Juvenile Osteochondritis Dissecans: A Case Report
Abstract
1. Introduction
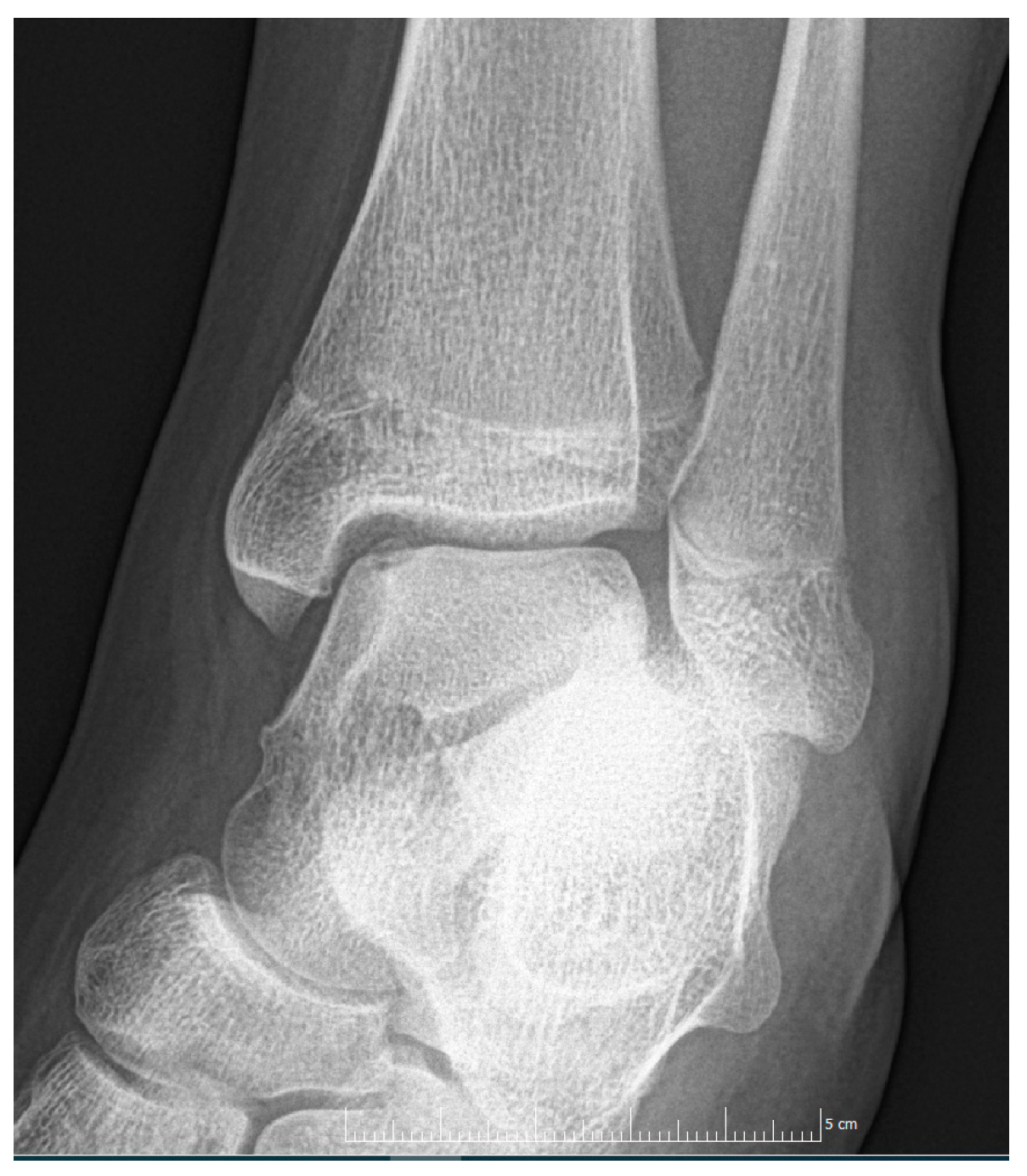
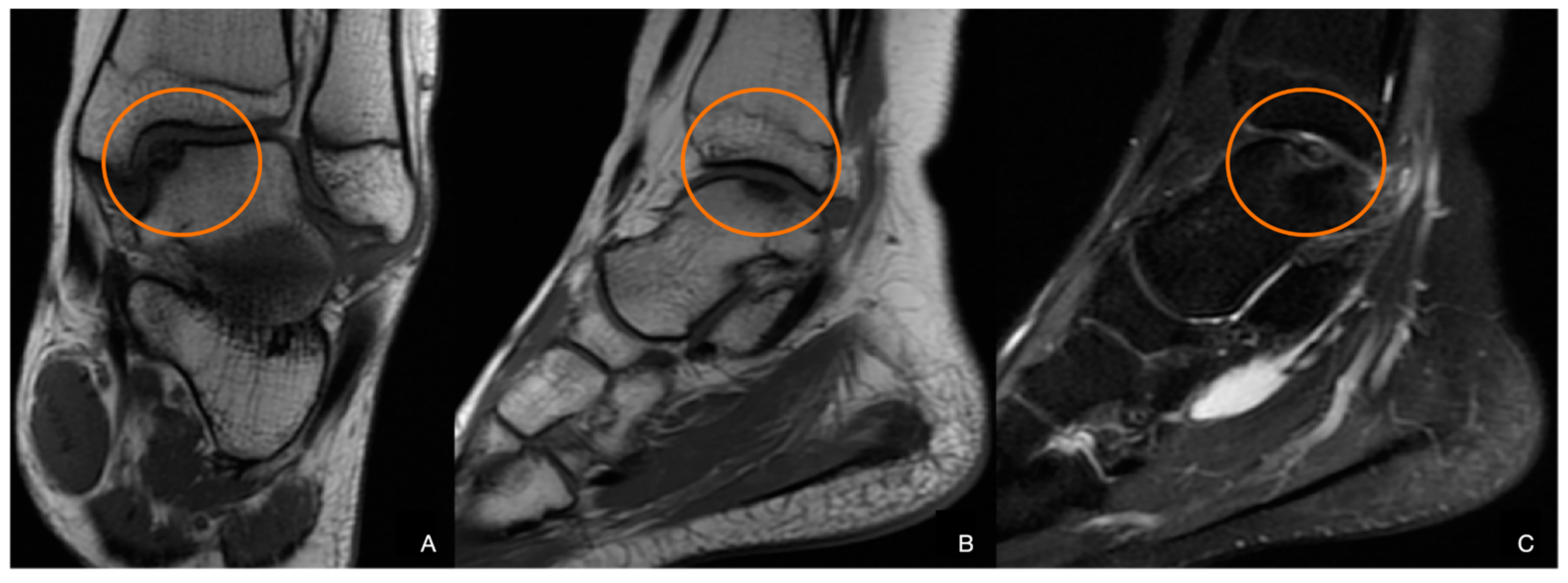
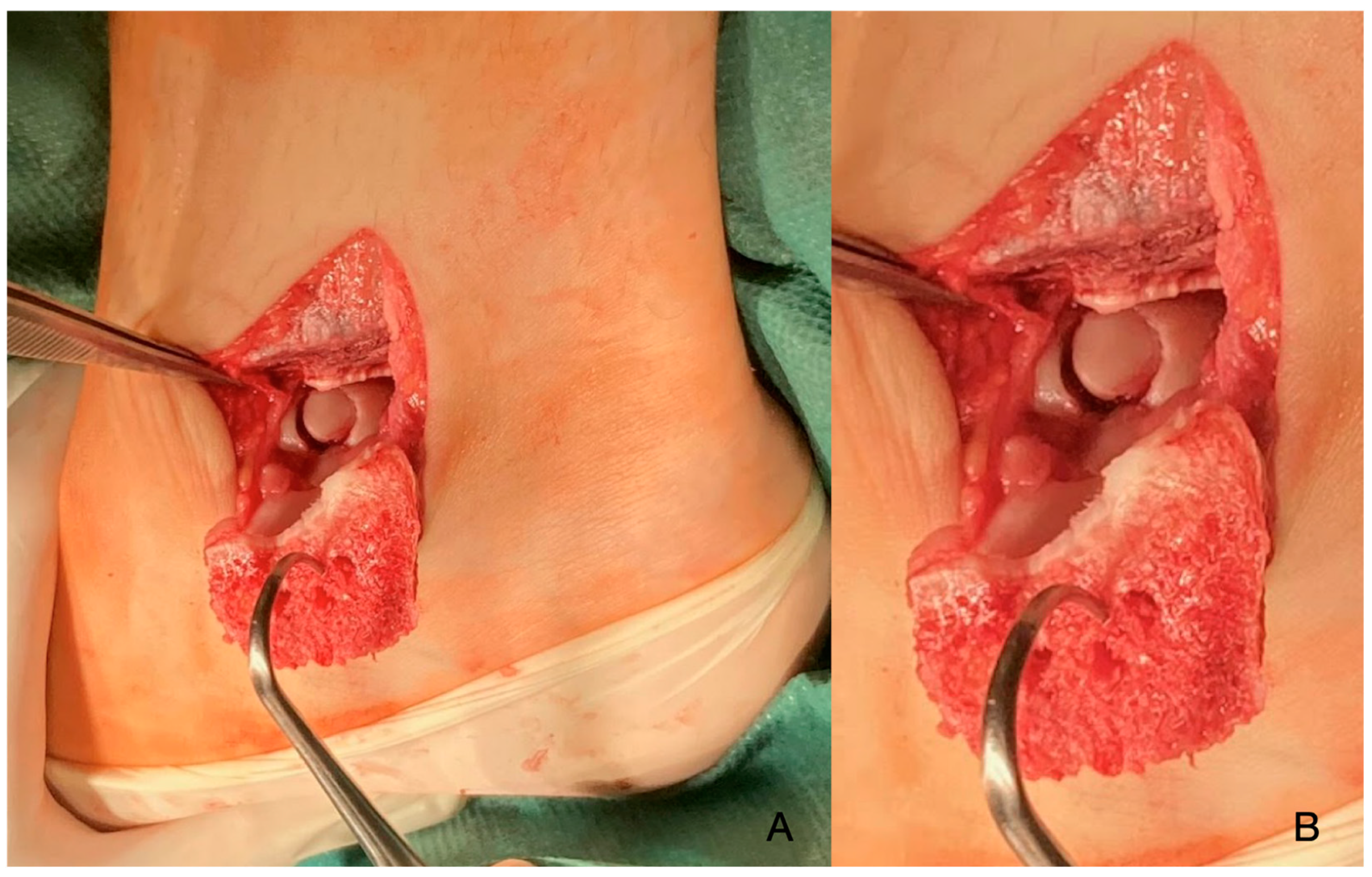
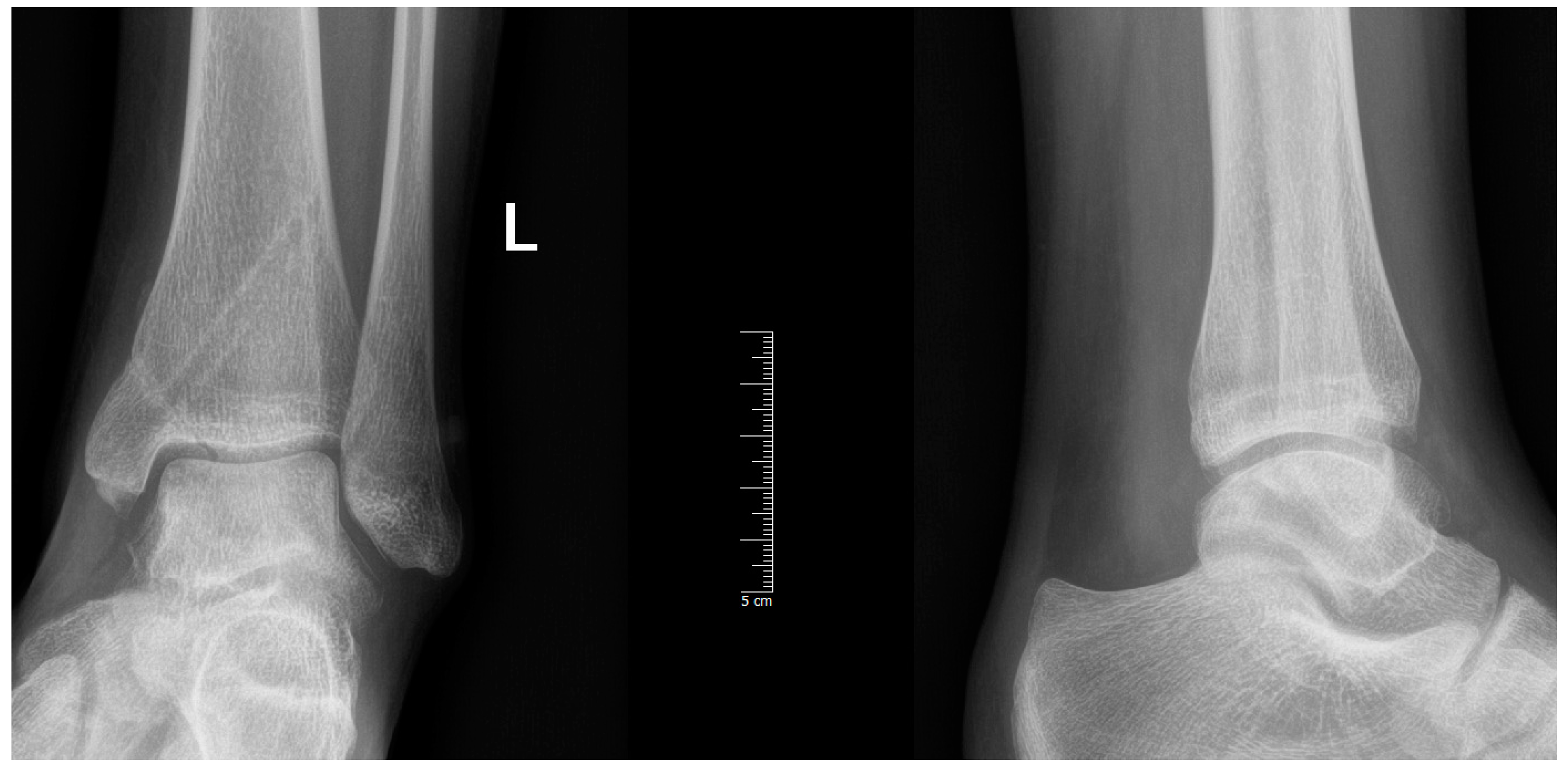
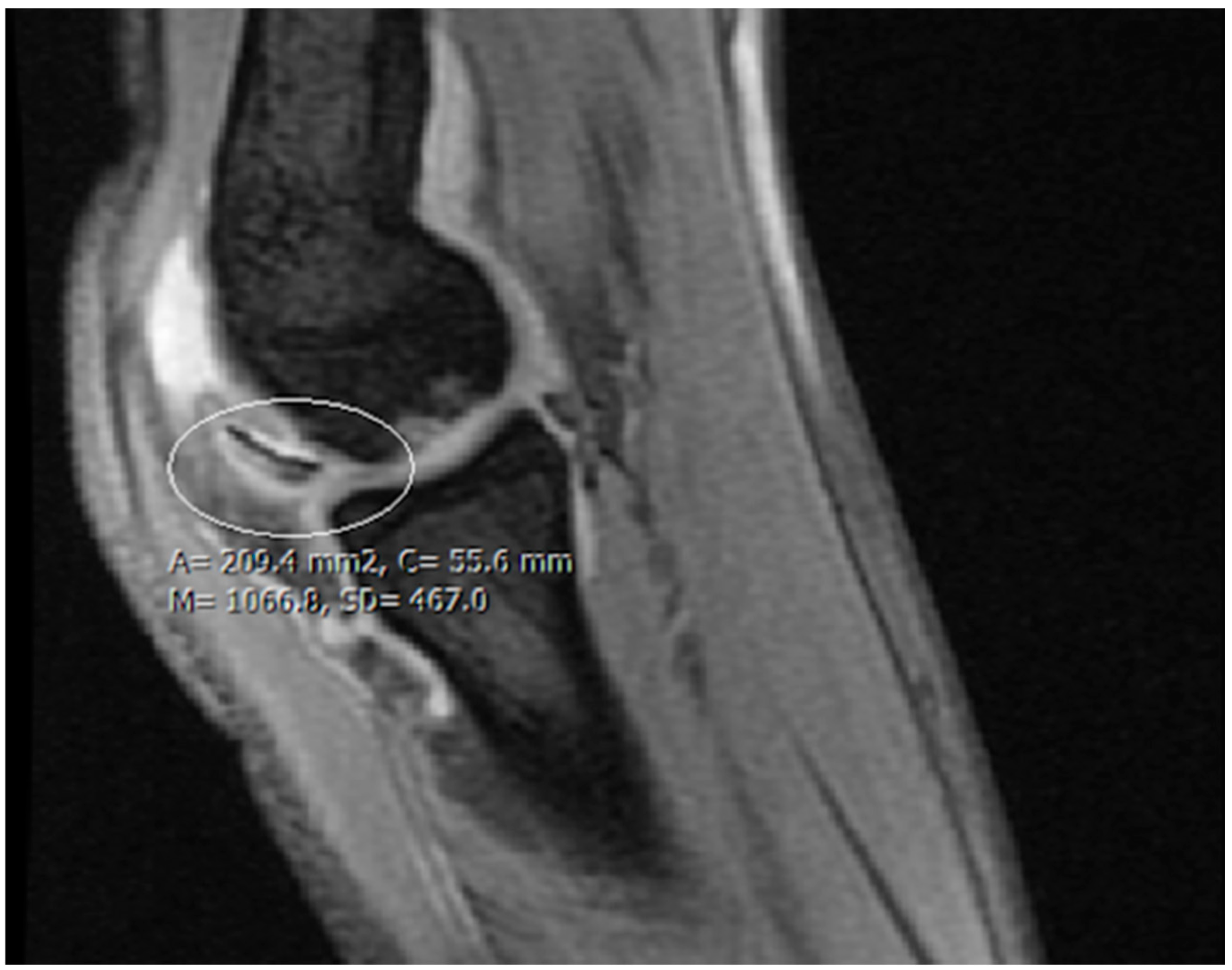
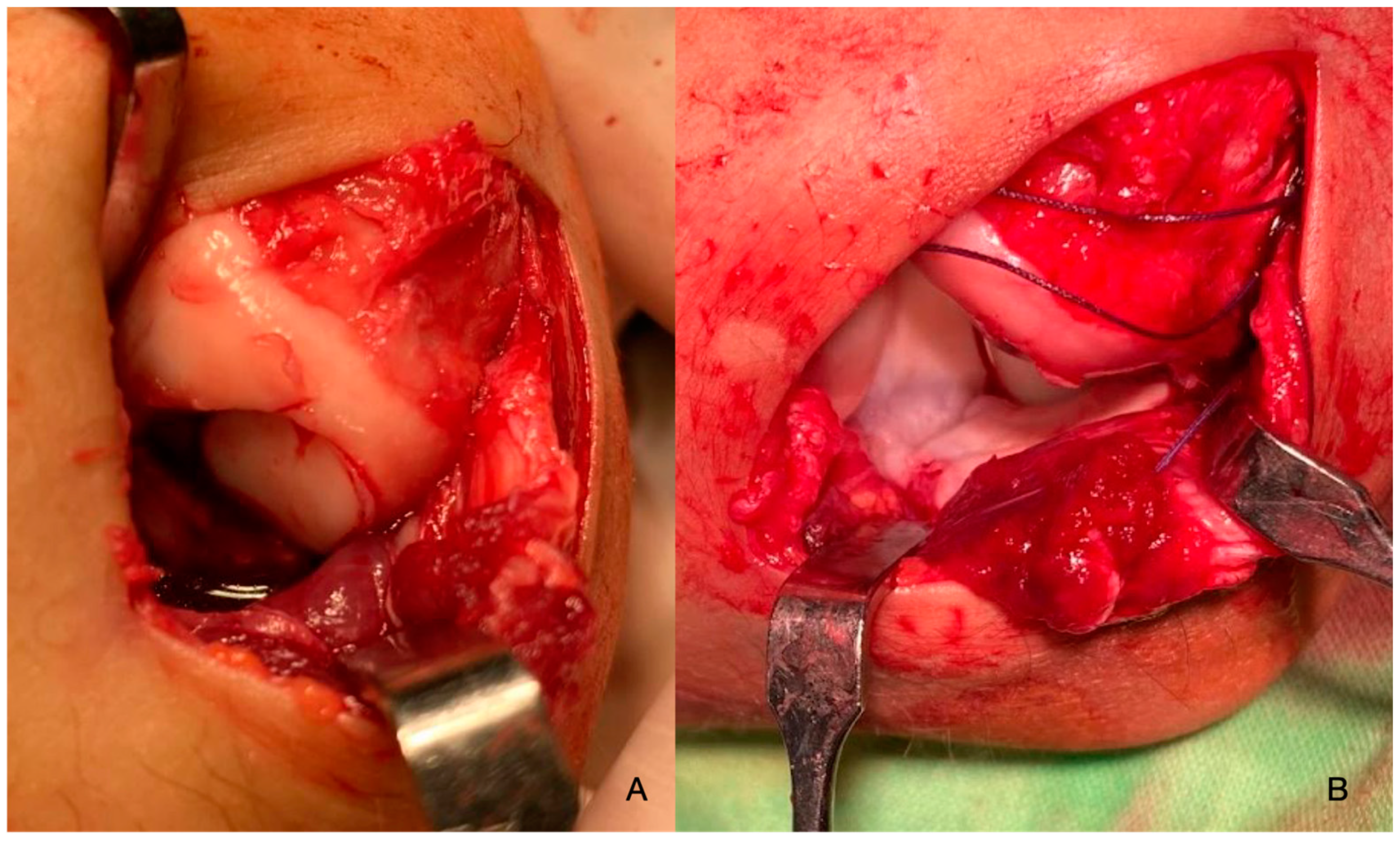
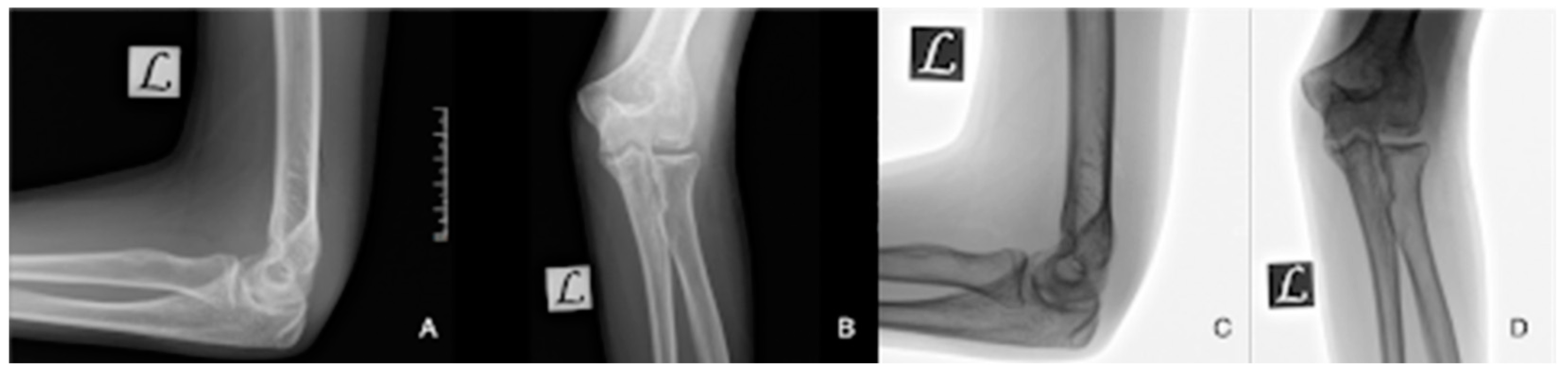
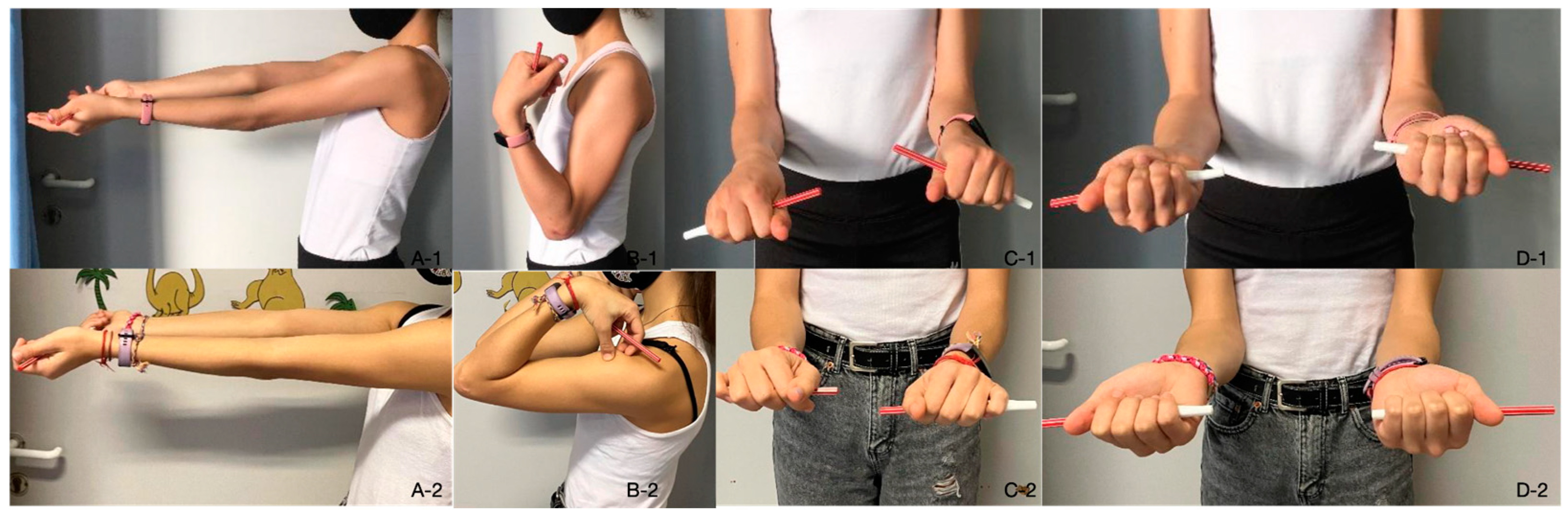
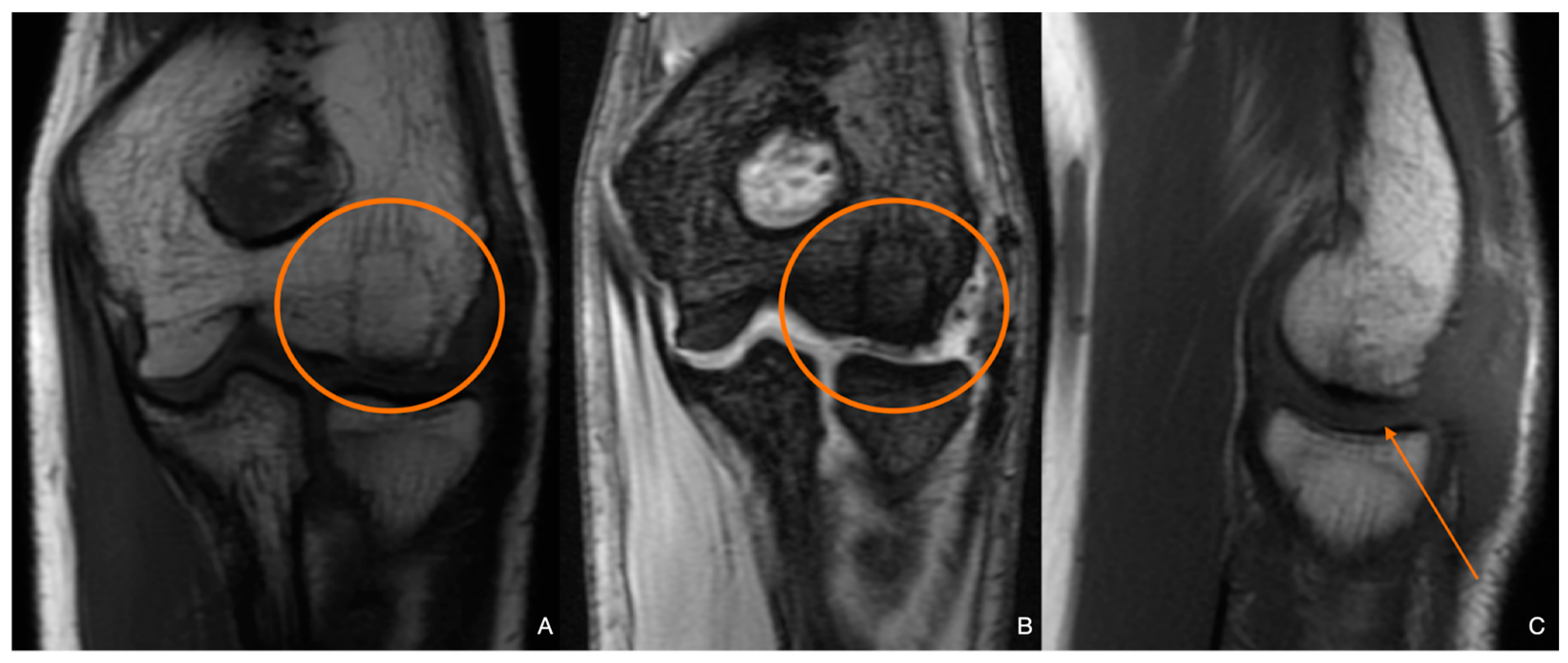
2. Materials and Methods
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bruns, J.; Werner, M.; Habermann, C. Osteochondritis Dissecans: Etiology, Pathology, and Imaging with a Special Focus on the Knee Joint. Cartilage 2018, 9, 346–362. [Google Scholar] [CrossRef] [PubMed]
- Tóth, F.; Nissi, M.J.; Ellermann, J.M.; Wang, L.; Shea, K.G.; Polousky, J.; Carlson, C.S. Novel Application of Magnetic Resonance Imaging Demonstrates Characteristic Differences in Vasculature at Predilection Sites of Osteochondritis Dissecans. Am. J. Sports Med. 2015, 43, 2522–2527. [Google Scholar] [CrossRef] [PubMed]
- Chau, M.M.; Klimstra, M.A.; Wise, K.L.; Ellermann, J.M.; Tóth, F.; Carlson, C.S.; Nelson, B.J.; Tompkins, M.A. Osteochondritis Dissecans. J. Bone Jt. Surg. Am. 2021, 103, 1132–1151. [Google Scholar] [CrossRef] [PubMed]
- Hefti, F.; Beguiristain, J.; Krauspe, R.; Möller-Madsen, B.; Riccio, V.; Tschauner, C.; Wetzel, R.; Zeller, R. Osteochondritis Dissecans: A Multicenter Study of the European Pediatric Orthopedic Society. J. Pediatr. Orthop. Part B 1999, 8, 231–245. [Google Scholar]
- Tóth, F.; Nissi, M.J.; Wang, L.; Ellermann, J.M.; Carlson, C.S. Surgical Induction, Histological Evaluation, and MRI Identification of Cartilage Necrosis in the Distal Femur in Goats to Model Early Lesions of Osteochondrosis. Osteoarthr. Cartil. OARS Osteoarthr. Res. Soc. 2015, 23, 300–307. [Google Scholar] [CrossRef]
- Kamei, G.; Adachi, N.; Deie, M.; Nakamae, A.; Nakasa, T.; Shibuya, H.; Okuhara, A.; Niimoto, T.; Kazusa, H.; Ohkawa, S.; et al. Characteristic Shape of the Lateral Femoral Condyle in Patients with Osteochondritis Dissecans Accompanied by a Discoid Lateral Meniscus. J. Orthop. Sci. 2012, 17, 124–128. [Google Scholar] [CrossRef]
- Ishikawa, M.; Adachi, N.; Yoshikawa, M.; Nakamae, A.; Nakasa, T.; Ikuta, Y.; Hayashi, S.; Deie, M.; Ochi, M. Unique Anatomic Feature of the Posterior Cruciate Ligament in Knees Associated With Osteochondritis Dissecans. Orthop. J. Sports Med. 2016, 4, 2325967116648138. [Google Scholar] [CrossRef]
- Takigami, J.; Hashimoto, Y.; Tomihara, T.; Yamasaki, S.; Tamai, K.; Kondo, K.; Nakamura, H. Predictive Factors for Osteochondritis Dissecans of the Lateral Femoral Condyle Concurrent with a Discoid Lateral Meniscus. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 799–805. [Google Scholar] [CrossRef]
- Gonzalez-Herranz, P.; Rodriguez, M.L.; de la Fuente, C. Femoral Osteochondritis of the Knee: Prognostic Value of the Mechanical Axis. J. Child. Orthop. 2017, 11, 1–5. [Google Scholar] [CrossRef]
- McElroy, M.J.; Riley, P.M.; Tepolt, F.A.; Nasreddine, A.Y.; Kocher, M.S. Catcher’s Knee: Posterior Femoral Condyle Juvenile Osteochondritis Dissecans in Children and Adolescents. J. Pediatr. Orthop. 2018, 38, 410–417. [Google Scholar] [CrossRef]
- Matching Osteochondritis Dissecans Lesions in Identical Twin Brothers—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/24025016/ (accessed on 11 July 2024).
- Oberti, V.; Sanchez Ortiz, M.; Allende, V.; Masquijo, J. Prevalencia de Hipovitaminosis D En Pacientes Con Osteocondritis Disecante Juvenil. Rev. Esp. Cir. Ortopédica Traumatol. 2021, 65, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Bruns, J.; Werner, M.; Soyka, M. Is Vitamin D Insufficiency or Deficiency Related to the Development of Osteochondritis Dissecans? Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1575–1579. [Google Scholar] [CrossRef] [PubMed]
- Maier, G.S.; Lazovic, D.; Maus, U.; Roth, K.E.; Horas, K.; Seeger, J.B. Vitamin D Deficiency: The Missing Etiological Factor in the Development of Juvenile Osteochondrosis Dissecans? J. Pediatr. Orthop. 2019, 39, 51–54. [Google Scholar] [CrossRef]
- Ellermann, J.M.; Ludwig, K.D.; Nissi, M.J.; Johnson, C.P.; Strupp, J.P.; Wang, L.; Zbýň, Š.; Tóth, F.; Arendt, E.; Tompkins, M.; et al. Three-Dimensional Quantitative Magnetic Resonance Imaging of Epiphyseal Cartilage Vascularity Using Vessel Image Features. JBJS Open Access 2019, 4, e0031. [Google Scholar] [CrossRef] [PubMed]
- Olstad, K.; Ekman, S.; Carlson, C.S. An Update on the Pathogenesis of Osteochondrosis. Vet. Pathol. 2015, 52, 785–802. [Google Scholar] [CrossRef] [PubMed]
- Tóth, F.; Tompkins, M.A.; Shea, K.G.; Ellermann, J.M.; Carlson, C.S. Identification of Areas of Epiphyseal Cartilage Necrosis at Predilection Sites of Juvenile Osteochondritis Dissecans in Pediatric Cadavers. J. Bone Jt. Surg. Am. 2018, 100, 2132–2139. [Google Scholar] [CrossRef]
- McCoy, A.M.; Toth, F.; Dolvik, N.I.; Ekman, S.; Ellermann, J.; Olstad, K.; Ytrehus, B.; Carlson, C.S. Articular Osteochondrosis: A Comparison of Naturally-Occurring Human and Animal Disease. Osteoarthr. Cartil. OARS Osteoarthr. Res. Soc. 2013, 21, 1638–1647. [Google Scholar] [CrossRef]
- Cabral, J.; Duart, J. Osteochondritis Dissecans of the Knee in Adolescents: How to Treat Them? J. Child. Orthop. 2023, 17, 54–62. [Google Scholar] [CrossRef]
- Takahara, M.; Ogino, T.; Tsuchida, H.; Takagi, M.; Kashiwa, H.; Nambu, T. Sonographic Assessment of Osteochondritis Dissecans of the Humeral Capitellum. AJR Am. J. Roentgenol. 2000, 174, 411–415. [Google Scholar] [CrossRef]
- Berndt, A.L.; Harty, M. Transchondral Fractures (Osteochondritis Dissecans) of the Talus. J. Bone Jt. Surg. Am. 1959, 41, 988–1020. [Google Scholar] [CrossRef]
- Guhl, J.F. Arthroscopic Treatment of Osteochondritis Dissecans. Clin. Orthop. 1982, 167, 65–74. [Google Scholar] [CrossRef]
- Brittberg, M.; Winalski, C.S. Evaluation of Cartilage Injuries and Repair. J. Bone Jt. Surg. Am. 2003, 85, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Dipaola, J.D.; Nelson, D.W.; Colville, M.R. Characterizing Osteochondral Lesions by Magnetic Resonance Imaging. Arthrosc. J. Arthrosc. Relat. Surg. 1991, 7, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Wall, E.J.; Vourazeris, J.; Myer, G.D.; Emery, K.H.; Divine, J.G.; Nick, T.G.; Hewett, T.E. The Healing Potential of Stable Juvenile Osteochondritis Dissecans Knee Lesions. J. Bone Jt. Surg. Am. 2008, 90, 2655–2664. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly(Lactic-Co-Glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Sanders, T.L.; Pareek, A.; Obey, M.R.; Johnson, N.R.; Carey, J.L.; Stuart, M.J.; Krych, A.J. High Rate of Osteoarthritis After Osteochondritis Dissecans Fragment Excision Compared With Surgical Restoration at a Mean 16-Year Follow-Up. Am. J. Sports Med. 2017, 45, 1799–1805. [Google Scholar] [CrossRef]
- Lyons, M.L.; Werner, B.C.; Gluck, J.S.; Freilich, A.M.; Dacus, A.R.; Diduch, D.R.; Chhabra, A.B. Osteochondral Autograft Plug Transfer for Treatment of Osteochondritis Dissecans of the Capitellum in Adolescent Athletes. J. Shoulder Elb. Surg. 2015, 24, 1098–1105. [Google Scholar] [CrossRef]
- Kennedy, J.G.; Murawski, C.D. The Treatment of Osteochondral Lesions of the Talus with Autologous Osteochondral Transplantation and Bone Marrow Aspirate Concentrate: Surgical Technique. Cartilage 2011, 2, 327–336. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, Y.; Zhou, X.; Fu, S.; Wang, G. Outcomes from Osteochondral Autograft Transplant or Mosaicplasty in 26 Patients with Type V Osteochondral Lesions of the Talus. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021, 27, e930527-1. [Google Scholar] [CrossRef]
- Tsuda, E.; Ishibashi, Y.; Sato, H.; Yamamoto, Y.; Toh, S. Osteochondral Autograft Transplantation for Osteochondritis Dissecans of the Capitellum in Nonthrowing Athletes. Arthrosc. J. Arthrosc. Relat. Surg. 2005, 21, 1270. [Google Scholar] [CrossRef]
- Dankelman, L.H.M.; Schilstra, S.; IJpma, F.F.A.; Doornberg, J.N.; Colaris, J.W.; Verhofstad, M.H.J.; Wijffels, M.M.E.; Prijs, J. Machine Learning Consortium Artificial Intelligence Fracture Recognition on Computed Tomography: Review of Literature and Recommendations. Eur. J. Trauma Emerg. Surg. 2023, 49, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.J.; Henderson, R.D.E.; Yi, X.; Babyn, P. Artificial Intelligence Solutions for Analysis of X-Ray Images. Can. Assoc. Radiol. J. 2021, 72, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Kraus, M.; Anteby, R.; Konen, E.; Eshed, I.; Klang, E. Artificial Intelligence for X-Ray Scaphoid Fracture Detection: A Systematic Review and Diagnostic Test Accuracy Meta-Analysis. Eur. Radiol. 2024, 34, 4341–4351. [Google Scholar] [CrossRef]
- Yoshizuka, M.; Sunagawa, T.; Nakashima, Y.; Shinomiya, R.; Masuda, T.; Makitsubo, M.; Adachi, N. Comparison of Sonography and MRI in the Evaluation of Stability of Capitellar Osteochondritis Dissecans. J. Clin. Ultrasound 2018, 46, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Flynn, S.; Ross, K.; Hannon, C.; Yasui, Y.; Newman, H.; Murawski, C.; Deyer, T.; Do, H.; Kennedy, J. Autologous Osteochondral Transplantation for Osteochondral Lesions of the Talus. Foot Ankle Int. 2016, 37, 363–372. [Google Scholar] [CrossRef]
- Hangody, L.; Füles, P. Autologous Osteochondral Mosaicplasty for the Treatment of Full-Thickness Defects of Weight-Bearing Joints: Ten Years of Experimental and Clinical Experience. J. Bone Jt. Surg. Am. 2003, 85, 25–32. [Google Scholar] [CrossRef]
- Hollander, J.J.; Dahmen, J.; Emanuel, K.S.; Stufkens, S.A.S.; Kennedy, J.G.; Kerkhoffs, G.M.M.J. The Frequency and Severity of Complications in Surgical Treatment of Osteochondral Lesions of the Talus: A Systematic Review and Meta-Analysis of 6962 Lesions. Cartilaget 2023, 14, 180–197. [Google Scholar] [CrossRef]



| Imaging: | X-ray | Arthroscopy | MRI | |||
|---|---|---|---|---|---|---|
| Classification | Berndt and Harty [21]—1959 | Guhl [22]—1982 | ICRS by Brittberg and Winalsky [23]—2003 | DiPaola [24]—1991 | Hefti [4]—1999 | Ellerman [15]—2019 |
| Stage 1 | Small subchondral compression | Intact Lesion | A stable lesion of the softened area covered by intact cartilage | Thickening of articular cartilage and low signal changes | Small change in signal, without clear fragment margins | Epiphyseal cartilage lesion with necrotic center |
| Stage 2 | Partially detached osteochondral fragment | A lesion with signs of early separation | Lesions with partial discontinuity which are stable when probed | Articular cartilage is breached, with a low signal rim behind the fragment indicating fibrous attachment | Osteochondral fragment with clear margins, without fluid in between | Epiphyseal cartilage lesion with complete or incomplete rim calcification |
| Stage 3 | Completely detached, non-displaced | Partially detached lesion | Lesions with complete discontinuity which are not dislocated (Dead in situ) | High signal changes behind the fragment indicate synovial fluid between the fragment and the underlying subchondral bone | Fluid is partially visible between the fragment and bone | Partially or completely ossified lesion |
| Stage 4 | Completely detached and displaced—loose body | Craters with loose bodies (salvageable or non-salvageable) | Empty defect bed with loose or dislocated fragment | Loose body | Fluid surrounds the fragment but it is still in situ | Healed osseous lesion with scar |
| Stage 5 | Scranton and McDermott modification: Subchondral Cyst | - | - | - | The fragment is completely detached and displaced | Unhealed, detached osseous lesion (Sequestrum) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nudelman, H.; Lőrincz, A.; Kassai, T.; Józsa, G. Juvenile Osteochondritis Dissecans: A Case Report. Diagnostics 2024, 14, 1931. https://doi.org/10.3390/diagnostics14171931
Nudelman H, Lőrincz A, Kassai T, Józsa G. Juvenile Osteochondritis Dissecans: A Case Report. Diagnostics. 2024; 14(17):1931. https://doi.org/10.3390/diagnostics14171931
Chicago/Turabian StyleNudelman, Hermann, Aba Lőrincz, Tamás Kassai, and Gergő Józsa. 2024. "Juvenile Osteochondritis Dissecans: A Case Report" Diagnostics 14, no. 17: 1931. https://doi.org/10.3390/diagnostics14171931
APA StyleNudelman, H., Lőrincz, A., Kassai, T., & Józsa, G. (2024). Juvenile Osteochondritis Dissecans: A Case Report. Diagnostics, 14(17), 1931. https://doi.org/10.3390/diagnostics14171931






