Clinical and Morphological Aspects of Aggressive Salivary Gland Mixed Tumors: A Narrative Review
Abstract
1. Introduction
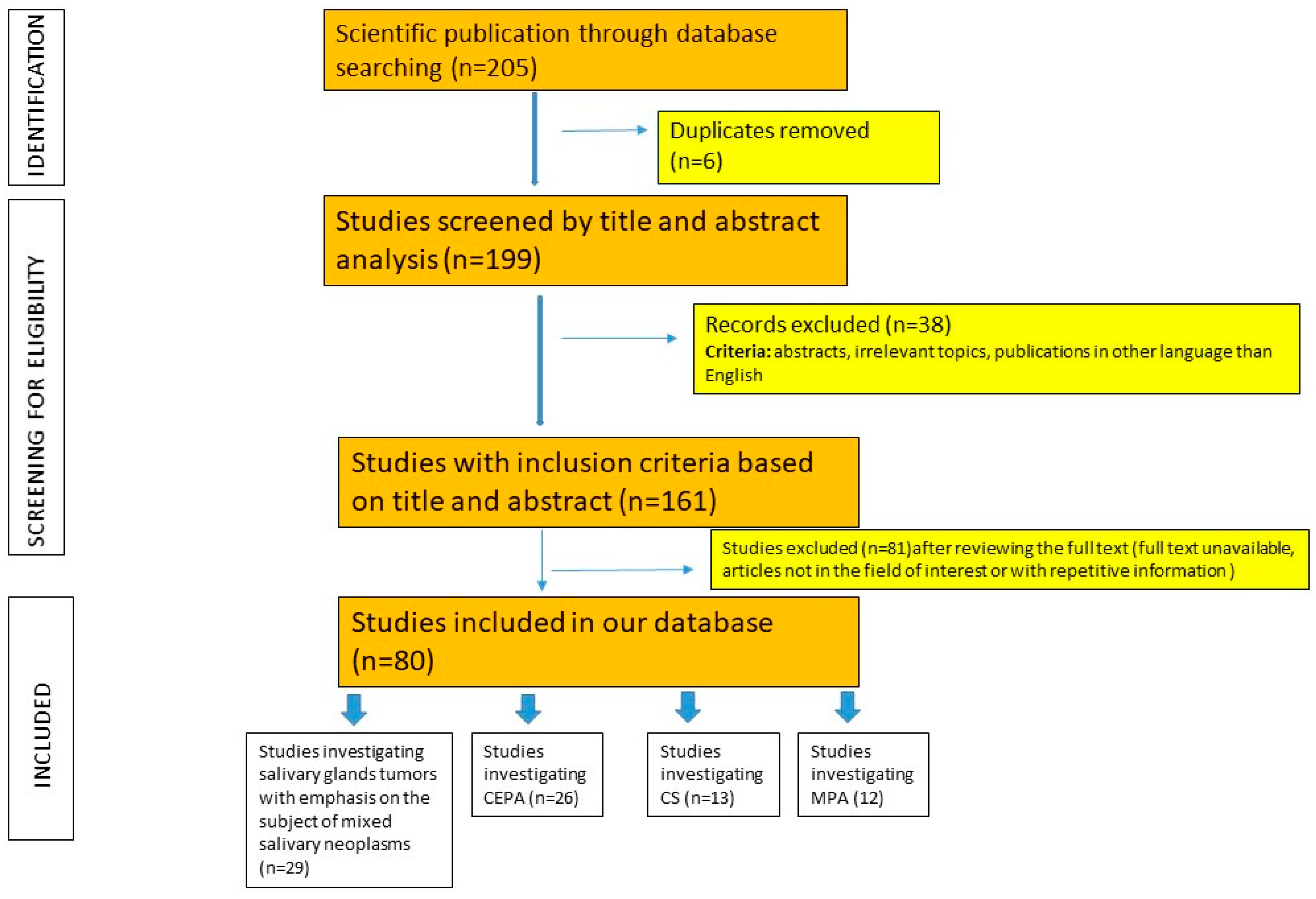
Materials and Methods
2. Clinico-Morphological Aspects of CEPA
2.1. Clinical Aspects
2.2. Gross Aspects
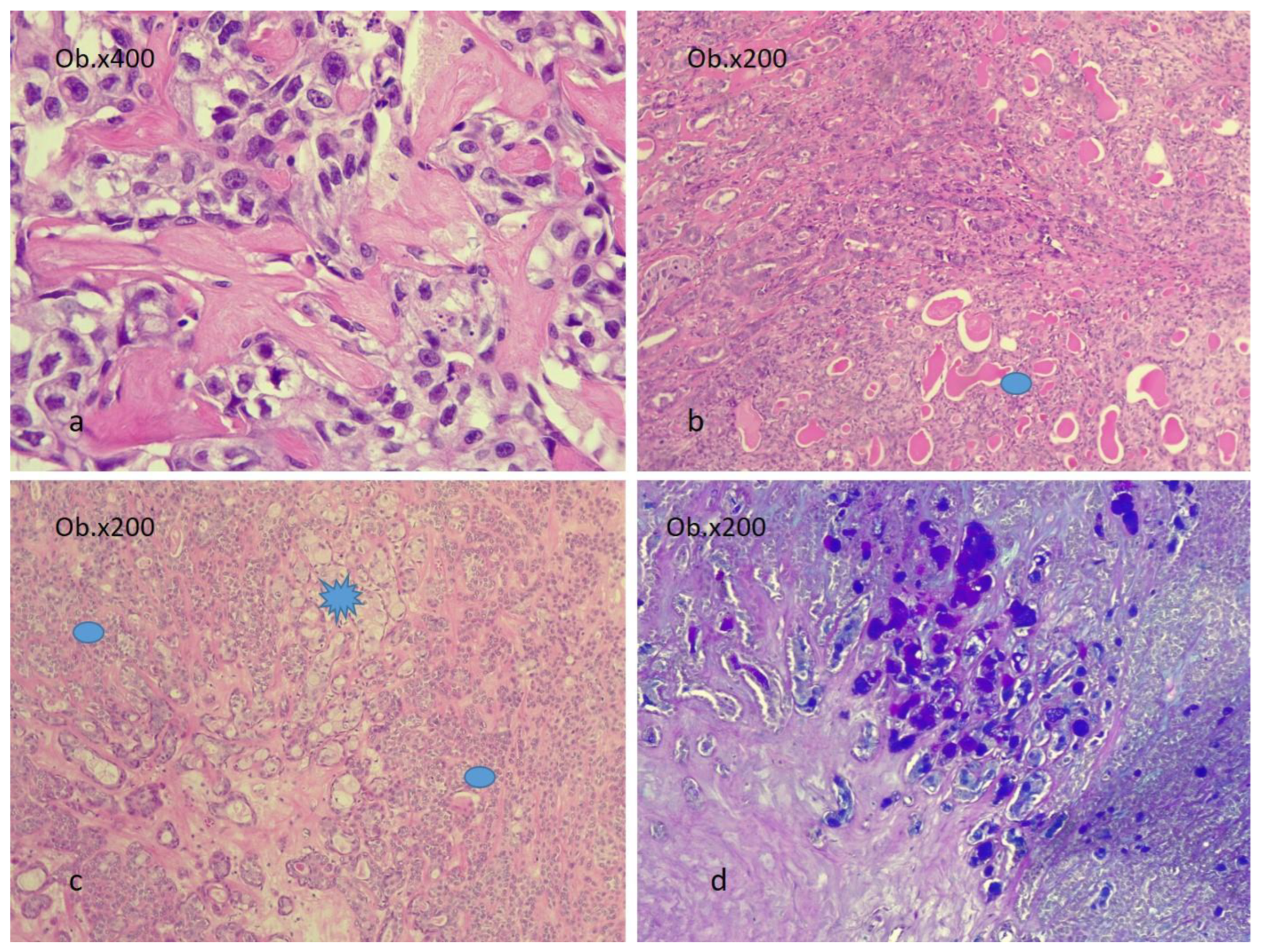
2.3. Microscopic Aspects
2.4. Patterns of Invasion in CEPA
2.5. The Evolutionary Course of CEPA
3. Clinico-Morphological Aspects in CS
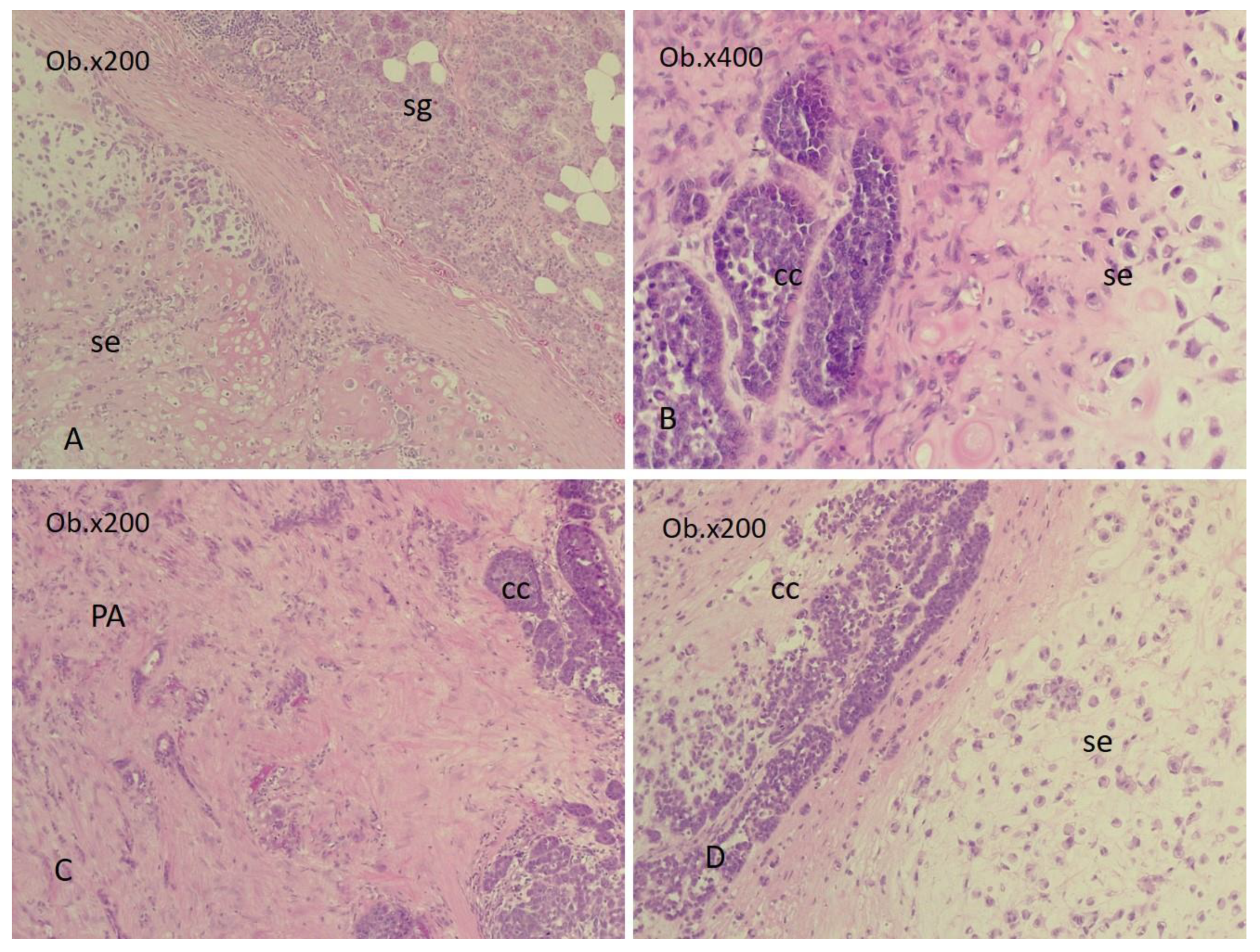
3.1. Gross and Microscopic Aspects
3.2. The Evolutionary Course of CS
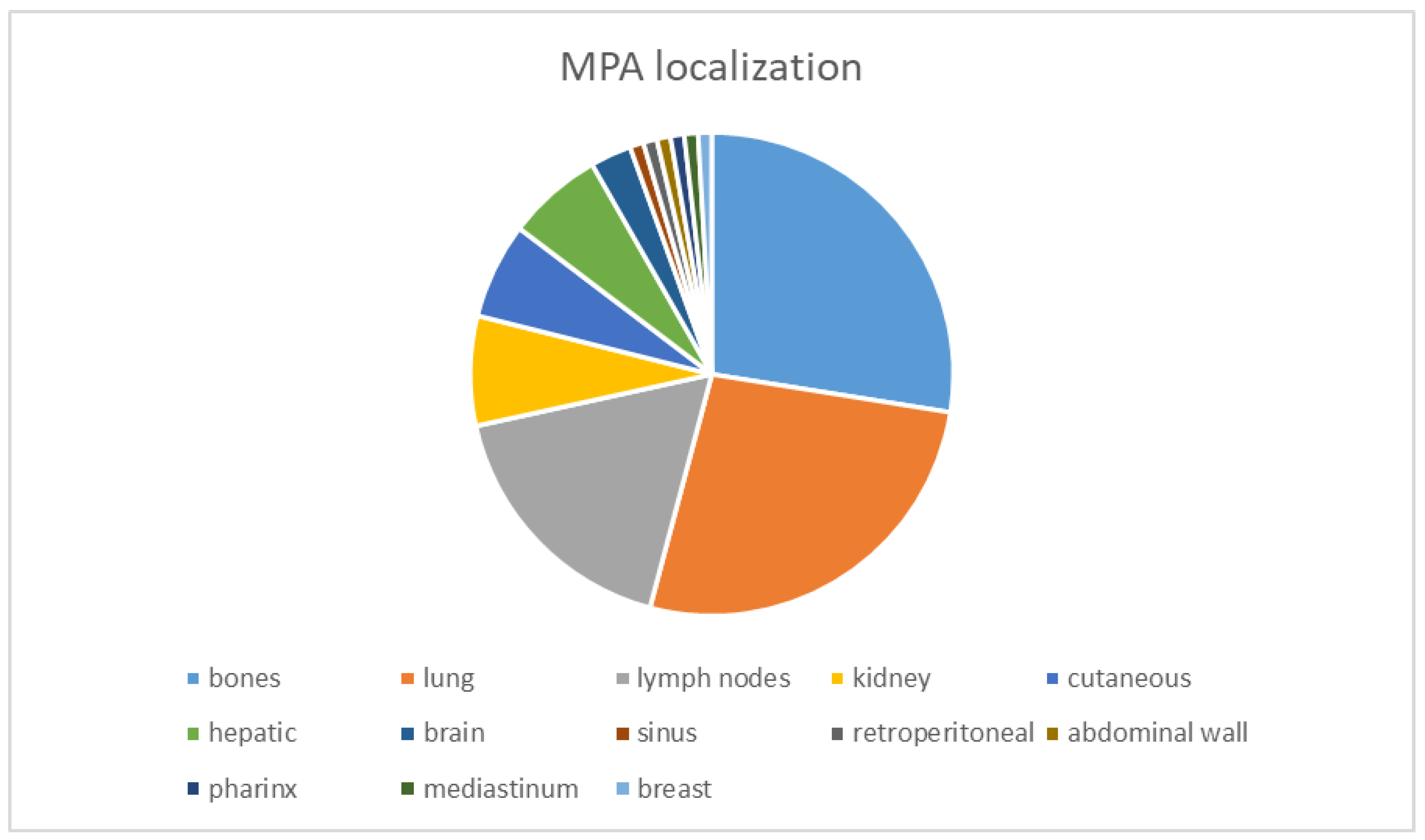
4. Clinico-Morphological Aspects of MPA
The Evolutionary Course of MPA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Locati, L.D.; Ferrarotto, R.; Licitra, L.; Benazzo, M.; Preda, L.; Farina, D.; Gatta, G.; Lombardi, D.; Nicolai, P.; Vander Poorten, V.; et al. Current management and future challenges in salivary glands cancer. Front. Oncol. 2023, 13, 1264287. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McHugh, J.B.; Visscher, D.W.; Barnes, E.L. Update on selected salivary gland neoplasms. Arch. Pathol. Lab. Med. 2009, 133, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Abdelwahed, M.; Chavarria, D.H.; Pereira, L.; Stone, G.; Johnson, A.; Li, J.Y. Carcinosarcoma of the parotid gland: A case report and review of the literature. J. Med. Case Rep. 2024, 18, 24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Knight, J.; Ratnasingham, K. Metastasising pleomorphic adenoma: Systematic review. Int. J. Surg. 2015, 19, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Alsanie, I.; Rajab, S.; Cottom, H.; Adegun, O.; Agarwal, R.; Jay, A.; Graham, L.; James, J.; Barrett, A.W.; van Heerden, W.; et al. Distribution and Frequency of Salivary Gland Tumours: An International Multicenter Study. Head Neck Pathol. 2022, 16, 1043–1054. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsushima, M.; Ohara, R.; Ishida, M.; Kanao, K.; Shimokava, R.; Nakajima, Y. Carcinoma ex pleomorphic adenoma of the submandibular gland with renal metastases composed exclusively of metastasizing pleomorphic adenoma. Int. Cancer Conf. J. 2012, 1, 116–120. [Google Scholar] [CrossRef]
- Gnepp, D.R.; Brandwein-Gensler, M.S.; El-Naggar, A.K.; Nagao, T. Carcinoma ex pleomorphic adenoma. In Pathology & Genetics of Head and Neck Tumours; Barnes, L., Eveson, J.W., Reichart, P., Sindranski, D., Eds.; World Health Organization Classification of Tumours; IARC Press: Lyon, France, 2005; Volume 5, pp. 242–243. [Google Scholar]
- Yamada, S.; Nabeshima, A.; Tabata, T.; Guo, X.; Tasaki, T.; Wang, K.Y.; Shimajiri, S.; Sasaguri, Y. Invasive salivary duct carcinoma ex pleomorphic adenoma of the parotid gland: A teaching case giving rise to the genuine diagnostic difficulty on an inadequate cytology specimen. Diagn. Pathol. 2012, 7, 61. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Antony, J.; Gopalan, V.; Smith, R.A.; Lam, A.K. Carcinoma ex pleomorphic adenoma: A comprehensive review of clinical, pathological and molecular data. Head Neck Pathol. 2012, 6, 1–9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cimino-Mathews, A.; Lin, B.M.; Chang, S.S.; Boahene, K.D.; Bishop, J.A. Carcinoma ex pleomorphic adenoma of the nasal cavity. Head Neck Pathol. 2011, 5, 405–409. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Nakamura, S.; Inui, M.; Matsumura, Y.; Takeoka, T.; Okumura, K.; Tagawa, T. A case of carcinoma ex pleomorphic adenoma in the buccal mucosa: Review of the literature. J. Maxillofac. Oral Surg. 2013, 12, 224–227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Zhao, J.; Wang, J.; Yu, C.; Guo, L.; Wang, K.; Liang, Z.; Lou, J. Prognostic factors affecting the clinical outcome of carcinoma ex pleomorphic adenoma in the major salivary gland. World J. Surg. Oncol. 2013, 11, 180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Palma, S. Carcinoma ex pleomorphic adenoma, with particular emphasis on early lesions. Head Neck Pathol. 2013, 7 (Suppl. S1), S68–S76. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Furukawa, M.; Suzuki, H.; Matsuura, K.; Takahashi, E.; Suzuki, H.; Tezuka, F. Carcinoma ex pleomorphic adenoma of the palatal minor salivary gland with extension into the nasopharynx. Auris Nasus Larynx 2001, 28, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Mitate, E.; Kawano, S.; Kiyoshima, T.; Kawazu, T.; Chikui, T.; Goto, Y.; Matsubara, R.; Nakamura, S. Carcinoma ex pleomorphic adenoma of the upper lip: A case of an unusual malignant component of squamous cell carcinoma. World J. Surg. Oncol. 2013, 11, 234. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reichart, P.A.; Kalz, S.; Rabel, A.; Bornstein, M.M. Carcinoma ex pleomorphic adenoma in a minor salivary gland: Report of a case. Oral Maxillofac. Surg. 2010, 14, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Kini, Y.K.; Halli, R.; Mishra, S.; Kalburge, J.V. Comprehensive management of a rare carcinoma ex pleomorphic adenoma of the lacrimal gland with a modified lateral orbitotomy access osteotomy. A case report. Oral Maxillofac. Surg. 2012, 16, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Tamiolakis, D.; Chimona, T.S.; Georgiou, G.; Proimos, E.; Nikolaidou, S.; Perogamvrakis, G.; Papadakis, C.E. Accessory parotid gland carcinoma ex pleomorphic adenoma. Case study diagnosed by fine needle aspiration. Stomatologija 2009, 11, 37–40. [Google Scholar] [PubMed]
- Yu, T.; Gao, Q.H.; Wang, X.Y.; Wen, Y.M.; Li, L.J. Malignant sublingual gland tumors: A retrospective clinicopathologic study of 28 cases. Oncology 2007, 72, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Kroul, R.; Dubey, A.; Binhamed, A.; Butler, J.; Cooke, A.; Abdoh, A.; Nason, R. Prognostic factors depicting overall survival in lesser major (submandibular, sublingual) and minor salivary gland tumors. Turk. J. Cancer 2008, 38, 159–166. [Google Scholar]
- Akan, H.; Yildiz, L.; Unal, R. Carcinoma ex pleomorphic adenoma of the minor salivary gland with pulmonary metastasis. Diagn. Interv. Radiol. 2008, 14, 3–5. [Google Scholar] [PubMed]
- Beckhardt, R.N.; Weber, R.S.; Zane, R.; Garden, A.S.; Wolf, P.; Carrillo, R.; Luna, M.A. Minor salivary gland tumors of the palate: Clinical and pathologic correlates of outcome. Laryngoscope 1995, 105, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Fidele, N.B.; Agustin, M.M.; Jian, G.; Bourleyi, S.I.; Augustin, L.; Olivier, N.K. Carcinosarcoma of parotid gland (malignant mixed tumor). Ann. Maxillofac. Surg. 2015, 5, 240–243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petersson, F.; Loh, K.S. Carcinosarcoma ex non-recurrent pleomorphic adenoma composed of TTF-1 positive large cell neuroendocrine carcinoma and myofibrosarcoma: Apropos a rare Case. Head Neck Pathol. 2013, 7, 163–170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andreadis, D.; Poulopoulos, A.; Epivatianos, A.; Nomikos, A.; Christidis, K.; Barbatis, C. Carcinosarcoma of the parotid gland: Immunohistochemical analysis with emphasis in cell cycle mitotic activity and cell adhesion molecules expression. Oral Oncol. 2006, 42, 140–143. [Google Scholar] [CrossRef]
- Kim, H.S.; Cho, H.J.; Chung, Y.T.; Park, S.A.; Cho, H.J.; Kim, J.M. Carcinosarcoma (true malignant mixed tumor) of the parotid gland –a report of a case with small cell carcinoma as the carcinomatous component. J. Pathol. Transl. Med. 2008, 42, 175–180. [Google Scholar]
- Lim, A.L.; Zahirrudin, Z.; Pua, K.C. A rare case of nasopharyngeal carcinosarcoma. Med. J. Malays. 2012, 67, 428–429. [Google Scholar] [PubMed]
- Seethala, R.R.; Stenman, G. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Tumors of the Salivary Gland. Head Neck Pathol. 2017, 11, 55–67. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, J.; Mateos-Micas, M.; Martínez-Tello, F.J.; Berjón, J.; Montalvo, J.J.; Forteza-González, G.; Galan-Hernández, R. Metastatic benign pleomorphic adenoma. Report of a case and review of the literature. Med. Oral Patol. Oral Cir. Bucal. 2008, 13, E193–E196. [Google Scholar] [PubMed]
- Marioni, G.; Marino, F.; Stramare, R.; Marchese-Ragona, R.; Staffieri, A. Benign metastasizing pleomorphic adenoma of the parotid gland: A clinicopathological puzzle. Head Neck 2003, 25, 1071–1076. [Google Scholar] [CrossRef]
- Fonseca, D.; Arya, S.S.; Kodandapani, S.; Chandini, A.; Kurapati, S.; Rao, C.; Gadepalli, T.; Ali, Z. Metastasising Pleomorphic Adenoma: A Rare Entity. Indian J. Otolaryngol. Head. Neck Surg. 2022, 74 (Suppl. S3), 6321–6323. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koyama, M.; Terauchi, T.; Koizumi, M.; Tanaka, H.; Sato, Y. Metastasizing pleomorphic adenoma in the multiple organs: A case report on FDG-PET/CT imaging. Medicine 2018, 97, e11077. [Google Scholar] [CrossRef] [PubMed]
- Santaliz-Ruiz, L.E.; Morales, G.; Santini, H.; Arroyo, A. Metastasizing pleomorphic adenoma: A fascinating enigma. Case Rep. Med. 2012, 148103. [Google Scholar] [CrossRef] [PubMed]
- Soltedo, J.; Aranaga, N. Metastasizing pleomorphic adenoma of the parotid gland. Ecancermedicalscience 2017, 11, 758. [Google Scholar]
- Catarzi, L.; Catarzi, L.; Gabriele, G.; Gabriele, G.; Pulli, B.; Pulli, B.; Cascino, F.; Cascino, F.; Gennaro, P.; Gennaro, P. Metastasizing Pleomorphic Adenoma of Parotid Gland: A Case Report and Literature Review. Indian J. Otolaryngol. Head. Neck Surg. 2024, 76, 1123–1125. [Google Scholar] [CrossRef]
- Nakai, A.; Suzuki, K.; Furuse, H.; Tsuda, T.; Masaki, Y.; Shinno, H.; Ito, Y.; Miyazawa, H.; Taniguchi, H. Multiple Metastasizing Pleomorphic Adenomas of the Lung. Intern. Med. 2017, 56, 691–694. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parums, D.V. Editorial: Review Articles, Systematic Reviews, Meta-Analysis, and the Updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 Guidelines. Med. Sci. Monit. 2021, 27, e934475. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aluffi Valletti, P.; Campagnoli, M.; Dell’Era, V.; Garzaro, M.; Boffano, P.; Neirotti, F.; Mazzer, A.M.; Brucoli, M. Oral and oropharyngeal malignant minor salivary gland tumors: A retrospective study. J. Stomatol. Oral. Maxillofac. Surg. 2024, 125, 101893. [Google Scholar] [CrossRef] [PubMed]
- Varghese, B.T.; Sebastian, P.; Abraham, E.K.; Mathews, A. Pleomorphic adenoma of minor salivary gland in the parapharyngeal space. World J. Surg. Oncol. 2003, 1, 2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sarmento, D.J.; Morais, M.L.; Costa, A.L.; Silveira, É.J. Minor intraoral salivary gland tumors: A clinical-pathological study. Einstein 2016, 14, 508–512. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hiyama, T.; Kuno, H.; Sekiya, K.; Oda, S.; Kobayashi, T. Imaging of Malignant Minor Salivary Gland Tumors of the Head and Neck. Radiographics 2021, 41, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Tondi-Resta, I.; Hobday, S.B.; Gubbiotti, M.A.; Jalaly, J.B.; Rassekh, C.H.; Montone, K.T.; Baloch, Z.W. Carcinoma Ex Pleomorphic Adenomas: An Institutional Experience and Literature Review. Am. J. Clin. Pathol. 2023, 159, 502–515. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.E.; Olsen, K.D.; Sebo, T.J. Carcinoma ex pleomorphic adenoma: Pathologic analysis of 73 cases. Hum. Pathol. 2001, 32, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Quereshi, A.; Barakzai, A.; Ui, N.; Gulzar, R.; Ahmad, Z.; Hasan, S. Spectrum of maignacy in mixed tumors nof salivary gland: A morphological and immunohistochemical review of 23 cases. Ind. J. Pathol. Microbiol. 2009, 52, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Klijanienko, J.; El Naggar, A.K.; Servois, V.; Rodriguez, J.; Validire, P.; Viehl, P. Mucoepidermoid carcinoma ex pleomorphic adenoma: Nonspecific preoperative cytologic findings in six cases. Cancer 1998, 84, 231–234. [Google Scholar] [CrossRef]
- Nishida, H.; Kusaba, T.; Kawamura, K.; Oyama, Y.; Daa, T. Histopathological Aspects of the Prognostic Factors for Salivary Gland Cancers. Cancers 2023, 15, 1236. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toluie, S.; Thompson, L.D.R. Sinonasal tract adenoid cystic carcinoma ex-pleomorphic adenoma: A clinicopathologic and immunophenotypic study of 9 cases combined with a comprehensive review of the literature. Head Neck Pathol. 2012, 6, 409–421. [Google Scholar] [CrossRef][Green Version]
- Iino, M.; Yamada, H.; Ishikawa, H.; Suzuki, M.; Shomura, E.; Ide, F.; Saito, I.; Mori, Y. Carcinoma ex pleomorphic adenoma of the submandibular gland: Report of a case with unusual malignant component of clear squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol Oral Radiol. Endod. 2008, 106, e30–e34. [Google Scholar] [CrossRef]
- Karpowicz, M.K.; Shalmon, B.; Molberg, K.H.; El-Naggar, A.K. Melanoma in a carcinoma ex pleomorphic adenoma of the parotid gland: A case report and putative histogenesis. Hum. Pathol 2011, 42, 1355–1358. [Google Scholar] [CrossRef]
- Krishna, P.S.; Sivapathasudharam, B. Acinic cell adenocarcinoma-a case report. J. Oral Maxillofac. Pathol. 2003, 7, 15–18. [Google Scholar] [CrossRef]
- Tawfik, O.; Namiq, A. Tumors of the salivary glands. In Cancer Grading Manual; Damjanov, I., Fang, F., Eds.; Springer: New York, NY, USA, 2007; pp. 13–18. [Google Scholar]
- Curran, A.E.; Allen, C.M.; Beck, F.M.; Damm, D.D.; Murrah, V.A. Distinctive pattern of glial fibrillary acidic protein immunoreactive useful in distinguishing fragmented pleomorphic adenoma, canalicular adenoma and polymorphous low grade adenocarcinoma of minor salivary glands. Head Neck Pathol. 2007, 1, 27–32. [Google Scholar] [CrossRef]
- Santos, G.C.; Carvalho, K.C.; Falzoni, R.; Simoes, A.C.; Rocha, R.M.; Lopes, A.; Vassallo, J.; Reis, L.F.; Soares, F.A.; da Cunha, I.W. Glial fibrillary acidic protein in tumor types with cartilaginous differentiation. Mod. Pathol. 2009, 22, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Nagao, T.; Sato, E.; Inoue, R.; Oshiro, H.; H Takahashi, R.; Nagai, T.; Yoshida, M.; Suzuki, F.; Obikane, H.; Yamashina, M.; et al. Immunohistochemical analysis of salivary gland tumors: Application for surgical pathology practice. Acta Histochem. Cytochem. 2012, 45, 269–282. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bussari, S.; Ganvir, S.M.; Sarode, M.; Jeergal, P.A.; Deshmukh, A.; Srivastava, H. Immunohistochemical Detection of Proliferative Marker Ki-67 in Benign and Malignant Salivary Gland Tumors. J. Contemp. Dent. Pract. 2018, 19, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Faur, A.C.; Buzaș, R.; Lăzărescu, A.E.; Ghenciu, L.A. Current Developments in Diagnosis of Salivary Gland Tumors: From Structure to Artificial Intelligence. Life 2024, 14, 727. [Google Scholar] [CrossRef]
- Żurek, M.; Fus, Ł.; Niemczyk, K.; Rzepakowska, A. Salivary gland pathologies: Evolution in classification and association with unique genetic alterations. Eur. Arch. Oto-Rhino-Laryngol. 2023, 280, 4739–4750. [Google Scholar] [CrossRef]
- Valstar, M.H.; Mast, H.; Ten Hove, I.; Moonen, L.R.; Balm, A.J.; Smeele, L.E.; Koljenović, S.; Dinjens, W.N.; van Velthuysen, M.F. Malignant transformation of salivary gland pleomorphic adenoma: Proof of principle. J. Pathol. Clin. Res. 2021, 7, 432–437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- LiVolsi, V.A.; Perzin, K.H. Malignant mixed tumors arising in salivary glands. I. Carcinomas arising in benign mixed tumors: A clinicopathologic study. Cancer 1977, 39, 2209–2230. [Google Scholar] [CrossRef] [PubMed]
- Tortoledo, M.E.; Luna, M.A.; Batsakis, J.G. Carcinomas ex pleomophic adenoma and malignant mixed tumors: Histomorphologic indexes. Arch. Otolaryngol 1984, 110, 172–176. [Google Scholar] [CrossRef]
- Brandwein, M.; Huvos, A.G.; Dardick, I.; Thomas, M.J.; Theise, N.D. Noninvasive and minimally invasive carcinoma ex mixed tumor: A clinicopathologic and ploidy study of 12 patients with major salivary tumors of lo (or no?) malignant potential. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1996, 81, 655–664. [Google Scholar] [CrossRef]
- Speight, P.M.; Barett, A.W. Prognostic factors in malignant tumours of the salivary glands. Br. J. Oral Maxillofac. Surg. 2009, 47, 587–593. [Google Scholar] [CrossRef]
- Speight, P.M.; Barett, A.W. Salivary gland tumors: Diagnostic challenges and an update on the latest WHO classification. Diagn. Histopathol. 2020, 26, 147–158. [Google Scholar] [CrossRef]
- Seethala, R.R.; Altemani, A.; Ferris, R.L.; Fonseca, I.; Gnepp, D.R.; Ha, P.; Nagao, T.; Skalova, A.; Stenman, G.; Thompson, L.D.R. Data Set for the Reporting of Carcinomas of the Major Salivary Glands: Explanations and Recommendations of the Guidelines from the International Collaboration on Cancer Reporting. Arch. Pathol. Lab. Med. 2019, 143, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Ettl, T.; Schartz-Furlan, S.; Gosau, M.; Reichert, T. Salivary gland carcinomas. Oral Maxillofac. Surg. 2012, 16, 267–283. [Google Scholar] [CrossRef]
- Felix, A.; Rosa, J.C.; Nunes, J.F.; Fonseca, I.; Cidado, A.; Soares, J. Hyalinizing clear cell carcinoma of salivary glands: A study of extracellular matrix. Oral Oncol. 2002, 38, 364–368. [Google Scholar] [CrossRef]
- Sheedy, S.P.; Welker, K.M.; DeLone, D.R.; Gilbertson, J.R. CNS metstasis of carcinoma ex pleomorphic adenoma of the parotid gland. Am. J. Neuroradiol. 2006, 27, 1483–1485. [Google Scholar]
- Peel, R.L.; Seethala, R.R. Pathology of salivary glands. In Salivary Glands Disorders; Myers, E., Ferris, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Chapter 3; pp. 33–104. [Google Scholar]
- Keh, S.M.; Tait, A.; Ahsan, F. Primary carcinosarcoma of the parotid gland. Clin. Pract. 2011, 1, e117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Woo, C.G.; Son, S.M. Carcinosarcoma of the parotid gland with abdominal metastasis: A case report and review of literature. World J. Surg. Oncol. 2018, 16, 103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gnepp, D.R. Malignant mixed tumors of the salivary glands: A review. Pathol. Annu. 1993, 28, 279–328. [Google Scholar]
- Kwon, M.Y.; Gu, M. True Malignant mixed tumor (carcinosarcoma) of the parotid gland with unusual Mesenchymal component. A case report and review of the literature. Arch. Pathol. Lab. Med. 2001, 125, 812–815. [Google Scholar] [CrossRef]
- Geraldes Filho, J.C.; Carvalho, L.G.M.; Pinheiro, N.F., Jr.; de Freitas, L.A.R.; Fontes Athanazio, P.R.; Abensur Athanazio, D. Carcinosarcoma of the parotid gland featuring foci of malignant giant cell tumor. J. Bras. Pathol. Med. Lab. 2012, 48, 129–134. [Google Scholar] [CrossRef]
- Jang, S.E.; Jun, Y.J.; Han, H.; Jang, K.S.; Paik, S.S. Parotid gland carcinosarcoma with osteosarcoma as sarcomatous component: A case report with fine needle aspiration cytologic findings. Korean J. Pathol. 2011, 45, 412–416. [Google Scholar] [CrossRef]
- Ghenciu, L.A.; Hațegan, O.A.; Bolintineanu, S.L.; Dănilă, A.-I.; Faur, A.C.; Prodan-Bărbulescu, C.; Stoicescu, E.R.; Iacob, R.; Șișu, A.M. Immune-Mediated Ocular Surface Disease in Diabetes Mellitus—Clinical Perspectives and Treatment: A Narrative Review. Biomedicines 2024, 12, 1303. [Google Scholar] [CrossRef] [PubMed]
- Septimiu-Radu, S.; Gadela, T.; Gabriela, D.; Oancea, C.; Rosca, O.; Lazureanu, V.E.; Fericean, R.M.; Bratosin, F.; Dumitrescu, A.; Stoicescu, E.R.; et al. A Systematic Review of Lung Autopsy Findings in Elderly Patients after SARS-CoV-2 Infection. J. Clin. Med. 2023, 12, 2070. [Google Scholar] [CrossRef]
- Georgescu, D.; Ancusa, O.E.; Azoulay, D.; Lascu, A.; Ionita, I.; Calamar-Popovici, D.; Ionita, M.; Rosca, C.I.; Brează, G.M.; Reisz, D.; et al. Portal Vein Thrombosis in Patients with Liver Cirrhosis: What Went Wrong? Int. J. Gen. Med. 2023, 16, 3889–3906. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Colizza, A.; Di Stadio, A.; Ralli, M.; De Luca, P.; Cavaliere, C.; Gilardi, A.; Zoccali, F.; Riminucci, M.; Greco, A.; Corsi, A.; et al. Systematic Review of Parotid Gland Sarcomas: Multi-Variate Analysis of Clinicopathologic Findings, Therapeutic Approaches and Oncological Outcomes That Affect Survival Rate. Cancers 2022, 14, 4862. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vékony, H.; Leemans, C.R.; Ylstra, B.; Meijer, G.A.; van der Waal, I.; Bloemena, E. Salivary gland carcinosarcoma: Oligonucleotide array CGH reveals similar genomic profiles in epithelial and mesenchymal components. Oral Oncol. 2009, 45, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Matsumiya-Matsumoto, Y.; Morita, Y.; Uzawa, N. Pleomorphic Adenoma of the Salivary Glands and Epithelial-Mesenchymal Transition. J. Clin. Med. 2022, 11, 4210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manucha, V.; Ioffe, O.B. Metastasizing pleomorphic adenoma of the salivary gland. Arch. Pathol. Lab. Med. 2008, 132, 1445–1447. [Google Scholar] [CrossRef]
- Watson, M.; McAllister, P.; Conn, B.; MacNeill, M.; Handley, T.P.B. Metastasising Pleomorphic Salivary Adenoma: A Rare Case Report of a Massive Untreated Minor Salivary Gland Pleomorphic Adenoma with Concurrent Ipsilateral Cervical Node Metastases. Head. Neck Pathol. 2019, 13, 500–506. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nouraei, S.A.R.; Ferguson, M.S.; Clarke, P.M.; Sandison, A.; Sadhu, G.S.; Michaels, L.; Rhys-Evans, P. Metastasizing pleomorphic salivary adenoma. Arch. Otolaryngol. Head. Neck Surg. 2006, 137, 788–793. [Google Scholar] [CrossRef]
- Manelli, G.; Rucci, L.; Gallo, O. Unusual metastases from a pleomorphic adenoma of the parotid gland: A case and literature review. Int. J. Otolaryngol. Head Neck Surg. 2013, 2, 232–235. [Google Scholar] [CrossRef][Green Version]
- Khan, M.N.; Raza, S.S.; Hussain Zaidi, S.A.; Haq, I.U.; Hussain, A.K.; Nadeem, M.D.; Farid, K. Pleomorphic Adenoma of Minor Salivary Glands. J. Ayub Med. Coll. Abbottabad 2016, 28, 620–622. [Google Scholar] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faur, A.C.; Șișu, A.M.; Ghenciu, L.A.; Iacob, R.; Stoicescu, E.R.; Hațegan, O.A.; Cornianu, M. Clinical and Morphological Aspects of Aggressive Salivary Gland Mixed Tumors: A Narrative Review. Diagnostics 2024, 14, 1942. https://doi.org/10.3390/diagnostics14171942
Faur AC, Șișu AM, Ghenciu LA, Iacob R, Stoicescu ER, Hațegan OA, Cornianu M. Clinical and Morphological Aspects of Aggressive Salivary Gland Mixed Tumors: A Narrative Review. Diagnostics. 2024; 14(17):1942. https://doi.org/10.3390/diagnostics14171942
Chicago/Turabian StyleFaur, Alexandra Corina, Alina Maria Șișu, Laura Andreea Ghenciu, Roxana Iacob, Emil Robert Stoicescu, Ovidiu Alin Hațegan, and Mărioara Cornianu. 2024. "Clinical and Morphological Aspects of Aggressive Salivary Gland Mixed Tumors: A Narrative Review" Diagnostics 14, no. 17: 1942. https://doi.org/10.3390/diagnostics14171942
APA StyleFaur, A. C., Șișu, A. M., Ghenciu, L. A., Iacob, R., Stoicescu, E. R., Hațegan, O. A., & Cornianu, M. (2024). Clinical and Morphological Aspects of Aggressive Salivary Gland Mixed Tumors: A Narrative Review. Diagnostics, 14(17), 1942. https://doi.org/10.3390/diagnostics14171942








