584. [Google Scholar] [CrossRef]
- Aggarwal, R.; Sounderajah, V.; Martin, G.; Ting, D.S.; Karthikesalingam, A.; King, D.; Ashrafian, H.; Darzi, A. Diagnostic accuracy of deep learning in medical imaging: A systematic review and meta-analysis. NPJ Digit. Med. 2021, 4, 65. [Google Scholar] [CrossRef]
- Khanna, N.N.; Maindarkar, M.A.; Viswanathan, V.; Puvvula, A.; Paul, S.; Bhagawati, M.; Ahluwalia, P.; Ruzsa, Z.; Sharma, A.; Kolluri, R. Cardiovascular/Stroke Risk Stratification in Diabetic Foot Infection Patients Using Deep Learning-Based Artificial Intelligence: An Investigative Study. J. Clin. Med. 2022, 11, 6844. [Google Scholar] [CrossRef]
- Suri, J.S.; Bhagawati, M.; Paul, S.; Protogerou, A.D.; Sfikakis, P.P.; Kitas, G.D.; Khanna, N.N.; Ruzsa, Z.; Sharma, A.M.; Saxena, S. A powerful paradigm for cardiovascular risk stratification using multiclass, multi-label, and ensemble-based machine learning paradigms: A narrative review. Diagnostics 2022, 12, 722. [Google Scholar] [CrossRef] [PubMed]
- Johri, A.M.; Singh, K.V.; Mantella, L.E.; Saba, L.; Sharma, A.; Laird, J.R.; Utkarsh, K.; Singh, I.M.; Gupta, S.; Kalra, M.S. Deep learning artificial intelligence framework for multiclass coronary artery disease prediction using combination of conventional risk factors, carotid ultrasound, and intraplaque neovascularization. Comput. Biol. Med. 2022, 150, 106018. [Google Scholar] [CrossRef] [PubMed]
- Konstantonis, G.; Singh, K.V.; Sfikakis, P.P.; Jamthikar, A.D.; Kitas, G.D.; Gupta, S.K.; Saba, L.; Verrou, K.; Khanna, N.N.; Ruzsa, Z. Cardiovascular disease detection using machine learning and carotid/femoral arterial imaging frameworks in rheumatoid arthritis patients. Rheumatol. Int. 2022, 42, 215–239. [Google Scholar] [CrossRef]
- Jamthikar, A.D.; Gupta, D.; Mantella, L.E.; Saba, L.; Laird, J.R.; Johri, A.M.; Suri, J.S. Multiclass machine learning vs. conventional calculators for stroke/CVD risk assessment using carotid plaque predictors with coronary angiography scores as gold standard: A 500 participants study. Int. J. Cardiovasc. Imaging 2021, 37, 1171–1187. [Google Scholar] [CrossRef] [PubMed]
- Johri, A.M.; Mantella, L.E.; Jamthikar, A.D.; Saba, L.; Laird, J.R.; Suri, J.S. Role of artificial intelligence in cardiovascular risk prediction and outcomes: Comparison of machine-learning and conventional statistical approaches for the analysis of carotid ultrasound features and intra-plaque neovascularization. Int. J. Cardiovasc. Imaging 2021, 37, 3145–3156. [Google Scholar] [CrossRef]
- Wilson, P.W.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of coronary heart disease using risk factor categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef]
- Conroy, R.M.; Pyörälä, K.; Fitzgerald, A.E.; Sans, S.; Menotti, A.; De Backer, G.; De Bacquer, D.; Ducimetiere, P.; Jousilahti, P.; Keil, U. Estimation of ten-year risk of fatal cardiovascular disease in Europe: The SCORE project. Eur. Heart J. 2003, 24, 987–1003. [Google Scholar] [CrossRef]
- Golf, D.C., Jr.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’agostino, R.B.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’donnell, C.J. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129 (Suppl. S2), S49–S73. [Google Scholar]
- Kumar, K.; Araki, T.; Rajan, J.; Saba, L.; Lavra, F.; Ikeda, N.; Sharma, A.M.; Shafique, S.; Nicolaides, A.; Laird, J.R. Accurate lumen diameter measurement in curved vessels in carotid ultrasound: An iterative scale-space and spatial transformation approach. Med. Biol. Eng. Comput. 2017, 55, 1415–1434. [Google Scholar] [CrossRef]
- Detrano, R.; Guerci, A.D.; Carr, J.J.; Bild, D.E.; Burke, G.; Folsom, A.R.; Liu, K.; Shea, S.; Szklo, M.; Bluemke, D.A. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N. Engl. J. Med. 2008, 358, 1336–1345. [Google Scholar] [CrossRef]
- Poplin, R.; Varadarajan, A.V.; Blumer, K.; Liu, Y.; McConnell, M.V.; Corrado, G.S.; Peng, L.; Webster, D.R. Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning. Nat. Biomed. Eng. 2018, 2, 158–164. [Google Scholar] [CrossRef]
- Escaned, J.; Baptista, J.; Di Mario, C.; Haase, J.R.; Ozaki, Y.; Linker, D.T.; De Feyter, P.J.; Roelandt, J.R.; Serruys, P.W. Significance of automated stenosis detection during quantitative angiography: Insights gained from intracoronary ultrasound imaging. Circulation 1996, 94, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Bourantas, C.V.; Kalatzis, F.G.; Papafaklis, M.I.; Fotiadis, D.I.; Tweddel, A.C.; Kourtis, I.C.; Katsouras, C.S.; Michalis, L.K. ANGIOCARE: An automated system for fast three-dimensional coronary reconstruction by integrating angiographic and intracoronary ultrasound data. Catheter. Cardiovasc. Interv. 2008, 72, 166–175. [Google Scholar] [CrossRef]
- Joseph, J.; Kiran, R.; Nabeel, P.; Shah, M.I.; Bhaskar, A.; Ganesh, C.; Seshadri, S.; Sivaprakasam, M. ARTSENS® Pen—Portable easy-to-use device for carotid stiffness measurement: Technology validation and clinical-utility assessment. Biomed. Phys. Eng. Express 2020, 6, 025013. [Google Scholar] [CrossRef]
- Daigle, R.J. Techniques in Noninvasive Vascular Diagnosis: An Encyclopedia of Vascular Testing; Summer Publishing LLC: Littleton, CO, USA, 2008. [Google Scholar]
- Nicolaides, A.N.; Kakkos, S.K.; Griffin, M.; Sabetai, M.; Dhanjil, S.; Thomas, D.J.; Geroulakos, G.; Georgiou, N.; Francis, S.; Ioannidou, E. Effect of image normalization on carotid plaque classification and the risk of ipsilateral hemispheric ischemic events: Results from the asymptomatic carotid stenosis and risk of stroke study. Vascular 2005, 13, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic minority over-sampling technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Elreedy, D.; Atiya, A.F. A comprehensive analysis of synthetic minority oversampling technique (SMOTE) for handling class imbalance. Inf. Sci. 2019, 505, 32–64. [Google Scholar] [CrossRef]
- Santoso, B.; Wijayanto, H.; Notodiputro, K.A.; Sartono, B. Synthetic over sampling methods for handling class imbalanced problems: A review. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2017. [Google Scholar]
- Fernández, A.; Garcia, S.; Herrera, F.; Chawla, N.V. SMOTE for learning from imbalanced data: Progress and challenges, marking the 15-year anniversary. J. Artif. Intell. Res. 2018, 61, 863–905. [Google Scholar] [CrossRef]
- Blagus, R.; Lusa, L. SMOTE for high-dimensional class-imbalanced data. BMC Bioinform. 2013, 14, 1–16. [Google Scholar] [CrossRef]
- Deepa, B.; Ramesh, K. Epileptic seizure detection using deep learning through min max scaler normalization. Int. J. Health Sci. 2022, 6, 10981–10996. [Google Scholar] [CrossRef]
- Sembiring, I.; Wahyuni, S.N.; Sediyono, E. LSTM algorithm optimization for COVID-19 prediction model. Heliyon 2024, 10, e26158. [Google Scholar] [CrossRef]
- O’Donncha, F.; Hu, Y.; Palmes, P.; Burke, M.; Filgueira, R.; Grant, J. A spatio-temporal LSTM model to forecast across multiple temporal and spatial scales. Ecol. Inform. 2022, 69, 101687. [Google Scholar] [CrossRef]
- Olhosseiny, H.H.; Mirzaloo, M.; Bolic, M.; Dajani, H.R.; Groza, V.; Yoshida, M. Identifying high risk of atherosclerosis using deep learning and ensemble learning. In Proceedings of the 2021 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Lausanne, Switzerland, 23–25 June 2021. [Google Scholar]
- An, Y.; Huang, N.; Chen, X.; Wu, F.; Wang, J. High-risk prediction of cardiovascular diseases via attention-based deep neural networks. IEEE ACM Trans. Comput. Biol. Bioinform. 2019, 18, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Baccouche, A.; Garcia-Zapirain, B.; Olea, C.C.; Elmaghraby, A. Ensemble deep learning models for heart disease classification: A case study from Mexico. Information 2020, 11, 207. [Google Scholar] [CrossRef]
- Bai, S.; Yan, M.; Wan, Q.; He, L.; Wang, X.; Li, J. DL-RNN: An accurate indoor localization method via double RNNs. IEEE Sens. J. 2019, 20, 286–295. [Google Scholar] [CrossRef]
- Dey, R.; Salem, F.M. Gate-variants of gated recurrent unit (GRU) neural networks. In Proceedings of the 2017 IEEE 60th International Midwest Symposium on Circuits and Systems (MWSCAS), Boston, MA, USA, 6–9 August 2017. [Google Scholar]
- Wang, J.J.; Yan, J.; Li, C.; Gao, R.X.; Zhao, R. Deep heterogeneous GRU model for predictive analytics in smart manufacturing: Application to tool wear prediction. Comput. Ind. 2019, 111, 1–14. [Google Scholar] [CrossRef]
- Creswell, A.; White, T.; Dumoulin, V.; Arulkumaran, K.; Sengupta, B.; Bharath, A.A. Generative adversarial networks: An overview. IEEE Signal Process. Mag. 2018, 35, 53–65. [Google Scholar] [CrossRef]
- Dhyani, M.; Kumar, R. An intelligent Chatbot using deep learning with Bidirectional RNN and attention model. Mater. Today Proc. 2021, 34, 817–824. [Google Scholar] [CrossRef]
- Cui, Z.; Ke, R.; Pu, Z.; Wang, Y. Stacked bidirectional and unidirectional LSTM recurrent neural network for forecasting network-wide traffic state with missing values. Transp. Res. Part C Emerg. Technol. 2020, 118, 102674. [Google Scholar] [CrossRef]
- Chen, D.; Yongchareon, S.; Lai, E.M.-K.; Yu, J.; Sheng, Q.Z.; Li, Y. Transformer with bidirectional GRU for nonintrusive, sensor-based activity recognition in a multiresident environment. IEEE Internet Things J. 2022, 9, 23716–23727. [Google Scholar] [CrossRef]
- Mostafa, A.L.; Abdel-Galil, H.; Belal, M. Ensemble Model-based Weighted Categorical Cross-entropy Loss for Facial Expression Recognition. In Proceedings of the 2021 Tenth International Conference on Intelligent Computing and Information Systems (ICICIS), Cairo, Egypt, 5–6 December 2021. [Google Scholar]
- Feng, L.; Shu, S.; Lin, Z.; Lv, F.; Li, L.; An, B. Can cross entropy loss be robust to label noise? In Proceedings of the Twenty-Ninth International Conference on International Joint Conferences on Artificial Intelligence, Online, 7–15 January 2021.
- Ruby, U.; Yendapalli, V. Binary cross entropy with deep learning technique for image classification. Int. J. Adv. Trends Comput. Sci. Eng. 2020, 9, 5393–5397. [Google Scholar]
- Hernández-Vázquez, M.A.; Hernández-Rodríguez, Y.M.; Cortes-Rojas, F.D.; Bayareh-Mancilla, R.; Cigarroa-Mayorga, O.E. Hybrid Feature Mammogram Analysis: Detecting and Localizing Microcalcifications Combining Gabor, Prewitt, GLCM Features, and Top Hat Filtering Enhanced with CNN Architecture. Diagnostics 2024, 14, 1691. [Google Scholar] [CrossRef]
- Suri, J.S.; Agarwal, S.; Carriero, A.; Paschè, A.; Danna, P.S.; Columbu, M.; Saba, L.; Viskovic, K.; Mehmedović, A.; Agarwal, S. COVLIAS 1.0 vs. MedSeg: Artificial intelligence-based comparative study for automated COVID-19 computed tomography lung segmentation in Italian and Croatian Cohorts. Diagnostics 2021, 11, 2367. [Google Scholar] [CrossRef]
- Daneshjou, R.; Smith, M.P.; Sun, M.D.; Rotemberg, V.; Zou, J. Lack of transparency and potential bias in artificial intelligence data sets and algorithms: A scoping review. JAMA Dermatol. 2021, 157, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.K.; Chabert, G.L.; Carriero, A.; Pasche, A.; Danna, P.S.; Agarwal, S.; Mohanty, L.; Nillmani; Sharma, N.; Yadav, S. Ensemble Deep Learning Derived from Transfer Learning for Classification of COVID-19 Patients on Hybrid Deep-Learning-Based Lung Segmentation: A Data Augmentation and Balancing Framework. Diagnostics 2023, 13, 1954. [Google Scholar] [CrossRef] [PubMed]
- Saba, L.; Than, J.C.; Noor, N.M.; Rijal, O.M.; Kassim, R.M.; Yunus, A.; Ng, C.R.; Suri, J.S. Inter-observer variability analysis of automatic lung delineation in normal and disease patients. J. Med. Syst. 2016, 40, 1–18. [Google Scholar] [CrossRef]
- Suri, J.S.; Agarwal, S.; Saba, L.; Chabert, G.L.; Carriero, A.; Paschè, A.; Danna, P.; Mehmedović, A.; Faa, G.; Jujaray, T. Multicenter study on COVID-19 lung computed tomography segmentation with varying glass ground opacities using unseen deep learning artificial intelligence paradigms: COVLIAS 1.0 validation. J. Med. Syst. 2022, 46, 62. [Google Scholar] [CrossRef]
- Unnikrishnan, P.; Kumar, D.K.; Arjunan, S.P.; Kumar, H.; Mitchell, P.; Kawasaki, R. Development of health parameter model for risk prediction of CVD using SVM. Comput. Math. Methods Med. 2016, 2016, 3016245. [Google Scholar] [CrossRef]
- Zhou, R.; Guo, F.; Azarpazhooh, M.R.; Hashemi, S.; Cheng, X.; Spence, J.D.; Ding, M.; Fenster, A. Deep learning-based measurement of total plaque area in B-mode ultrasound images. IEEE J. Biomed. Health Inform. 2021, 25, 2967–2977. [Google Scholar] [CrossRef]
- Jain, P.K.; Sharma, N.; Giannopoulos, A.A.; Saba, L.; Nicolaides, A.; Suri, J.S. Hybrid deep learning segmentation models for atherosclerotic plaque in internal carotid artery B-mode ultrasound. Comput. Biol. Med. 2021, 136, 104721. [Google Scholar] [CrossRef]
- Jain, P.K.; Sharma, N.; Saba, L.; Paraskevas, K.I.; Kalra, M.K.; Johri, A.; Laird, J.R.; Nicolaides, A.N.; Suri, J.S. Unseen artificial intelligence—Deep learning paradigm for segmentation of low atherosclerotic plaque in carotid ultrasound: A multicenter cardiovascular study. Diagnostics 2021, 11, 2257. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Sharma, N.; Saba, L.; Paraskevas, K.I.; Kalra, M.K.; Johri, A.; Nicolaides, A.N.; Suri, J.S. Automated deep learning-based paradigm for high-risk plaque detection in B-mode common carotid ultrasound scans: An asymptomatic Japanese cohort study. Int. Angiol. 2021, 41, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.; Agarwal, S.; Saba, L.; Chabert, G.L.; Gupta, S.; Carriero, A.; Pasche, A.; Danna, P.; Mehmedovic, A.; Faa, G. Eight pruning deep learning models for low storage and high-speed COVID-19 computed tomography lung segmentation and heatmap-based lesion localization: A multicenter study using COVLIAS 2.0. Comput. Biol. Med. 2022, 146, 105571. [Google Scholar] [CrossRef]
- Suri, J.S.; Bhagawati, M.; Paul, S.; Protogeron, A.; Sfikakis, P.P.; Kitas, G.D.; Khanna, N.N.; Ruzsa, Z.; Sharma, A.M.; Saxena, S. Understanding the bias in machine learning systems for cardiovascular disease risk assessment: The first of its kind review. Comput. Biol. Med. 2022, 142, 105204. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; Werahera, P.; Barqawi, A.; Crawford, E.; Shinohara, K.; Simoneau, A.; Suri, J. Adaptation of a 3D prostate cancer atlas for transrectal ultrasound guided target-specific biopsy. Phys. Med. Biol. 2008, 53, N397. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Goyal, P.; Girshick, R.; He, K.; Dollár, P. Focal loss for dense object detection. In Proceedings of the IEEE International Conference on Computer Vision, Venice, Italy, 22–29 October 2017. [Google Scholar]
- Bartlett, P.L.; Wegkamp, M.H. Classification with a Reject Option using a Hinge Loss. J. Mach. Learn. Res. 2008, 9, 1823–1840. [Google Scholar]
- Bénédict, G.; Koops, V.; Odijk, D.; de Rijke, M. SigmoidF1: A smooth F1 score surrogate loss for multilabel classification. arXiv 2021, arXiv:2108.10566. [Google Scholar]
- Kyriacou, E.C.; Petroudi, S.; Pattichis, C.S.; Pattichis, M.S.; Griffin, M.; Kakkos, S.; Nicolaides, A. Prediction of high-risk asymptomatic carotid plaques based on ultrasonic image features. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 966–973. [Google Scholar] [CrossRef]
- El-Barghouty, N.; Nicolaides, A.; Bahal, V.; Geroulakos, G.; Androulakis, A. The identification of the high risk carotid plaque. Eur. J. Vasc. Endovasc. Surg. 1996, 11, 470–478. [Google Scholar] [CrossRef][Green Version]
- Stoitsis, J.; Golemati, S.; Nikita, K.; Nicolaides, A. Characterization of carotid atherosclerosis based on motion and texture features and clustering using fuzzy c-means. In Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–5 September 2004. [Google Scholar]
- Loizou, C.P.; Petroudi, S.; Pantziaris, M.; Nicolaides, A.N.; Pattichis, C.S. An integrated system for the segmentation of atherosclerotic carotid plaque ultrasound video. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2014, 61, 86–101. [Google Scholar] [CrossRef]
- Stoitsis, J.; Golemati, S.; Kendros, S.; Nikita, K. Automated detection of the carotid artery wall in B-mode ultrasound images using active contours initialized by the Hough transform. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008. [Google Scholar]
- Matsakou, A.I.; Golemati, S.; Stoitsis, J.S.; Nikita, K.S. Automated detection of the carotid artery wall in longitudinal B-mode images using active contours initialized by the Hough transform. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–2 September 2011. [Google Scholar]
- Suri, J.S. Two-dimensional fast magnetic resonance brain segmentation. IEEE Eng. Med. Biol. Mag. 2001, 20, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, M.J.; Al Mukaddim, R.; Mitchell, C.C.; Maybock, J.; Wilbrand, S.M.; Dempsey, R.J.; Varghese, T. Lumen segmentation using a Mask R-CNN in carotid arteries with stenotic atherosclerotic plaque. Ultrasonics 2024, 137, 107193. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Guo, F.; Azarpazhooh, M.R.; Spence, J.D.; Gan, H.; Ding, M.; Fenster, A. Carotid vessel-wall-volume ultrasound measurement via a UNet++ ensemble algorithm trained on small data sets. Ultrasound Med. Biol. 2023, 49, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- El-Baz, A.; Suri, J.S. Big Data in Multimodal Medical Imaging; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Rumsfeld, J.S.; Joynt, K.E.; Maddox, T.M. Big data analytics to improve cardiovascular care: Promise and challenges. Nat. Rev. Cardiol. 2016, 13, 350–359. [Google Scholar] [CrossRef]
- Krittanawong, C.; Johnson, K.W.; Hershman, S.G.; Tang, W.W. Big data, artificial intelligence, and cardiovascular precision medicine. Expert Rev. Precis. Med. Drug Dev. 2018, 3, 305–317. [Google Scholar] [CrossRef]
- Hulsen, T.; Friedecký, D.; Renz, H.; Melis, E.; Vermeersch, P.; Fernandez-Calle, P. From big data to better patient outcomes. Clin. Chem. Lab. Med. CCLM 2023, 61, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Dabla, P.K. Unlocking new potential of clinical diagnosis with artificial intelligence: Finding new patterns of clinical and lab data. World J. Diabetes 2024, 15, 308. [Google Scholar] [CrossRef]
- Wang, S.; Xie, T.; Liu, H.; Zhang, X.; Cheng, J. PSE-Net: Channel pruning for Convolutional Neural Networks with parallel-subnets estimator. Neural Netw. 2024, 174, 106263. [Google Scholar] [CrossRef]
- Louati, H.; Louati, A.; Bechikh, S.; Kariri, E. Embedding channel pruning within the CNN architecture design using a bi-level evolutionary approach. J. Supercomput. 2023, 79, 16118–16151. [Google Scholar] [CrossRef]
- Hong, W.; Li, G.; Liu, S.; Yang, P.; Tang, K. Multi-objective evolutionary optimization for hardware-aware neural network pruning. Fundam. Res. 2022, 4, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, N.; Müller, S.; Eggensperger, K.; Hutter, F. Tabpfn: A transformer that solves small tabular classification problems in a second. arXiv 2022, arXiv:2207.01848. [Google Scholar]
- Huang, X.; Khetan, A.; Cvitkovic, M.; Karnin, Z. Tabtransformer: Tabular data modeling using contextual embeddings. arXiv 2020, arXiv:2012.06678. [Google Scholar]
- Somepalli, G.; Goldblum, M.; Schwarzschild, A.; Bruss, C.B.; Goldstein, T. Saint: Improved neural networks for tabular data via row attention and contrastive pre-training. arXiv 2021, arXiv:2106.01342. [Google Scholar]
- Makris, G.C.; Lavida, A.; Griffin, M.; Geroulakos, G.; Nicolaides, A.N. Three-dimensional ultrasound imaging for the evaluation of carotid atherosclerosis. Atherosclerosis 2011, 219, 377–383. [Google Scholar] [CrossRef]
- Kyriacou, E.C.; Pattichis, C.; Pattichis, M.; Loizou, C.; Christodoulou, C.; Kakkos, S.K.; Nicolaides, A. A review of noninvasive ultrasound image processing methods in the analysis of carotid plaque morphology for the assessment of stroke risk. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 1027–1038. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, M.; Chiu, B. Unsupervised shape-and-texture-based generative adversarial tuning of pre-trained networks for carotid segmentation from 3D ultrasound images. Med. Phys. 2024. early view. [Google Scholar] [CrossRef]




| CVP | Models | ACC (%) | SPEC (%) | SEN (%) | p-Value | AUC (0–1) |
|---|---|---|---|---|---|---|
| K2 | RNN | 80.11 | 82.96 | 81.87 | <0.001 | 0.883 |
| GRU | 81.12 | 83.32 | 82.16 | <0.001 | 0.892 | |
| LSTM | 81.81 | 85.67 | 83.36 | <0.001 | 0.907 | |
| K4 | RNN | 82.52 | 81.97 | 83.88 | <0.001 | 0.850 |
| GRU | 83.13 | 82.33 | 84.17 | <0.001 | 0.897 | |
| LSTM | 84.82 | 84.68 | 85.37 | <0.001 | 0.908 | |
| K5 | RNN | 83.50 | 82.95 | 84.86 | <0.001 | 0.895 |
| GRU | 84.11 | 83.31 | 85.15 | <0.001 | 0.900 | |
| LSTM | 85.80 | 85.66 | 86.35 | <0.001 | 0.916 | |
| K10 | RNN | 84.20 | 83.15 | 83.86 | <0.001 | 0.896 |
| GRU | 85.12 | 84.11 | 86.15 | <0.001 | 0.902 | |
| LSTM | 86.80 | 86.67 | 87.35 | <0.001 | 0.918 |
| CVP. | Models | ACC (%) | SPEC (%) | SEN (%) | p-Value | AUC (0–1) |
|---|---|---|---|---|---|---|
| K2 | BiRNN | 82.22 | 80.16 | 82.34 | <0.001 | 0.891 |
| BiGRU | 83.42 | 81.17 | 83.18 | <0.001 | 0.905 | |
| BiLSTM | 84.32 | 83.29 | 84.12 | <0.001 | 0.913 | |
| K4 | BiRNN | 83.28 | 82.66 | 83.94 | <0.001 | 0.910 |
| BiGRU | 84.24 | 83.12 | 84.28 | <0.001 | 0.915 | |
| BiLSTM | 85.13 | 85.68 | 86.52 | <0.001 | 0.923 | |
| K5 | BiRNN | 84.28 | 83.66 | 84.94 | <0.001 | 0.920 |
| BiGRU | 85.24 | 84.12 | 85.28 | <0.001 | 0.925 | |
| BiLSTM | 86.13 | 86.68 | 87.52 | <0.001 | 0.933 | |
| K10 | BiRNN | 85.27 | 84.65 | 85.93 | <0.001 | 0.920 |
| BiGRU | 86.23 | 85.11 | 86.27 | <0.001 | 0.925 | |
| BiLSTM | 87.00 | 86.67 | 88.51 | <0.001 | 0.935 |
| CVP | Models | ACC (%) | SPEC (%) | SEN (%) | p-Value | AUC (0–1) |
|---|---|---|---|---|---|---|
| K2 | RNN + GRU | 85.00 | 82.76 | 82.62 | <0.001 | 0.930 |
| RNN + LSTM | 85.91 | 85.37 | 83.56 | <0.001 | 0.939 | |
| LSTM + GRU | 88.02 | 88.16 | 84.62 | <0.001 | 0.943 | |
| BiRNN + BiGRU | 90.53 | 89.75 | 88.32 | <0.001 | 0.954 | |
| BiRNN + BiLSTM | 91.84 | 90.18 | 88.32 | <0.001 | 0.968 | |
| BiLSTM + BiGRU | 94.14 | 92.22 | 89.12 | <0.001 | 0.974 | |
| K4 | RNN + GRU | 86.66 | 83.76 | 83.62 | <0.001 | 0.931 |
| RNN + LSTM | 86.02 | 86.37 | 84.56 | <0.001 | 0.940 | |
| LSTM + GRU | 89.02 | 89.16 | 85.62 | <0.001 | 0.944 | |
| BiRNN + BiGRU | 91.53 | 90.75 | 87.32 | <0.001 | 0.955 | |
| BiRNN + BiLSTM | 92.84 | 91.18 | 89.32 | <0.001 | 0.969 | |
| BiLSTM + BiGRU | 95.14 | 93.22 | 90.12 | <0.001 | 0.975 | |
| K5 | RNN + GRU | 86.45 | 82.76 | 84.62 | <0.001 | 0.947 |
| RNN + LSTM | 86.50 | 85.37 | 86.56 | <0.001 | 0.957 | |
| LSTM + GRU | 91.02 | 88.16 | 87.62 | <0.001 | 0.959 | |
| BiRNN + BiGRU | 93.53 | 84.75 | 87.32 | <0.001 | 0.972 | |
| BiRNN + BiLSTM | 94.84 | 86.18 | 87.32 | <0.001 | 0.976 | |
| BiLSTM + BiGRU | 96.14 | 92.22 | 93.12 | <0.001 | 0.982 | |
| K10 | RNN + GRU | 88.14 | 82.75 | 84.61 | <0.001 | 0.948 |
| RNN + LSTM | 88.24 | 84.36 | 85.55 | <0.001 | 0.958 | |
| LSTM + GRU | 92.01 | 87.15 | 86.61 | <0.001 | 0.960 | |
| BiRNN + BiGRU | 94.52 | 88.74 | 87.31 | <0.001 | 0.975 | |
| BiRNN + BiLSTM | 95.83 | 89.17 | 87.31 | <0.001 | 0.979 | |
| BiLSTM + BiGRU | 97.25 | 92.21 | 93.11 | <0.001 | 0.985 |
| SN | PCA | CST | RFR |
|---|---|---|---|
| 1 | Age | Age | Age |
| 2 | Diabetes T1D | Diabetes T1D | Diabetes T1 D |
| 3 | Avg Sys before angio | Avg Sys before angio | Avg Sys before angio |
| 4 | IPN | IPN | IPN |
| 5 | Creatinine | Creatinine | Creatinine |
| 6 | Hyperlipidemia | Hyperlipidemia | Hyperlipidemia |
| 7 | Alpha-Blockers | Alpha-Blockers | Alpha-Blockers |
| 8 | Insulin | Family Hx of CVD | Current Smoker |
| 9 | Angina | Current Smoker | BMI |
| 10 | Anti-Platelet/Anti-Coagulants | Anti-Platelet/Anti-Coagulants | TPA |
| SN | M1 (a) | M2 (b) | % Increase (a − b/a) × 100 |
|---|---|---|---|
| 1 | ML | HDL | 30.20% |
| 2 | UniDL | HDL | 8.72% |
| 3 | BiDL | HDL | 7.26% |
| Seen Data | Unseen Data | |||||
|---|---|---|---|---|---|---|
| SN | Models | Mean AUC (0–1) | p-Value | Mean AUC (0–1) | p-Value | Difference (%) |
| 1 | ML | 0.702 | <0.005 | 0.683 | <0.005 | 2.78% |
| 2 | UniDL | 0.910 | <0.001 | 0.884 | <0.001 | 2.94% |
| 3 | BiDL | 0.931 | <0.001 | 0.905 | <0.001 | 2.87% |
| 4 | HDL | 0.956 | <0.001 | 0.939 | <0.001 | 1.79% |
| SN | Model1 | Model2 | Mann-Whitney |
|---|---|---|---|
| 1 | BiRNN | RNN | p < 0.05 |
| 2 | BiGRU + BiRNN | RNN | p < 0.05 |
| 3 | BiRNN + BiLSTM | BiRNN | p < 0.05 |
| 4 | BiRNN + BiLSTM | RNN | p < 0.05 |
| 5 | BiRNN + BiGRU | BiRNN | p < 0.05 |
| 6 | BiRNN + BiGRU | RNN | p < 0.05 |
| 7 | BiLSTM + BiGRU | RNN | p < 0.05 |
| 8 | BiLSTM + BiGRU | BiRNN | p < 0.05 |
| C0 | C1 | C2 | C3 | C4 | C5 | C6 | C7 |
|---|---|---|---|---|---|---|---|
| SN | Authors | NOF | ML/DL Models Used | #Patients/#Images | CV | SV | Results Obtained |
| R1 | Unnikrishnan et al. [96] | 09 | SVM | 2.4 K | K5 | 🗴 | AUC for ML = 0.71; for CCVRC = 0.57 |
| R2 | Jamthikar et al. [56] | 39 | RF, SVM, XGBoost | 500 | K10 | ✓ | AUC for ML = 0.95; for CCVRC = 0.50 |
| R3 | Alaa et al. [19] | 473 | SVM, GBM, RF, AdaBoost | 423.6 K | K10 | 🗴 | AUC for ML = 0.724; for CCVRC = 0.774 |
| R4 | Zhou et al. [97] | 🗴 | UNet++ | 144/510 497/638 | K5 | 🗴 | TPA error = 5.55 ± 4.34 mm2; DSC = 83.3–85.7% |
| R5 | Jain et al. [98] | 🗴 | UNet+, UNet, SegNetUNet, SegNet, Unet + SegNet | 97/970 | K5 | 🗴 | AUC for UNet = 0.91, for UNet + = 0.91, for SegNet-UNet = 0.908, for SegNet = 0.905, and for SegNetUNet+ = 0.898 (using CE-loss models) and 0.883, 0.889, 0.905, 0.889, and 0.907 (using DSC-loss models); PA error = 3.49 mm2 for SDL and 4.21 mm2 for HDL |
| R6 | Jain et al. [99] | 24 | UNet | 379, 300 | K10 | 🗴 | Unseen FoM: 70.96 and 91.14 Seen FoM: 97.57, 88.89, and 99.14 |
| R7 | Jain et al. [100] | 24 | 🗴 AtheroEdge 2.0, UNet, UNetSegNet | 379 | K10 | 🗴 | AUC for UNet = 0.93, for SegNet-UNet = 0.94; for AtheroEdge™ 2.0 = 0.95, respectively; SDL PA error = 9.9 mm2; HDL PA error = 8 mm2; AtheroEdge™ = 2.0 mm2; PA error = 9.6 mm2 |
| R8 | Johri et al. [54] | 39 | RF, SVM, RNN, LSTM | 500 | K10 | ✓ | AUC for DL AUC = 0.99, for ML = 0.89, for CCVRC = 0.50 |
| R9 | Akari et al. [48] | 56 | SVM | 4004 | K10 | ✓ | ACC = 98.43; reliability index = 97.32% |
| R10 | Proposed method | 39 | SVM, RF, XGBoost, UniDL, BiDL, HDL | 500 | K2, K4, K5, K10 | ✓ | AUC for ML = 0.702, for UniDL = 0.910, for BiDL = 0.931, and for HDL = 0.956 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhagawati, M.; Paul, S.; Mantella, L.; Johri, A.M.; Gupta, S.; Laird, J.R.; Singh, I.M.; Khanna, N.N.; Al-Maini, M.; Isenovic, E.R.; et al. Cardiovascular Disease Risk Stratification Using Hybrid Deep Learning Paradigm: First of Its Kind on Canadian Trial Data. Diagnostics 2024, 14, 1894. https://doi.org/10.3390/diagnostics14171894
Bhagawati M, Paul S, Mantella L, Johri AM, Gupta S, Laird JR, Singh IM, Khanna NN, Al-Maini M, Isenovic ER, et al. Cardiovascular Disease Risk Stratification Using Hybrid Deep Learning Paradigm: First of Its Kind on Canadian Trial Data. Diagnostics. 2024; 14(17):1894. https://doi.org/10.3390/diagnostics14171894
Chicago/Turabian StyleBhagawati, Mrinalini, Sudip Paul, Laura Mantella, Amer M. Johri, Siddharth Gupta, John R. Laird, Inder M. Singh, Narendra N. Khanna, Mustafa Al-Maini, Esma R. Isenovic, and et al. 2024. "Cardiovascular Disease Risk Stratification Using Hybrid Deep Learning Paradigm: First of Its Kind on Canadian Trial Data" Diagnostics 14, no. 17: 1894. https://doi.org/10.3390/diagnostics14171894
APA StyleBhagawati, M., Paul, S., Mantella, L., Johri, A. M., Gupta, S., Laird, J. R., Singh, I. M., Khanna, N. N., Al-Maini, M., Isenovic, E. R., Tiwari, E., Singh, R., Nicolaides, A., Saba, L., Anand, V., & Suri, J. S. (2024). Cardiovascular Disease Risk Stratification Using Hybrid Deep Learning Paradigm: First of Its Kind on Canadian Trial Data. Diagnostics, 14(17), 1894. https://doi.org/10.3390/diagnostics14171894








